Abstract
This experiment aimed to evaluate the capacities of two types of chitooligosaccharides (COS) with different molecular weights for the ability to eliminate lipid accumulation in hepatocytes. We have established a lipid accumulation model in HepG2 cells for these studies in vitro, which was established by induction with oleic acid. The capacity of COS to eliminate lipid accumulation was evaluated using three metrics: the thiazolyl blue dye absorbance (MTT value), the morphology of intracellular lipid droplets and the triglyceride level (TG). Two types of COS with different molecular weights (1000 Da and 3000 Da) can significantly reduce intracellular lipid accumulation and decrease TG content in HepG2 cells, in a dose-dependent fashion. We found that low molecular weight COS is more efficacious than high molecular weight COS. Two types of COS can eliminate lipid accumulation induced by oleic acid in HepG2 cells, leading to an obvious hypolipidemic effect in vitro. These results suggest that COS may be effective preventive agents in fatty liver disease.
Keywords: Chitooligosaccharides, Oleic acid, Lipid accumulation, HepG2 cells
1. Introduction
Fatty liver diseases are divided into two categories: alcoholic fatty liver disease (AFLD) and nonalcoholic fatty liver disease (NAFLD) (Montilla et al., 2013). NAFLD is more common in clinical practice and can seriously impact the quality of a patient’s life. The current clinical treatments for NAFLD include insulin sensitization agents, lipids, diet pills, antihypertensive agents, cell protective agents, anti-inflammatory cytokine antioxidants and other types (McCullough, 2004, Cordeiro et al., 2015). In recent years, with improvements of the living standards and the changes of the diet structure of the people, the incidence of NAFLD has increased year by year, but the pathogenesis is not entirely clear. Drug treatment is not perfect; therefore, there is an urgent need for new drugs that can enhance prevention and treatment of NAFLD (Torres et al., 2012).
Chitosan (CTS), a linear biopolymer comprising glucosamine and N-acetyl-glucosamine residues, is an N-deacetylated product of chitin and one of the most abundant polysaccharides in nature (Lin et al., 2007). CTS is non-toxic, biocompatible, biodegradable and highly soluble in acidic solutions, and it has been widely used in the chemical industry, environmental protection, food, pharmaceutics, medicine, cosmetics, agriculture and many other fields (Lin et al., 2007, Qin et al., 2006). However, its high molecular weight has limited its applications. Fortunately, CTS can be hydrolyzed enzymatically by specific chitosanases and non-specific enzymes. The degradation products of CTS, COS, not only can be used as drug carrier (Tan et al., 2014, Chen et al., 2013), but also has better biological activity owing to the rise in the water soluble (Chae et al., 2005). COS has also been implicated in many biological activities, such as lowering blood lipids, controlling blood pressure and blood glucose, and producing other antidiabetic actions (Choi et al., 2012a, Choi et al., 2012b, Ju et al., 2010). In addition, COS has been also widely used in the field of functional food (Je and Kim, 2012, Choi et al., 2012a, Choi et al., 2012b). Increasing researchers have devoted themselves to the study on the molecular mechanisms on the various physiological functions of COS and related products aforesaid, especially its anti-obesity and hypolipidemic capacity (Huang et al., 2015, Zhang et al., 2011, Zhang et al., 2012, Tao et al., 2013). However, there has been very little research on the use of COS for NAFLD; therefore, the goal of this study was to simulate the pathological characteristics of clinical NAFLD. Oleic acid-induction of HepG2 cells leads to a significant increase in lipid droplets. This system can thus be used as a lipid accumulation model. The actions of two types of COS with different molecular weights are evaluated for their efficacy at reducing intracellular lipid accumulation. This study can be used to test their potential value in the prevention and treatment of fatty liver disease.
