Abstract
Purpose
To evaluate the visual outcome and intraocular pressure changes after Visian Implantable Collamer Lens (ICL) implantation V4b and V4c (with central hole) for correction of high myopia.
Methods
A prospective, consecutive, interventional comparative case series of V4b and V4c ICL implantation was done in high myopic patients who were unsuitable for laser vision. The main outcome measures studied were uncorrected and corrected distant visual acuity (UDVA, CDVA), ICL vault, intraocular pressure (IOP), endothelial cell count (ECC), and development of subcapsular lens opacities. The patients were evaluated at postoperative 1,3,6, and 9 months.
Results
A total of 62 eyes of 32 patients (24.56 ± 4.8 years) underwent V4b ICL implantation (21 non-toric, 41 toric ICL-TICL) with intraoperative peripheral iridectomy (PI), and 10 eyes of 5 patients (26.13 ± 3.8 years) had implantation of V4c ICL (4 non-toric, 6 TICL). The mean preoperative manifest spherical equivalent (MSE) was −9.98 ± 2.8 D and −9.14 ± 2.4 D in the V4b and V4c groups, respectively, which reduced to postoperative values of −0.24 ± 1.3 D and −0.2 ± 1.18 D, respectively. At the end of 9 months follow-up, mean ECC loss was 6.4% and 6.1%, mean vault was 573.13 ± 241.13 μ, and 612 ± 251.14 μ, respectively, in the V4b and V4c groups. Anterior subcapsular opacities were present in 6.9% and 3.14% of eyes with V4b and V4c groups, respectively. Four eyes from V4b (9.75%) and 1 eye from V4c (16.66%) had rotation of more than 30° and required realignment surgery, which was done successfully. Two eyes (3.22%) with V4b ICL implantation had high postoperative IOP (>35 mm Hg) due to blocked PI and required Nd:Yag laser iridotomy, which was done with successful control of IOP. The safety indices were 1.11 and 1.14, and efficacy indices were 1.4 and 1.5 in the V4b and V4c groups, respectively, at the end of 9 months.
Conclusion
ICL implantation is a safe and effective surgery for correction of high myopia. Implantation of ICL with a central hole showed negligible postoperative IOP fluctuations without a peripheral iridectomy.
Keywords: Implantable collamer lens, Intraocular pressure, High myopia
Introduction
Phakic intraocular lens (pIOL) provides internal compensation of the dysfunctional refractive condition of the phakic eye and reduces or eliminates the dependence on glasses or contact lens.1 An implantable lens consisting of a biocompatible collagen copolymer (Visian Implantable Collamer lens [ICL]; STAAR Surgical, Nidau, Switzerland) was developed in 1993 as a posterior chamber pIOL and was called the implantable contact lens, as initially it was thought that it would come into contact with the anterior surface of the crystalline lens.2 Staar (Monrovia, CA, USA) patented this material made of 60% poly-hydroxyethylmethacrylate – HEMA, water (36%), benzophenone (3.8%), and 0.2% porcine collagen, and called it the Collamer (collagen-copolymer).1, 2 ICL is a posterior chamber phakic IOL which is a soft, flexible gel-lens ushering an era of reversible refractive surgery. ICLs are ciliary sulcus placed posterior chamber pIOLs that can be implanted through a small (3.0 mm), self-sealing limbal/clear corneal incision. In contrast with refractive lens exchange, ICL implantation does not impair natural accommodation or increase the risk of retinal detachment above the background rate for untreated patients with high myopia, and have a good safety profile.3
ICL is a boon in achieving spectacle independence in patients who are unsuitable for laser refractive procedures like those with high myopia (>−13diopter D), thin corneas, those with expected residual stromal bed thickness less than 300 μ, and severe dry eye. With its increasing acceptance and establishment of safety profile, ICL implantation has become an increasingly popular choice for the correction of moderate to high myopia.4, 5
The convexo-concave design of the ICL creates a vault between it and the anterior lens surface. However, the previous V4b ICL Model is known to cause pupillary block, and so either a preoperative/intraoperative laser/surgical peripheral iridotomy/iridectomy (PI) is required. To overcome this additional step, the V4c model with a central hole (0.36 mm) was developed in 2011. It has a central hole in addition to two additional holes outside the optic facilitating aqueous outflow and removal of ophthalmic viscosurgical device (OVD) during surgery. It also helps maintain the lens nutrition.6
However, complications of ICL implantation such as cataract formation (anterior subcapsular lens opacities-typical butterfly cataract), endothelial cell loss, pigment dispersion, intraocular pressure (IOP) elevation, and secondary glaucoma have been reported, and these complications are expected to increase with time.4, 5 Studies have shown good acceptance profile of both the ICL models.4, 5, 7
In view of the increasing prevalence of this surgical procedure, we conducted this study to evaluate the visual outcome, complication rate and safety indices of both the V4b and V4c ICL models for the correction of high myopia in a tertiary eye care center in South India over a follow-up period of 9 months.
