Abstract
Purpose
To determine the distribution of Zernike coefficients and higher order aberrations in a normal population and its relationship with age, gender, biometric components, and spherical equivalent.
Methods
During the first phase of the Shahroud cohort study, 6311 people of the 40-64-year-old population of Shahroud city were selected through random cluster sampling. A subsample of participants was examined with Zywave aberrometer (The Bausch & Lomb, Rochester, NY) to measure aberrations. Measurements of aberrations were done before cycloplegic refraction, and values generated from a minimum pupil diameter of 5 mm were reported in this analysis.
Results
After applying exclusion criteria, 904 eyes of 577 people were analyzed in this study and mean age in this study was 49.5 ± 5.7 years and 62.9% were female. Mean root-mean-square (RMS) of the third−, fourth−, and fifth-order aberrations was 0.194 μm (95%CI: 0.183 to 0.204), 0.115 μm (95%CI: 0.109 to 0.121), and 0.041 μm (95%CI: 0.039 to 0.043), respectively. Total RMS coma (Z3−1, Z31, Z5−1, Z51), Total RMS trefoil (Z3−3, Z33, Z5−3, Z53), and spherical aberration (Z40) in the studied population was 0.137 μm (95% CI:0.129–0.145), 0.132 μm (95% CI: 0.123–0.140), and −0.161 μm (95%CI:−0.174 to −0.147), respectively. Mean higher-order Zernike RMS in this study was 0.306 (95% CI: 0.295–0.318) micrometer, and in the multiple model, it significantly correlated with older age and short axial length. The highest amounts of higher-order RMS were observed in hyperopes, and the smallest in emmetropes. Increased nuclear opacity was associated with a significant increase in HO RMS (p < 0.001). Analysis of Zernike coefficients demonstrated that spherical aberration (Z40) significantly correlated with nuclear cataract only (age-adjusted Coef = 0.37 and p = 0.012).
Conclusion
This report is the first to describe the distribution of higher-order aberrations in an Iranian population. Higher-order aberrations in this study were on average higher that those reported in previous studies.
Keywords: Zernike coefficients, Higher order aberrations, Population based study, Adult
Introduction
Higher-order aberrations (HOAs), one of the important subjects in the science of vision and optics, received very little attention before 2000. However, advances in diagnostic and therapeutic methods in recent years have brought them to the attention of ophthalmologists and optometrists.1, 2 As we know, HOAs are part of the refractive errors which are not correctable with sphere and cylinder corrections. They are also among errors of the optical system of the eye which can deteriorate the quality of the retinal image.3, 4 Since HOAs can impact visual performance and contrast sensitivity, they are considered important indices in the field of quality of vision and deserve attention.5, 6, 7 In addition, today, attention to HOAs after laser refractive surgery has become one of the important issues in the assessment of the quality of laser refractive methods.6, 8, 9, 10 Implantation of intraocular lenses has caused many studies to demonstrate changes in HOAs after surgery.11, 12, 13 There has been more attention to HOAs among cataract patients and myopes compared to other ocular conditions.14, 15, 16 The decision to correct HOAs or not is a challenging one for which no definite answer has been found. Therefore, knowledge of the distribution of these errors in the normal population can be helpful in more accurate corrections using novel techniques in refractive surgery or customized contact lenses. Knowledge of the normal values of HOAs in the normal population can also be helpful in early diagnosis of pathologic conditions such as keratoconus. To date, few studies have examined the distribution of HOAs in different races.15, 17, 18, 19, 20 Cervino et al21 and Lim et al17 have shown the differences among some ethnic groups. Considering changes in HOAs in different ethnicities, describing their distribution and other components in different populations provides valuable information for each geographic region. Knowledge of the normal distribution of these values can be very useful for developing nomograms for refractive surgery. Previously, some studies described differences in ophthalmic indices such as corneal thickness, keratometry, anterior chamber depth, pupil diameter, corneal diameter, and even distribution of refractive errors in Iranian populations.22, 23, 24, 25, 26, 27, 28, 29, 30, 31 Nonetheless, no study has yet reported the distribution of HOAs in an Iranian population. The aim of this report is to determine the distribution of HOAs and their relationship with other components in a normal Iranian population. The results of this report can also be used as a baseline for the Middle East region. This report also studies the relationship between HOAs and variables of age, gender, ocular biometrics, and refractive errors.