2. Materials and methods
2.1. Materials
The human hepatocellular carcinoma cell line HepG2 was provided by the Institute of Chinese Medical Science of Guangdong Pharmaceutical University. Drugs and reagents used in this study include the following: Chitooligosaccharides I and II with average molecular weights of 1000 and 3000, Shandong Laizhou Haili Biological Products Co. Ltd., Shangdong, China, GIBCO Dulbecco’s Modified Eagle Medium (DMEM), Invitrogen Co. Ltd., New York, American, Fetal bovine serum (FBS), Hyclone Co. Ltd., Utah, American, Trypsin, Amresco Co. Ltd., Ohio, American, Sodium oleate, Tokyo chemical industry Co. Ltd., Tokyo, Japan, Oil red O staining solution, Zhuhai Beisuo Biological Technology Co. Ltd., Guangdong, China, Triglyceride kits, Biosino Biotechnology & Science Inc., Beijing, China, and Thiazolyl blue tetrazolium bromide (MTT), Sigma Co. Ltd., San Francisco, American. All other reagents and solvents were of analytical reagent grade.
2.2. Preparation of drug solution
Preparation of the induction solution for lipid accumulation was performed as follows: sodium oleate was dissolved in phosphate buffer salt (PBS) to produce a 50 mmol L−1 stock solution using a shocking water bath at 55 °C, and then dropped into PBS containing 10% FBS for a 5 mmol L−1 working solution. The solution was then filtered through the 0.2 μm to remove bacteria in the solution and cryopreserved at 4 °C.
Preparation of COS I solution was performed as follows: COS I was dissolved in PBS to produce a 100 mg ml−1 stock solution, and then dropped into PBS containing 10% fetal bovine serum to produce a 10 mg ml−1 working solution. The solution was then filtered to remove bacteria in the solution and cryopreserved at 4 °C.
Preparation of the COS II solution was performed in the same manner as the COS I solution.
2.3. HepG2 cell culture
HepG2 cells were cultured using 13.5 g L−1 DMEM culture medium containing high glucose, 10% fetal bovine serum and double-antibiotic solution (penicillin 100 μmol·ml−1 and streptomycin 100 μg ml−1) in an incubator providing 5% CO2 at 37 °C. Subculture or subsequent trials were conducted when cell confluence reached 80–90% (Nair et al., 2014).
2.4. HepG2 cell viability with the MTT method
HepG2 cells were inoculated into 96-well culture plates with 1.0 × 104 cells per well and cultured immediately. After 24 h incubation in an incubator containing 5% CO2 at 37 °C, the culture medium was replaced and injected with COS I and COS II at different concentrations. The final concentrations were 1.0 × 107, 3.0 × 106, 1.0 × 106, 3.0 × 105, 1.0 × 105, 3.0 × 104, 1.0 × 104, and 3.0 × 103 μg L−1, and were prepared alongside the no-induction and no-COS control groups. The final volumes of the six parallel wells in each group were 200 μl. After 24 h incubation in an incubator containing 5% CO2 at 37 °C, the experiment was conducted according to the following steps: 20 μl of 0.5 mg mL−1 MTT solution was injected into each well of 96-well culture plates and incubated for 4 h in the dark, culture medium was removed, 150 μl of DMSO was injected into each well of a 96-well culture plate, the plates were simultaneously shocked on the cell oscillator, the absorbance was read at 485 nm (OD485), and the cell survival rate (CSR) was calculated on the basis of OD485 to screen the suitable concentration range of the COS I and COS II (Mohd et al., 2014).
Aa, As and A0 represent the absorbance of the experiment group, no-COS control group and no-induction control group, respectively, in the formula.
2.5. Oil red O staining for HepG2 cells
HepG2 cells were subcultured with 2.5 × 105 cells per well in six-well culture dishes following 24 h incubation in the 37 °C incubator containing 5% CO2, and then were subsequently divided into nine groups (with six parallel wells in each group) as the blank control group, model group, berberine (Ber) control group due to its prevention of lipid accumulation in vitro and in vivo demonstrated in many studies (Zhou et al., 2014, Hu et al., 2012), and COS I and COS II groups with high (3.0 × 105 μg L−1), middle (1.0 × 105 μg L−1) and low (3.0 × 104 μg L−1) concentrations, respectively. The ingredients in each group are shown as follows:
Blank control group: high glucose DMEM culture medium containing 10% FBS.