Methods
This is a prospective, consecutive, comparative, interventional case series. All patients undergoing ICL implantation for the correction of high myopia (manifest spherical equivalent – MSE ≥ −6 D) were included in the study. The following were the inclusion criteria: (a) age between 21 and 45 years, (b) stable refraction within the past 1 year, (c) patients not suitable for corneal-based laser refractive procedures – those with abnormal corneal topography and keratoconus, predicted thin residual stromal bed thickness of less than 300 μ, high refractive errors of >−13 D, severe dry eye, (d) corneal diameter > 11 mm, (e) internal anterior chamber depth – ACD (measured from endothelium) > 2.9 mm. Bilateral implantation of the same ICL model for the correction of bilateral moderate-high myopia was preferred in an individual patient. A detail preoperative assessment was carried out including uncorrected distant visual acuity (UDVA), corrected distant visual acuity (CDVA), IOP measurement with non-contact tonometry – NCT (NT-510 NIDEK technologies, Japan) and a gonioscopy to ensure wide open angles. A detailed slit-lamp examination to rule out any ocular pathology was done. A detailed fundus examination to rule out any myopia-related or other fundus pathology was done, and prophylactic barrier laser, if required, was given. Automated and manual keratometry values were recorded using Topcon KR-8800 and ultrasound pachymetry using Tomey Pachymeter SP2000. Corneal topography was performed using Optikon 2000 Keratron Scout topographer (Optikon, Italy) and axial length and anterior chamber depth (ACD) by Sonomed PacScan 300A (Sonomed, INC., USA). The White-to-White (WTW) diameter was measured using a digital biometric ruler-digital calipers. Endothelial cell count (ECC) was measured using Tomey EM-3000 specular microscope (Tomey corporation, Japan). The ICL power was calculated using the Staar Surgical Customer Service Department formula that uses the ACD, mean keratometry or simulated keratometry values, central corneal pachymetry, horizontal WTW distance, and refraction 12 mm from the corneal vertex. The horizontal axis was marked with the patient sitting at the slit lamp prior to the surgery. Two dots were placed on the corneal-limbal area with a surgical marker indicating 0 and 180 meridians as reference for later toric ICL (TICL) alignment.
Surgical technique
Pupillary dilatation was achieved with a combination eye-drop containing 1% tropicamide and 2.5% phenylephrine. 0.5% proparacaine was the topical anesthetic used. With a temporal approach, two 1 mm paracenteses were made using angled keratome or 15° side port knife at 12 and 6 o'clock positions. Hypromellose 2% (Viscomet PF, Unimed technologies) viscoelastic was injected into the anterior chamber taking care not to overfill the chamber. A temporal 3 mm clear corneal incision was made. The ICL was loaded into the cartridge. The paracenteses were used to position the footplates under the iris using the special manipulating instruments like Vukich's manipulator. It was ensured that all haptics were posterior to the iris. In case of V4b ICL, the pupil was constricted with carbachol (MIO-CHOL, 0.01% preservative free, USA, marketed in India by Appasamy associates), and a single PI at 1 the o'clock position was done with vitrector under viscoelastic cover. In case of V4c ICL, this step was skipped. In case of TICL proper alignment was ensured. At the end of surgery, viscoelastic was cleared from the AC.