Methods
The present report is part of the first phase of the Shahroud cohort study in which data was collected cross-sectionally. Details of the sampling strategy and methodology have been published elsewhere, and given here only in brief.32
In this study, the 40- to 64-year-old citizens of Shahroud, a city in the north of Iran, were selected as the target population. 300 clusters were randomly selected in the city using multistage sampling. Clusters were selected proportionate to the population of the 9 health care centers of Shahroud. After selecting samples in each cluster, a total of 6311 people were invited to participate in the study. In light of time and cost considerations, some of the examinations were conducted for a subsample of participants. After enrollment and obtaining written informed consent, each participant had complete eye examinations at the study clinic, and their demographics and medical and ophthalmic history were recorded through interviews.
Examinations
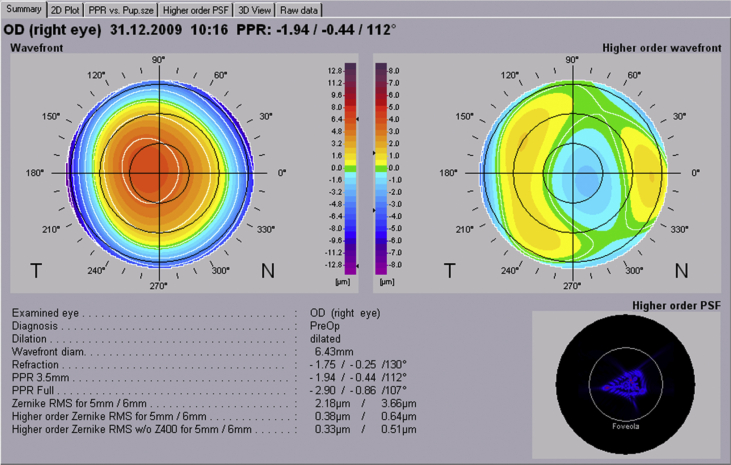
Participants had various examinations including optometry tests, ophthalmic examinations, corneal imaging, and biometry. Optometry tests included vision test using the LogMAR chart, as well as subjective, cycloplegic, and manifest refraction. Ophthalmic examinations were done in two stages before and after pupil dilation. Before dilation, slit lamp biomicroscopy and measurement of intraocular pressure was done. After pupil dilation, clinical lens opacities grading, assessment of vitreous opacities with the slit lamp, and retinal examinations using direct and indirect ophthalmoscopy was conducted. Measurement of HOAs in the subsample was also done before instilling cycloplegic drops. In these samples, the Zywave aberrometer (The Bausch & Lomb, Rochester, NY) was used to assess HOAs and Zernike indices. The accuracy of this device in measuring aberrations has been studied before.33, 34 Those images without error by the device were included in this report. Fig. 1 illustrates a sample of the device output and HOA values.
Fig. 1.
Zywave™ aberrometer output view for higher order aberrations.
To determine lens opacity, slit lamp examination was. An ophthalmologist conducted lens opacity grading with a slit lamp, and graded any nuclear, posterior subcapsular (PSC), and cortical opacity by making comparisons against standard photographs of the Lens Opacities Classification System III (LOCS III).
Exclusion criteria
Since certain aberrations tend to change as an effect of surgery, cases with any history of surgery were excluded. Cases with a pupil diameter less than 5.0 mm were also excluded from the analysis. The higher order aberrations were reported for 5 mm.
Statistical analysis
In this study, since the correlation between eyes was low in terms of root mean square (RMS) of HOAs (r = 0.277), data from both eyes was used in the analysis. For descriptive values, the mean and 95% confidence intervals (CI) were determined. The correlation between the two eyes of each case was accounted for in calculating the standard deviation and 95% CIs. To examine relationships between Zernike coefficients and RMS of HOAs with variables of age, gender, spherical equivalent, axial length, corneal power, central corneal thickness, intraocular pressure, and different types of cataract, multiple Generalized Estimation Equation linear models were used.