Model group: high glucose DMEM culture medium containing 10% FBS and oleic acid with a final concentration of 0.2 mmol L−1.
Ber control group: high glucose DMEM culture medium containing 10% FBS, oleic acid and Ber with a final concentration of 0.2 mmol L−1 and 10 μmol L−1, respectively.
COS I group: high glucose DMEM culture medium containing 10% FBS and oleic acid with a final concentration of 0.2 mmol L−1, COS I with final concentration of 3.0 × 105 (high concentration), 1.0 × 105 (middle concentration) and 3 × 104 (low concentration) μg L−1.
COS II group: high glucose DMEM culture medium containing 10% FBS and oleic acid with a final concentration of 0.2 mmol L−1, COS II with final concentration of 3.0 × 105 (high concentration), 1.0 × 105 (middle concentration), 3.0 × 104 (low concentration) μg L−1.
After 24 h incubation, the culture medium was removed, and the experiment was conducted according to the following steps: 1 ml PBS was injected into each well and wells were then rinsed three times repeatedly, 500 μl of 4% paraformaldehyde was injected into each well for a 20 min fixation and then cleaned with 1 ml PBS three times repeatedly, 500 μl of Oil red O working solution was injected into each well and stained for 15 min in room temperature, 60% isopropanol was injected into each well for 1 min fixation and wells were cleaned with three rinses of isopropanol to produce a white transparent background, cell morphology and the distribution of lipid droplets were observed with the inverted microscope, and recorded as pictures, 500 μl of 60% isopropanol was injected into each well and incubated for 15 min at room temperature, after the extraction, 200 μl of leaching liquor was injected into 96-well culture plates, and absorbance of each well was determined at 485 nm (Kim et al., 2013).
2.6. The determination of the intracellular TG
HepG2 cells were inoculated into 24-well culture plates at 5.0 × 104 cells per well and taken out after 24 h of incubation to set up the experimental groups as the blank control group, model group, Ber control group, and COS I and COS II groups with three different concentrations, 3.0 × 105, 1.0 × 105, and 3.0 × 104 μg L−1, respectively, the ingredients in each group refer to 2.4. After 24 h incubation of the 24-well culture plates as described above, the culture solution was removed, they were cleaned with precooled PBS for three times and then blotted up, RIPA lysate (107 cell ml−1) was injected in each group to produce sufficient lysis, the pyrolysis products of cells were scraped gently with cell scrapers and transferred into 1.5 ml Eppendorf (EP) tubes, respectively, they were shocked for 30 min and simultaneously centrifuged for 15 min (12,000 r min−1) at 4 °C, supernatant was collected, 10 μl supernatant in each EP tube was used for protein quantification by the BCA protein concentration determination kit method, the remaining supernatant was extracted by methanol-chloroform (V/V = 2:1), allowed to stand for 30 min, centrifuged for 15 min (12,000 r min−1) at 4 °C, the supernatant solution was removed and the bottom solution dried at 70 °C, 20 μl of PBS was injected for the dissolution of lipid (Kuo et al., 2012), and intracellular concentration of TG was determined according to the instructions of triglyceride determination kits.
2.7. Statistical analysis
The experimental data are represented as average value ± standard deviation (X ± S) and analyzed by SPSS 17.0 statistical software. The significance analysis is conducted by one-way ANOVA, with the statistical significance assigned to P < 0.05.