A standard postoperative regime consisting of topical prednisolone acetate 1% (Pred forte, Allergan, USA) 4 times a day for 5 days tapering over 2 weeks and topical gatifloxacin 0.3% (Zymaxid, Allergan Las, Irvine, USA) 4 times a day for 2 weeks was started. Timolol maleate eyedrops 0.5% (Timolet, Sun Pharmaceuticals, India) was also started 2 times per day for 3 days. Postoperatively, the patient was examined at 4 h to check for proper ICL positioning and vaulting on slit lamp, and IOP was checked. The patient was then followed on postoperative day 1, 1 week, 1 month, 3 months, 6 months, and 9 months. The main surgical outcomes were evaluated at 1, 3, 6, and 9 months follow-up. At each of these visits, UDVA, CDVA, MSE, IOP, ICL vaulting, and ECC were evaluated. ICL vault was measured by anterior segment optical coherence tomography (AS-OCT) RTVue (Model-RT100 Version 6.9, Fremont, USA). Patients were asked about subjective symptoms of glare and haloes at the end of follow-up period of 9 months.
The safety index was calculated by dividing the postoperative CDVA (in decimal) at 9 months by the preoperative CDVA (in decimal). The efficacy index was calculated by dividing the postoperative UDVA (in decimal) by the preoperative UDVA (in decimal).
The data were analyzed using SPSS version 20 (Statistical Package for Social Sciences) with paired t-test for intragroup comparison and Mann Whitney U value test for intergroup comparisons. A p value less than 0.05 was considered significant.
Results
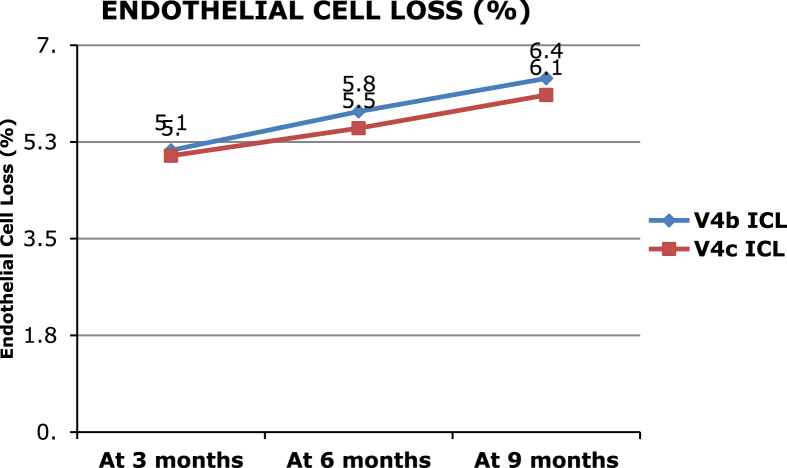
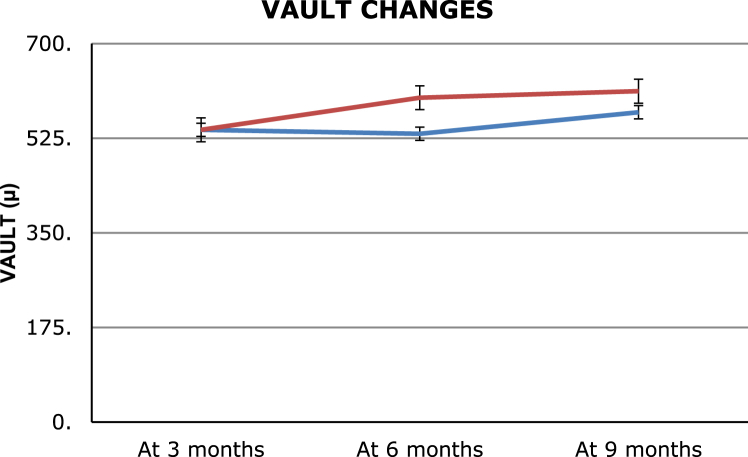
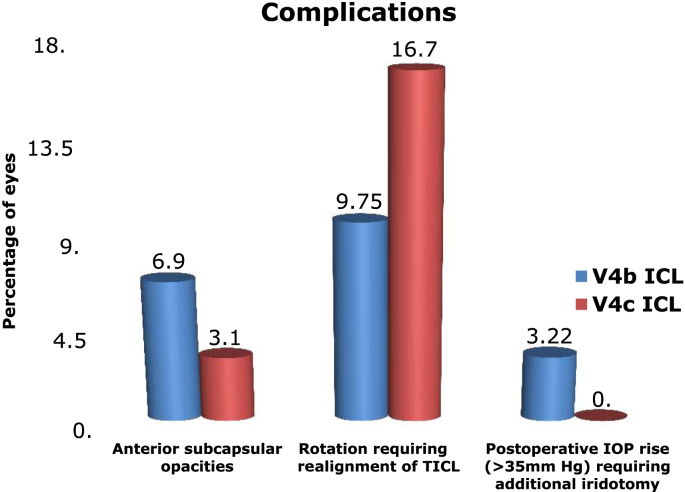
A total of 62 eyes of 32 patients with a mean ± SD age of 24.56 ± 4.8 years underwent V4b ICL implantation (21 non-toric, 41 toric ICL-TICL) with intraoperative peripheral iridectomy (PI), and 10 eyes of 5 patients with a mean ± SD age of 26.13 ± 3.8 years had implantation of V4c ICL with central hole (p = 0.81) (4 non-toric, 6 TICL). The large difference in the number of eyes in the two groups is due to the later development of the V4c model. The mean preoperative manifest spherical equivalent (MSE) was −9.98 ± 2.8 D and −9.14 ± 2.4 D in the V4b and V4c groups, respectively (p = 0.51), which reduced to postoperative values of −0.24 ± 1.3 D and −0.2 ± 1.18 D, respectively (p = 0.09). The MSE reduced significantly in the two groups (p < 0.001 in both the groups). The mean preoperative