Results
Of the 6311 people selected for this study, 5190 participated, and 1017 were selected as the subsample. Aberrations were measured in 749 people in the subsample group. After applying exclusion criteria, 904 eyes of 577 people were analyzed. The mean age in this group was 49.5 ± 5.7 years, and 62.9% were female.
In this study, we found a mean AL of 23.11 ± 0.82 (20.91–26.26) mm. The prevalence of nuclear, cortical and PSC cataract was 0.7%, 3.9% and 1.0%; respectively.
Zernike coefficients
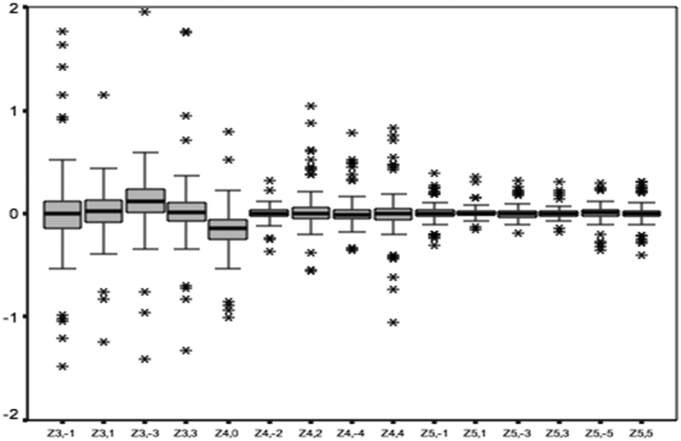
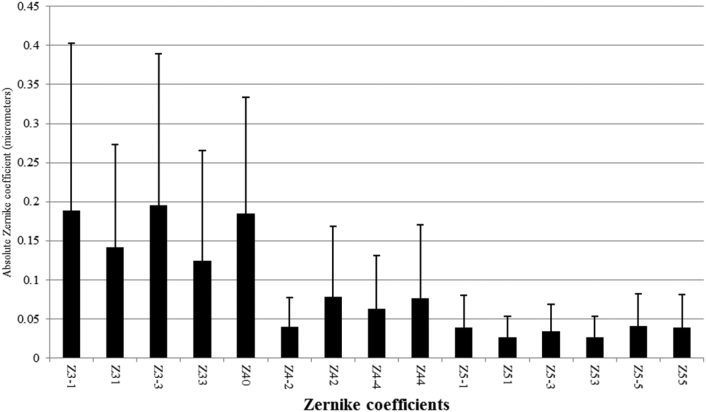
Table 1, Table 2 summarize the mean and 95% CIs of the Zernike coefficients in all studied eyes in different age and gender groups. Fig. 2 also shows the distribution of Zernike coefficients in the studied population; third order aberrations comprise a major portion of these coefficients. Mean total RMS coma (Z3−1, Z31, Z5−1, Z51) and total RMS trefoil (Z3−3, Z33, Z5−3, Z53) in the studied population was 0.137 μm (95% CI: 0.129–0.145) and 0.132 μm (95% CI: 0.123–0.140). Mean and 95% CIs of third−, fourth−, and fifth-order aberrations by age and gender is demonstrated in Table 1. Mean and standard deviation of absolute values of Zernike coefficients in demonstrated in Fig. 3.
Table 1.
Mean and 95% confidence interval (CI) for each Zernicke and root mean square values of total−, third−, fourth−, and fifth-order, higher order aberrations, total coma (Z31, Z3−1, Z51, Z5−1), and total trefoil (Z33, Z3−3, Z53, Z5−3) in all eyes by gender.