3. Results
3.1. Toxicological evaluation of COS I and COS II to HepG2 cells
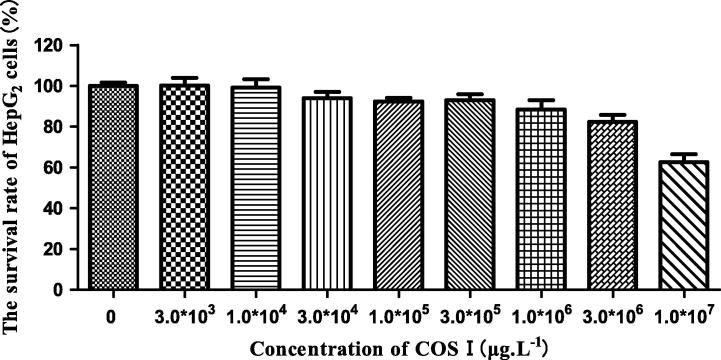
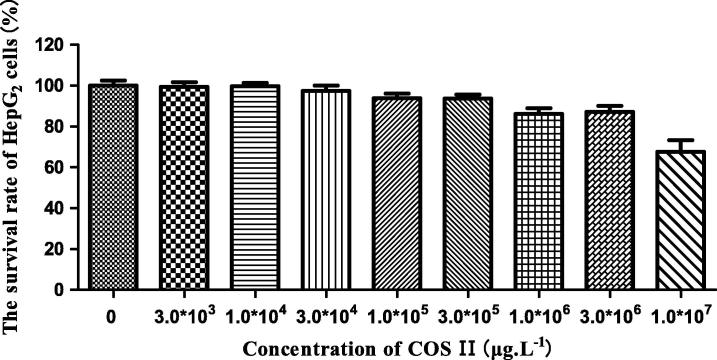
COS I and COS II elicit some cytotoxicity at high concentrations. We explored the effects of COS I and COS II in different concentrations on normal HepG2 cell viability with the MTT method to choose a drug concentration characterized by the least toxicity to the cells. The results are shown in Figure 1, Figure 2. The cell viability decreases gradually with an increased concentration of COS I and COS II, suggesting that COS I and COS II in high concentration have some toxicity to HepG2 cells. COS I and COS II have the least toxicity to HepG2 cells at concentrations less than 3.0 × 105 μg L−1. At this concentration, the cell viability is more than 90%, indicating that the concentration of COS I and COS II should be less than 3.0 × 105 μg L−1 (The high, middle and low concentrations of COS I and COS II are 3.0 × 105 μg L−1, 1.0 × 105 μg L−1 and 3.0 × 104 μg L−1, respectively).
Figure 1.
Effects of COS I on proliferation of HepG2 cells.
Figure 2.
Effects of COS II on proliferation of HepG2 cells.
3.2. HepG2 cell morphology and oil red O staining
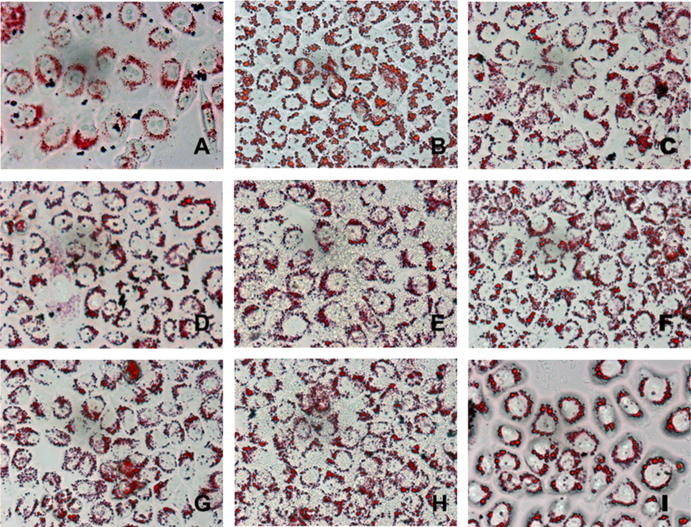
The results of oil red O staining for HepG2 cellular morphology by the inverted microscope are displayed in Fig. 3, and HepG2 cells in the induced group contain a large number of red lipid droplets fused to each other, indicating that the HepG2 cell lipid accumulation model is established successfully. HepG2 red lipid droplets show a dose-dependent trend that is characterized by decreasing number, decreasing volume and shallow color. Compared with the induced group, the COS I and COS II middle and low concentration groups show obviously improved lipid accumulation and alleviated lipid fusion in the HepG2 cells. The lipid characteristics of the middle groups are comparable to those of the Ber positive group. The COS I and COS II high concentration groups show the effects described above even more obviously.