astigmatism was −1.7 ± 1.5 diopter cylinder (Dcyl) and −1.8 ± 1.5 Dcyl which respectively reduced to −0.7 ± 0.7 Dcyl and −0.8 ± 0.4 Dcyl at 9 months (p < 0.001 in both the groups) (Table 1). A gain of 1 line of CDVA was seen in 10% and 11.76% eyes in V4b and V4c groups, respectively (p = 0.08), while no change in CDVA was seen in 90% and 88.24% of eyes (p = 0.07). No eye had loss of lines of CDVA post surgery. At the end of 9 months follow-up, mean ECC loss was 6.4% and 6.1% (Fig. 1, Fig. 2) (p = 0.08), mean vault was 573.13 ± 241.13 μ, and 612 ± 251.14 μ, respectively, in the V4b and V4c groups (p = 0.02) (Fig. 3). Anterior subcapsular opacities were present in 6.9% and 3.14% of eyes with V4b and V4c groups, respectively (p < 0.01). Four eyes from V4b (9.75%) and 1 eye from V4c (16.66%) groups had rotation of more than 30° and required re-alignment surgery which was done successfully (Fig. 4). Two eyes (3.22%) with V4b ICL implantation had high postoperative IOP (>35 mm Hg) due to blocked PI and required Nd:Yag laser iridotomy which was done with successful control of IOP. The safety indices were 1.11 and 1.14 and efficacy indices were 1.4 and 1.5 in the V4b and V4c groups, respectively, at the end of 9 months. At the end of 9 months, on questioning, the most common subjective symptom reported was glare and haloes in 23% and 25% in the two groups, respectively (p = 0.09). However, they were not annoying enough to cause visual disability. Due to the large difference in the sample size between the two groups, the results of the intergroup comparisons done by the Mann Whitney U value test with p values is limited and need to be considered accordingly.
Table 1.
Demographics.
| V4b ICL group (n = 62) | p Value V4b groupa | V4c ICL group (n = 10) | p Value V4c groupa | ||
|---|---|---|---|---|---|
| Age (years) | 24.56 ± 4.8 | 26.13 ± 3.8 | |||
| Sex | Males (n) | 21 | 4 | ||
| Females (n) | 11 | 1 | |||
| TICL (n) | 41 | 6 | |||
| Preoperative MSE (D) | −9.98 ± 2.8 | <0.001 | −9.14 ± 2.4 | <0.001 | |
| Postoperative MSE (D) at 9 months | −0.24 ± 1.3 | −0.2 ± 1.18 | |||
| Preoperative astigmatism (Dcyl) | −1.7 ± 1.5 | <0.001 | −1.8 ± 1.5 | <0.001 | |
| Postoperative astigmatism (Dcyl) at 9 months | −0.7 ± 0.7 | −0.8 ± 0.4 | |||
MSE – manifest spherical equivalent, D – diopter, cyl – cylinder.
Paired t test.
Fig. 1.
Showing endothelial cell loss over the follow-up period in both the ICL groups.