| All |
Female |
Male |
|
|---|---|---|---|
| Mean (95%CI) | Mean (95%CI) | Mean (95%CI) | |
| Z3−1 | −0.003 (−0.025 to 0.019) | 0.014 (−0.012 to 0.041) | −0.030 (−0.068 to 0.008) |
| Z31 | 0.024 (0.015 to 0.034) | 0.023 (0.010 to 0.035) | 0.027 (0.013 to 0.042) |
| Z3−3 | 0.130 (0.112 to 0.148) | 0.108 (0.088 to 0.127) | 0.166 (0.131 to 0.201) |
| Z33 | 0.017 (0.006 to 0.027) | 0.011 (−0.002 to 0.024) | 0.023 (0.005 to 0.042) |
| Z40 | −0.161 (−0.174 to −0.147) | −0.174 (−0.191 to −0.156) | −0.138 (−0.16 to −0.116) |
| Z4−2 | 0.001 (−0.002 to 0.004) | 0.001 (−0.004 to 0.005) | 0.003 (−0.003 to 0.008) |
| Z42 | 0.010 (0.001 to 0.019) | 0.011 (−0.001 to 0.022) | 0.007 (−0.008 to 0.022) |
| Z4−4 | −0.003 (−0.009 to 0.002) | 0.001 (−0.007 to 0.008) | −0.010 (−0.018 to −0.001) |
| Z44 | −0.003 (−0.012 to 0.005) | −0.004 (−0.015 to 0.006) | 0.001 (−0.015 to 0.016) |
| Z5−1 | 0.003 (−0.001 to 0.007) | 0.006 (0.001 to 0.011) | −0.001 (−0.008 to 0.006) |
| Z51 | 0.008 (0.005 to 0.010) | 0.005 (0.003 to 0.008) | 0.011 (0.007 to 0.015) |
| Z5−3 | −0.005 (−0.008 to −0.002) | −0.006 (−0.010 to −0.002) | −0.004 (−0.01 to 0.002) |
| Z53 | 0.002 (0.000 to 0.004) | 0.003 (0.001 to 0.006) | 0 (−0.004 to 0.004) |
| Z5−5 | 0.006 (0.002 to 0.009) | 0.007 (0.002 to 0.012) | 0.004 (−0.003 to 0.011) |
| Z55 | 0.001 (−0.002 to 0.005) | −0.002 (−0.007 to 0.002) | 0.007 (0.001 to 0.013) |
| Tcoma | 0.137 (0.129 to 0.145) | 0.139 (0.129 to 0.150) | 0.134 (0.12 to 0.148) |
| Ttrefoil | 0.132 (0.123 to 0.140) | 0.124 (0.115 to 0.133) | 0.145 (0.129 to 0.160) |
| 3rd Order | 0.194 (0.183 to 0.204) | 0.191 (0.178 to 0.203) | 0.200 (0.181 to 0.220) |
| 4rd Order | 0.115 (0.109 to 0.121) | 0.118 (0.110 to 0.126) | 0.109 (0.099 to 0.12) |
| 5rd Order | 0.041 (0.039 to 0.043) | 0.040 (0.038 to 0.043) | 0.042 (0.038 to 0.046) |
| RMS HOA | 0.306 (0.295 to 0.318) | 0.305 (0.290 to 0.319) | 0.309 (0.290 to 0.329) |
| RMS HOA without Z40 | 0.282 (0.271 to 0.293) | 0.277 (0.263 to 0.292) | 0.291 (0.271 to 0.31) |
Tcoma: Total Coma (Z31, Z3−1, Z51, Z5−1).
Ttrefoil: Total Trefoil (Z33, Z3−3, Z53, Z5−3).
RMS HOA: Root mean square higher order aberration.
Table 2.
Mean and 95% confidence interval (CI) for each Zernicke and root mean square values of total−, third−, fourth−, and fifth-order, Higher Order Aberrations, Total Coma (Z31, Z3−1, Z51, Z5−1), and total Trefoil (Z33, Z3−3, Z53, Z5−3) by age.