Figure 3.
Oil red O staining maps of HepG2 cells (10 × 40). (A) Stained control group cells, (B) Stained induced group cells, (C) Stained 10 μmol L−1 berberine-positive cells, (D) Stained 3.0 × 105 μg L−1 COS I-treated cells, (E) Stained 1.0 × 105 μg L−1 COS I-treated cells, (F) Stained 3.0 × 104 μg L−1 COS I-treated cells, (G) Stained 3.0 × 105 μg L−1 COS II-treated cells, (H) Stained 1.0 × 105 μg L−1 COS II-treated cells, (I) Stained 3.0 × 104 μg L−1 COS II-treated cells.
3.3. The effects of COS I and COS II in different concentrations on the HepG2 lipid accumulation model
The absorbances for HepG2 cells in each group are shown below (Fig. 4) after the process of oil red O staining and extraction with isopropanol. It is observed that there is a significantly increasing lipid accumulation in HepG2 cells in the model group compared with blank group (P < 0.001). Therefore, the effects of COS I and COS II in the high and middle concentration groups on the reduction in lipid accumulation are comparable to those of the Ber-positive control group.
Figure 4.
Effect of different experiments on lipid accumulation in HepG2 cells.
3.4. The effects of COS I and COS II in different concentration on the content of TG in HepG2 lipid accumulation models
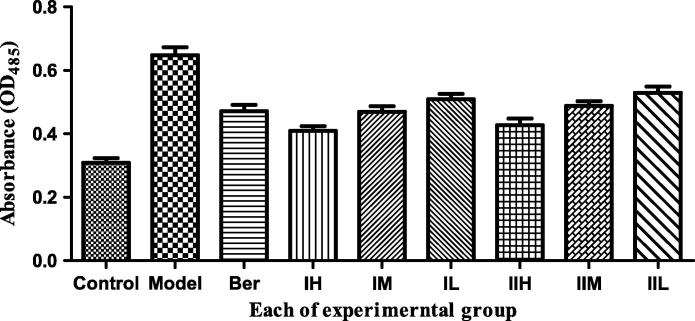
We observed the effects of COS I and COS II in the high, middle and low concentration groups (which are less toxic to HepG2 cells) on the content of TG in the HepG2 lipid accumulation model (Figure 3, Figure 5).
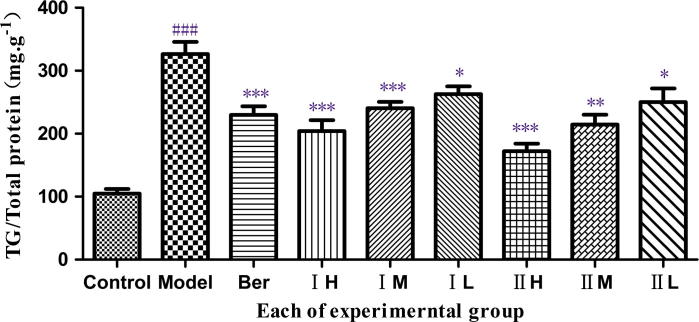
Figure 5.
Effect of COS I and COS II on the content of TG. Note: COS I and COS II groups with high (3.0 × 105 μg L−1), middle (1.0 × 105 μg L−1) and low (3.0 × 104 μg L−1) concentrations, which also can be called IH, IM, IL and IIH, IIM, IIL for short respectively. ###P < 0.001, compared with the control group, *P < 0.05, **P < 0.01, ***P < 0.001, compared with the model group.