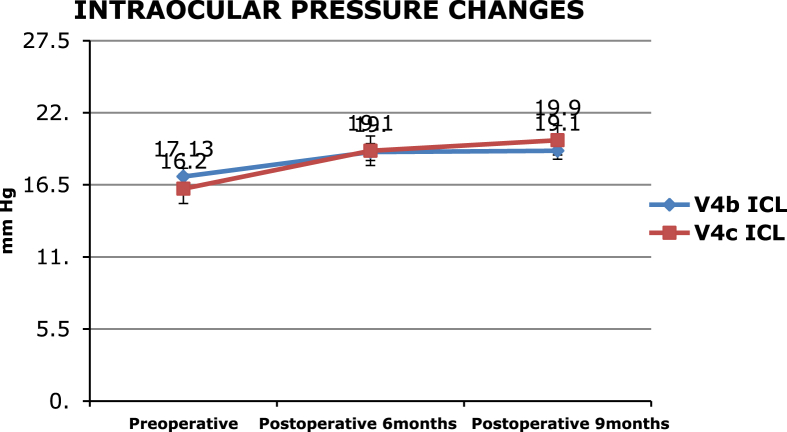
Fig. 2.
Showing intraocular pressure changes preoperatively and postoperatively in both the ICL groups.
Fig. 3.
Showing central vault changes in both the ICL groups during the follow-up period.
Fig. 4.
Showing complications noted in both the ICL groups during the follow-up period.
Discussion
Phakic intraocular lens implantation is so far the only refractive treatment for high myopia that offers preservation of accommodation and potential reversibility.
In our study, we found that both the types of ICL with or without the central hole showed a satisfactory visual outcome which was maintained at the end of 9 months follow-up period.
Huseynova et al.8 and Ferrer-Blasco et al.9 also found similar results with the two models with both providing good visual outcome and no difference in the objective scatter index and higher order aberrations.
ICLs have emerged as a successful and promising modality for the treatment of moderate to high myopia especially in candidates unsuitable for laser refractive procedures.10, 11 Though being an intraocular procedure, it provides the advantage of reversibility and an acceptable safety profile. With the advent of toric ICL, a significant amount of astigmatism can be corrected.12 The TICL have shown to be stable over a long term period with the haptics enforcing stable lens position in the ciliary sulcus.13 The TICL is fundamentally different from toric intraocular lenses as it is not subject to contraction of the capsular bag. The soft footplates of the ICL conform to the normal undulating contours of the ciliary sulcus with a kind of lock-and-key situation where the footplates will drape over and into the tiny irregular features of the sulcus. This prevents excessive lens movement. In our study, we had 4 from V4b (9.75%) and 1 eye from V4c (16.66%) groups requiring realignment surgery with successful outcomes. Lee et al. found an incidence of 1.7% of rotation in excess of 10° with 98.3% showing excellent rotational stability without decrease in visual acuity.14
There were no reports of excessive pigment dispersion or secondary glaucoma in our study. The IOP was maintained below 21 mm of Hg in both the groups over 9 months.
Higueras-Esteban et al.7 found no significant changes between the V4b and V4c models with respect to IOP stability. Kawamorita et al.6 studied the fluid dynamics of aqueous humor in V4c model and suggested that Hole-ICLs improve the circulation of aqueous humor to the anterior surface of the crystalline lens. Sanders15 reported approximately 6–7% of eyes developing anterior subcapsular opacities at 7 year following ICL implantation but only 1–2% had progressed to clinically significant cataract in the same period, especially in high myopes and older patients.12 Fernandes et al. also found cataract as the major complication.16 In our study, none of the eyes had visually significant cataract at the end of 9 months follow-up period.