There is a significantly increasing content of TG in the model group compared with the blank control group (P < 0.001), suggesting that the lipid accumulation model in HepG2 cells is established successfully. Compared with model groups, COS II in the high and middle concentrations and COS I in the high concentrations extremely decrease the TG content (P < 0.001). An extremely significant difference exists between COS I at the middle concentration and the model group (P < 0.01) and between the COS I, the COS II and the model groups. Notably, the decrease in TG accumulation is linearly correlated with an increasing concentration of COS I and COS II in the range from 3.0 × 104 to 3.0 × 105 μg L−1. Furthermore, the two COS have the strongest suppressive effects on TG content in HepG2 cells at 1.0 × 105 μg L−1.
4. Conclusion and discussion
NAFLD has gradually become a widespread and serious disease since the first official report of NAFLD in 1958, and the average incidence of NAFLD is approximately 20%, with a yearly increase. As the leading cause of hepatic dysfunction worldwide, the typical characteristic of NAFLD is intracellular lipid accumulation in hepatocytes (Ogawa et al., 2013). The main lipid accumulating in liver of NAFLD patients is TG, and its accumulation results from an imbalance between its synthesis and transformation (Sun et al., 2015, Rouabhia et al., 2014). Genetic factors combined with the external environment and metabolic stress result in the pathogenesis of NAFLD, and the overwhelming theory for the molecular mechanism of NAFLD is the ‘two-hit theory’ as shown in a recent study (Ogawa et al., 2013, Sun et al., 2015, Rouabhia et al., 2014). The ‘first’ hit of the ‘two-hit theory’ is the lipid accumulation in the liver. Fatty degeneration of hepatocytes is more susceptible to ‘second’ hit (oxidative stress, etc) than normal one, which further promote the generation of inflammation that is the development of NAFLD. Based on this theory, the ‘first’ hit becomes an important target for prevention and treatment of NAFLD. Considering from this target, the experiment simulates lipid accumulation in hepatic cells, and then investigates the effect of drugs on the lipid accumulation model, which aims to screen the active substances in the prevention and treatment of NAFLD.
HepG2 cells have been successfully used for the establishment of a fatty liver cell model and because of their stable cell characteristics such as ease of cultivation, these cells can be used to screen for preventive and therapeutic drugs and to explore fatty liver pathogenesis (Kim et al., 2013, Kuo et al., 2012). Long-chain fatty acids can form into lipid droplets in cells, which lead to lipid accumulation (Montilla et al., 2013). As the foremost component of long-chain free fatty acids, oleic acid can be selected for the establishment of a lipid accumulation model in hepatocytes, which relates to the pathophysiological process of NAFLD (Torres et al., 2012, Chen et al., 2015). The establishment of a hepatocyte lipid accumulation model can be achieved through induction by 1 mmol L−1 oleic acid for 24 h in HepG2 cells. After induction by oleic acid (0.1–2 mmol L−1) in a series of gradient concentrations in HepG2 cells, the level of intracellular lipid accumulation is positively correlated with oleic acid concentration.
For this study, we chose oleic acid as the 24 h continuous inducer for the establishment of lipid accumulating model, which can effectively simulate the pathological process of NAFLD, and we evaluated the roles of COS I and COS II in eliminating lipid accumulation in hepatocytes by detecting the conventional indexes of NAFLD such as the content of TG and lipid droplets in hepatocytes, simultaneously. The results show that the inhibition of TG accumulation by COS I and COS II in the high-dose groups is the most remarkable, with significant dose-dependent responses. Consequently, COS I and COS II present a potential medicinal value in the prevention and treatment of fatty liver. Nevertheless, the molecular mechanisms of inhibiting lipid accumulation in response to COS I and COS II have not been confirmed and will require further exploration in the future.