In a study conducted by Pothireddy et al.17 in India, the safety index was 0.75, and the efficacy index was 1.04 twelve months postoperatively. ICL was thus evaluated to be a safe and effective procedure in terms of visual outcome. Pineda-Fernandez et al. reported MSE in 61.1% and 22% of eyes within ±1.00 D and ±0.50 D of emmetropia.18 In their study, the mean residual sphere was −0.25 D, and mean residual cylinder was −0.12 Dcyl. Insignificant refractive change during follow-up after ICL implantation with similar results was obtained by Igarashi et al.19
A study by Dejaco-Ruhswurm et al. demonstrated rapid cell loss until 1 year postoperatively, after which the rate of loss was no longer statistically significant.20, 21 In our study, the vitrector PI had clean cuts with minimum pigment dispersion and a chance of being incomplete or getting blocked. IOP was stable throughout the 9 months of follow-up in both the groups. In the V4c group, this could be attributed not just to the centraflow technology, but also to the negligible pigment dispersion due to avoidance of PI. None of our cases developed secondary glaucoma following excessive vault or pigment dispersion during the follow-up. In our study, the ICL vault was maintained at 9 months follow-up period.22, 23 Kamiya et al.24 found the vault of the new central hole pIOL to be essentially equivalent to the vault of the conventional pIOL, suggesting that the presence of the central hole did not significantly affect the vault or the refractive accuracy.
Kamiya et al.25, 26 found the V4c ICL with aquaport essentially equivalent in the optical quality variables to conventional ICL implantation. They suggested that the presence of the central artificial hole does not significantly affect the optical quality and the intraocular scattering after surgery. Other studies27, 28, 29, 30 have also found good optical quality results with V4b and V4c ICLs. Maroccos et al.31 studied pIOLs and found that V4b ICL implantation leads to decreased night vision performance with glare and haloes. Lyu et al.32 found a common incidence of glare and haloes after ICL implantation. Many studies33, 34, 35, 36, 37, 38 have shown good visual performance and quality of life after ICL implantation. In our study, patients in both groups experienced glare and haloes, but they were visually insignificant and non-annoying.
ICL thus offers a safe, effective, and reversible option for correction of high myopia. However, evaluation of the incidence of cataract formation and endothelial cell loss over a decade should be carried out.
This study has several limitations. The small sample size and unequal number of patients in the two groups precludes definite conclusion. Also, including both eyes of one patient in the study, limits the conclusion. ICL with a central hole offers an added advantage of annulling a PI and providing a stable IOP. However, a larger sample size undergoing V4c implantation with a longer follow-up is required for confirming the results.
Footnotes
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Lovisolo C.F., Reinstein D.Z. Phakic intraocular lenses. Surv Ophthalmol. 2005;50:549–587. doi: 10.1016/j.survophthal.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Rosen E., Gore C. Staar Collamer posterior chamber phakic intraocular lens to correct myopia and hyperopia. J Cataract Refract Surg. 1998;24:596–606. doi: 10.1016/s0886-3350(98)80253-8. [DOI] [PubMed] [Google Scholar]
- 3.Chun Y.S., Lee J.H., Lee J.M. Outcomes after implantable contact lens for moderate to high myopia. J Kor Ophthalmol Soc. 2004;45:480–489. [Google Scholar]
- 4.Sanders D.R., Vukich J.A., Doney K., Gaston M., Implantable Contact Lens in Treatment of Myopia Study Group U.S. Food and Drug Administration clinical trial of the implantable contact lens for moderate to high myopia. Ophthalmology. 2003;110:255–266. doi: 10.1016/s0161-6420(02)01771-2. [DOI] [PubMed] [Google Scholar]
- 5.Kamiya K., Shimizu K., Igarashi A., Hikita F., Komatsu M. Four-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Arch Ophthalmol. 2009;127:845–850. doi: 10.1001/archophthalmol.2009.67. [DOI] [PubMed] [Google Scholar]
- 6.Kawamorita T., Uozato H., Shimizu K. Fluid dynamics simulation of aqueous humour in a posterior-chamber phakic intraocular lens with a central perforation. Graefes Arch Clin Exp Ophthalmol. 2012;250:935–939. doi: 10.1007/s00417-011-1850-2. [DOI] [PubMed] [Google Scholar]