Acknowledgments
This project was financially supported by the National Science Foundation of China (no. 81173107), the Science and Technology Planning Project of Guangdong, China (2013B021100018, 2013B090600050, 2015A010101318).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Jiao Guo, Email: wshxalb@163.com.
Zhengquan Su, Email: suzhq@scnu.edu.cn.
References
- Chae S.Y., Jang M.K., Nah J.W. Influence of molecular weight on oral absorption of water soluble chitosans. J. Control. Release. 2005;102(2):383–394. doi: 10.1016/j.jconrel.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Chen J., Huang G.D., Tan S.R., Guo J., Su Z.Q. The preparation of capsaicin-chitosan microspheres (CCMS) enteric coated tablets. Int. J. Mol. Sci. 2013;14(12):24305–24319. doi: 10.3390/ijms141224305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Xu M., Wang T. Advanced fibrosis associates with atherosclerosis in subjects with nonalcoholic fatty liver disease. Atherosclerosis. 2015;241(1):145–150. doi: 10.1016/j.atherosclerosis.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Choi C.R., Kim E.K., Kim Y.S., Je J.Y., An S.H., Lee J.D., Wang J.H., Ki S.S., Jeon B.T., Moon S.H., Park P.J. Chitooligosaccharides decreases plasma lipid levels in healthy men. Int. J. Food Sci. Nutr. 2012;63(1):103–106. doi: 10.3109/09637486.2011.602051. [DOI] [PubMed] [Google Scholar]
- Choi E.H., Yang H.P., Chun H.S. Chitooligosaccharide ameliorates diet-induced obesity in mice and affects adipose gene expression involved in adipogenesis and inflammation. Nutr. Res. 2012;32(3):218–228. doi: 10.1016/j.nutres.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Cordeiro A., Pereira S.E., Saboya C.J., Ramalho A. Nonalcoholic fatty liver disease relationship with metabolic syndrome in class III obesity individuals. Biomed Res. Int. 2015;2015:839253. doi: 10.1155/2015/839253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Ehli E.A., Kittelsrud J. Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine. 2012;19(10):861–867. doi: 10.1016/j.phymed.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Huang L., Chen J., Cao P., Pan H., Ding C., Xiao T., Zhang P., Guo J., Su Z. Anti-obese effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Mar. Drugs. 2015;13(5):2732–2756. doi: 10.3390/md13052732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Je J.Y., Kim S.K. Chitooligosaccharides as potential nutraceuticals: production and bioactivities. Adv. Food Nutr. Res. 2012;65:321–336. doi: 10.1016/B978-0-12-416003-3.00021-4. [DOI] [PubMed] [Google Scholar]
- Ju C., Yue W., Yang Z., Zhang Q., Yang X., Liu Z., Zhang F. Antidiabetic effect and mechanism of chitooligosaccharides. Biol. Pharm. Bull. 2010;33(9):1511–1516. doi: 10.1248/bpb.33.1511. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Kang S.I., Shin H.S. Sasa quelpaertensis and p-coumaric acid attenuate oleic acid-induced lipid accumulation in HepG2 cells. Biosci. Biotechnol. Biochem. 2013;77(7):1595–1598. doi: 10.1271/bbb.130167. [DOI] [PubMed] [Google Scholar]
- Kuo Y.T., Lin T.H., Chen W.L., Lee H.M. Alpha-lipoic acid induces adipose triglyceride lipase expression and decreases intracellular lipid accumulation in HepG2 cells. Eur. J. Pharmacol. 2012;692(1–3):10–18. doi: 10.1016/j.ejphar.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Lin C.W., Chen L.J., Lee P.L., Lee C.I., Lin J.C., Chiu J.J. The inhibition of TNF-alpha-induced E-selectin expression in endothelial cells via the JNK/NF-kappaB pathways by highly N-acetylated chitooligosaccharides. Biomaterials. 2007;28(7):1355–1366. doi: 10.1016/j.biomaterials.2006.11.006. [DOI] [PubMed] [Google Scholar]
- McCullough A.J. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin. Liver Dis. 2004;8(3):521–533. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Mohd G.M., Al-Naqeb G., Krishnan S.K., Hazizul H.M., Adam A. Apoptosis Induction by Polygonum minus is related to antioxidant capacity, alterations in expression of apoptotic-related genes, and S-phase cell cycle arrest in HepG2 cell line. Biomed Res. Int. 2014;2014:539607. doi: 10.1155/2014/539607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montilla A., Ruiz-Matute A.I., Corzo N., Giacomini C., Irazoqui G. Enzymatic generation of chitooligosaccharides from chitosan using soluble and immobilized glycosyltransferase (Branchzyme) J. Agric. Food Chem. 2013;61(43):10360–10367. doi: 10.1021/jf403321r. [DOI] [PubMed] [Google Scholar]
- Nair S.V., Hettihewa M., Rupasinghe H.P. Apoptotic and inhibitory effects on cell proliferation of hepatocellular carcinoma HepG2 cells by methanol leaf extract of Costus speciosus. Biomed Res. Int. 2014;2014:637098. doi: 10.1155/2014/637098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y., Imajo K., Yoneda M., Nakajima A. Pathophysiology of NAsh/NAFLD associated with high levels of serum triglycerides. Nihon Rinsho. 2013;71(9):1623–1629. [PubMed] [Google Scholar]
- Qin C., Gao J., Wang L., Zeng L., Liu Y. Safety evaluation of short-term exposure to chitooligomers from enzymic preparation. Food Chem. Toxicol. 2006;44(6):855–861. doi: 10.1016/j.fct.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Rouabhia S., Milic N., Abenavoli L. Metformin in the treatment of non-alcoholic fatty liver disease: safety, efficacy and mechanism. Expert Rev. Gastroenterol. Hepatol. 2014;8(4):343–349. doi: 10.1586/17474124.2014.894880. [DOI] [PubMed] [Google Scholar]
- Sun C., Fan J.G., Qiao L. Potential epigenetic mechanism in non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2015;16(3):5161–5179. doi: 10.3390/ijms16035161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Zhang H.L., Hu Y.M., Wan S., Su Z.Q. Preparation of chitosan and water-soluble chitosan microspheres via spray-drying method to lower blood lipids in rats fed with high-fat diets. Int. J. Mol. Sci. 2013;14(2):4174–4184. doi: 10.3390/ijms14024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S., Gao B., Tao Y., Guo J., Su Z.Q. Antiobese effects of capsaicin–chitosan microsphere (CCMS) in obese rats induced by high fat diet. J. Agric. Food Chem. 2014;62(8):1866–1874. doi: 10.1021/jf4040628. [DOI] [PubMed] [Google Scholar]
- Torres D.M., Williams C.D., Harrison S.A. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2012;10(8):837–858. doi: 10.1016/j.cgh.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Zhang H.L., Tao Y., Guo J., Hu Y.M., Su Z.Q. Hypolipidemic effects of chitosan nanoparticles in hyperlipidemia rats induced by high fat diet. Int. Immunopharmacol. 2011;11(4):457–461. doi: 10.1016/j.intimp.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Zhang H.L., Zhong X.B., Tao Y., Wu S.H., Su Z.Q. Effects of chitosan and water-soluble chitosan micro- and nanoparticles in obese rats fed a high-fat diet. Int. J. Nanomedicine. 2012;7:4069–4076. doi: 10.2147/IJN.S33830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Cao S., Wang Y., Xu P., Yan J., Bin W., Qiu F., Kang N. Berberine metabolites could induce low density lipoprotein receptor up-regulation to exert lipid-lowering effects in human hepatoma cells. Fitoterapia. 2014;92:230–237. doi: 10.1016/j.fitote.2013.11.010. [DOI] [PubMed] [Google Scholar]