- 7.Higueras-Esteban A., Ortiz-Gomariz A., Gutiérrez-Ortega R. Intraocular pressure after implantation of the Visian Implantable Collamer Lens with CentraFLOW without iridotomy. Am J Ophthalmol. 2013;156(4):800–805. doi: 10.1016/j.ajo.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Huseynova T., Ozaki S., Ishizuka T., Mita M., Tomita M. Comparative study of 2 types of implantable collamer lenses, 1 with and 1 without a central artificial hole. Am J Ophthalmol. 2014;157(6):1136–1143. doi: 10.1016/j.ajo.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Ferrer-Blasco T., García-Lázaro S., Belda-Salmerón L., Albarrán-Diego C., Montés-Micó R. Intra-eye visual function comparison with and without a central hole contact lens-based system: potential applications to ICL design. J Refract Surg. 2013;29(10):702. doi: 10.3928/1081597X-20130919-03. [DOI] [PubMed] [Google Scholar]
- 10.Sanders D.R., Doney K., Poco M., ICL in Treatment of Myopia Study Group United States Food and Drug Administration clinical trial of the Implantable Collamer Lens (ICL) for moderate to high myopia: three-year follow-up. Ophthalmology. 2004;111:1683–1692. doi: 10.1016/j.ophtha.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 11.Han S.Y., Lee K.H. Long term effect of ICL implantation to treat high myopia. J Kor Ophthalmol Soc. 2007;48:465–472. [Google Scholar]
- 12.Chang J., Lau S. Toric implantable collamer lens for high myopic astigmatic Asian eyes. Ophthalmology. 2009;116:2340–2347. doi: 10.1016/j.ophtha.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 13.Mori T., Yokoyama S., Kojima T. Factors affecting rotation of a posterior chamber collagen copolymer toric phakic intraocular lens. J Cataract Refract Surg. 2012;38:568–573. doi: 10.1016/j.jcrs.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 14.Lee D., Lee S.J., Kyung H. Rotational stability after toric implantable collamer lens implantation. J Kor Ophthalmol Soc. 2015;56(4):477–484. [Google Scholar]
- 15.Sanders D.R. Anterior subcapsular opacities and cataracts 5 years after surgery in the Visian Implantable Collamer Lens FDA trial. J Refract Surg. 2008;24:566–570. doi: 10.3928/1081597X-20080601-04. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes P.R., González-Méijome J.M., Madrid-Costa D., Ferrer-Blasco T., Jorge J., Montés-Micó R. Implantable collamer posterior chamber intraocular lenses: a review of potential complications. J Refract Surg. 2011 Oct;27(10):765–776. doi: 10.3928/1081597X-20110617-01. [DOI] [PubMed] [Google Scholar]
- 17.Pothireddy R., Reddy K.P., Senthil S., Rao H.L. Posterior chamber toricphakic intraocular lenses for myopic astigmatism first experience in India. J Cataract Refract Surg. 2012;38:1583–1589. doi: 10.1016/j.jcrs.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Pineda-Fernandez A., Jaramillo J., Vargas J., Jaramillo M., Jaramillo J., Galındez A. Phakic posterior chamber intraocular lens for high myopia. J Cataract Refract Surg. 2004;30:2277–2283. doi: 10.1016/j.jcrs.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 19.Igarashi A., Shimizu K., Kamiya K. Eight-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Am J Ophthalmol. 2014;157:532–539. doi: 10.1016/j.ajo.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Dejaco-Ruhswurm I., Scholz U., Pieh S. Long-term endothelial changes in phakic eyes with posterior chamber intraocular lenses. J Cataract Refract Surg. 2002;28:1589–1593. doi: 10.1016/s0886-3350(02)01210-5. [DOI] [PubMed] [Google Scholar]
- 21.Edelhauser H.F., Sanders D.R., Azar R., Lamielle H., ICL in Treatment of Myopia Study Group Corneal endothelial assessment after ICL implantation. J Cataract Refract Surg. 2004;30:576–583. doi: 10.1016/j.jcrs.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 22.Kamiya K., Shimizu K., Kawamorita T. Changes in vaulting and the effect on refraction after posterior chamber phakic intraocular lens implantation. J Cataract Refract Surg. 2009;35:1582–1586. doi: 10.1016/j.jcrs.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 23.Lee H., Kang S.Y., Seo K.Y., Chung B., Choi J.Y., Kim K.S. Dynamic vaulting changes in V4c versus V4 posterior chamber phakic lenses under differing lighting conditions. Am J Ophthalmol. 2014;158(6):1199–1204. doi: 10.1016/j.ajo.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Kamiya K., Shimizu K., Ando W., Igarashi A., Iijima K., Koh A. Comparison of vault after implantation of posterior chamber phakic intraocular lens with and without a central hole. J Cataract Refract Surg. 2015;41(1):67–72. doi: 10.1016/j.jcrs.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya K., Shimizu K., Saito A., Igarashi A., Kobashi H. Comparison of optical quality and intraocular scattering after posterior chamber phakic intraocular lens with and without a central hole (hole ICL and conventional ICL) implantation using the double-pass instrument. PLoS One. 2013;8(6):e66846. doi: 10.1371/journal.pone.0066846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamiya K., Shimizu K., Igarashi A., Kobashi H., Ishii R., Sato N. Clinical evaluation of optical quality and intraocular scattering after posterior chamber phakic intraocular lens implantation. Invest Ophthalmol Vis Sci. 2012;53:3161–3166. doi: 10.1167/iovs.12-9650. [DOI] [PubMed] [Google Scholar]
- 27.Domínguez-Vicent A., Ferrer-Blasco T., Pérez-Vives C., Esteve-Taboada J.J., Montés-Micó R. Optical quality comparison between 2 collagen copolymer posterior chamber phakic intraocular lens designs. J Cataract Refract Surg. 2015;41(6):1268–1278. doi: 10.1016/j.jcrs.2014.09.050. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Vives C., Domínguez-Vicent A., Ferrer-Blasco T., Pons Á.M., Montés-Micó R. Optical quality of the Visian Implantable Collamer Lens for different refractive powers. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1423–1429. doi: 10.1007/s00417-012-2200-8. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Vives C., Ferrer-Blasco T., Madrid-Costa D., García-Lázaro S., Montés-Micó R. Optical quality comparison of conventional and hole-visian implantable collamer lens at different degrees of decentering. Am J Ophthalmol. 2013;156(1):69–76. doi: 10.1016/j.ajo.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Uozato H., Shimizu K., Kawamorita T., Ohmoto F. Modulation transfer function of intraocular collamer lens with a central artificial hole. Graefe's Arch Clin Exp Ophthalmol. 2011;249(7):1081–1085. doi: 10.1007/s00417-010-1602-8. [DOI] [PubMed] [Google Scholar]
- 31.Maroccos R., Vaz F., Marinho A., Guell J., Lohmann C.P. Glare and halos after “phakic IOL”. Surgery for the correction of high myopia. Der Ophthalmol: Zeit Deutsch Ophthalmol Gesellsch. 2001;98(11):1055–1059. doi: 10.1007/s003470170024. [DOI] [PubMed] [Google Scholar]
- 32.Lyu I.J., Kim C., Chung E.S., Chung T.Y. Risk factors associated with glare and halo after ICL implantation. Invest Ophthalmol Vis Sci. 2011;52(14):6204. [Google Scholar]
- 33.Alfonso J.F., Lisa C., Cueto L.F.V., Belda-Salmerón L., Madrid-Costa D., Montés-Micó R. Clinical outcomes after implantation of a posterior chamber collagen copolymer phakic intraocular lens with a central hole for myopic correction. J Cataract Refract Surg. 2013;39(6):915–921. doi: 10.1016/j.jcrs.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 34.Alfonso J.F., Baamonde B., Fernández-Vega L., Fernandes P., González-Méijome J.M., Montés-Micó R. Posterior chamber collagen copolymer phakic intraocular lenses to correct myopia: five-year follow-up. J Cataract Refract Surg. 2011;37:873–880. doi: 10.1016/j.jcrs.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 35.Kamiya K., Shimizu K., Aizawa D., Igarashi A., Komatsu M., Nakamura A. One-year follow-up of posterior chamber toric phakic intraocular lens implantation for moderate to high myopic astigmatism. Ophthalmology. 2010;117:2287–2294. doi: 10.1016/j.ophtha.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 36.Bechmann M., Ullrich S., Thiel M.J., Kenyon K.R., Ludwig K. Imaging of posterior chamber phakic intraocular lens by optical coherence tomography. J Cataract Refract Surg. 2002;28:360–363. doi: 10.1016/s0886-3350(01)00978-6. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu K., Kamiya K., Igarashi A., Shiratani T. Intraindividual comparison of visual performance after posterior chamber phakic intraocular lens with and without a central hole implantation for moderate to high myopia. Am J Ophthalmol. 2012;154:486–494. doi: 10.1016/j.ajo.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Ieong A., Hau S.C., Rubin G.S., Allan B.D. Quality of life in high myopia before and after implantable Collamer lens implantation. Ophthalmology. 2010;117:2295–2300. doi: 10.1016/j.ophtha.2010.03.055. [DOI] [PubMed] [Google Scholar]