| Mean (95%CI) |
Mean (95%CI) |
Mean (95%CI) |
Mean (95%CI) |
Mean (95%CI) |
|
|---|---|---|---|---|---|
| 40 to 44 | 45 to 49 | 50 to 54 | 55 to 59 | >=60 | |
| Z3−1 | −0.0055(−0.0492 to 0.0381) | 0.0017 (−0.0343 to 0.0376) | 0.0288 (−0.0082 to 0.0657) | −0.0415 (−0.1042 to 0.0213) | −0.0536 (−0.2456 to 0.1385) |
| Z31 | 0.02(0.0046 to 0.0353) | 0.0203(0.0021 to 0.0384) | 0.038(0.0198 to 0.0563) | 0.0108(−0.0183 to 0.0398) | 0.0436(−0.0116 to 0.0989) |
| Z3−3 | 0.1075(0.076 to 0.139) | 0.1322(0.0999 to 0.1645) | 0.1094(0.0777 to 0.141) | 0.1445(0.0987 to 0.1902) | 0.2661(0.1081 to 0.4242) |
| Z33 | 0.0111(−0.0059 to 0.028) | 0.0168(−0.0032 to 0.0368) | 0.0161(−0.0053 to 0.0374) | 0.0157(−0.019 to 0.0504) | 0.0302(−0.0077 to 0.0682) |
| Z40 | −0.1235(−0.1484 to −.0986) | −0.1491(−0.174 to −0.1243) | −0.1791(−0.2031 to −0.1551) | −0.1871(−0.2312 to −0.1429) | −0.214(−0.2988 to −0.1291) |
| Z4−2 | −0.0018(−0.0077 to 0.0041) | −0.0032(−0.0093 to 0.0029) | 0.0069(0.0007 to 0.0131) | 0.0042(−0.0063 to 0.0147) | 0.0062(−0.0145 to 0.0269) |
| Z42 | 0.0029(−0.0125 to 0.0183) | 0.0061(−0.0087 to 0.0209) | 0.0024(−0.0136 to 0.0185) | 0.022(−0.0061 to 0.05) | 0.0581(−0.0186 to 0.1348) |
| Z4−4 | 0.0006(−0.009 to 0.0102) | 0.0025(−0.0081 to 0.0131) | −0.0064(−0.0177 to 0.0048) | −0.0106(−0.0266 to 0.0053) | −0.0183(−0.0491 to 0.0125) |
| Z44 | −0.0039(−0.0171 to 0.0093) | 0.0042(−0.0094 to 0.0178) | −0.0077(−0.0216 to 0.0062) | 0.0079(−0.022 to 0.0379) | −0.0441(−0.1205 to 0.0323) |
| Z5−1 | 0.0052(−0.0032 to 0.0137) | 0.0032(−0.0036 to 0.01) | 0.0087(0.0005 to 0.017) | 0.0005(−0.0106 to 0.0117) | −0.0213(−0.0468 to 0.0043) |
| Z51 | 0.0076(0.0037 to 0.0114) | 0.0087(0.0038 to 0.0135) | 0.0076(0.0037 to 0.0115) | 0.0035(−0.0024 to 0.0095) | 0.0137(0.0014 to 0.026) |
| Z5−3 | −0.0024(−0.0088 to 0.004) | −0.0056(−0.0112 to 0.0001) | −0.0087(−0.0147 to −0.0027) | −0.0064(−0.0162 to 0.0034) | 0.0057(−0.0181 to 0.0295) |
| Z53 | 0.001(−0.004 to 0.006) | 0.0039(−0.0006 to 0.0083) | 0.0048(0.0008 to 0.0088) | −0.0047(−0.0106 to 0.0013) | 0.003(−0.0076 to 0.0136) |
| Z5−5 | 0.0026(−0.0035 to 0.0086) | 0.0054(−0.0022 to 0.0129) | 0.0107(0.0041 to 0.0173) | 0.0068(−0.0053 to 0.0188) | −0.0024(−0.0226 to 0.0177) |
| Z55 | −0.0022(−0.009 to 0.0046) | −0.0013(−0.007 to 0.0045) | 0.0022(−0.0046 to 0.0091) | 0.0129(0.0002 to 0.0256) | −0.0102(−0.0317 to 0.0113) |
| Tcoma | 0.119(0.104 to 0.133) | 0.134(0.121 to 0.146) | 0.131(0.117 to 0.144) | 0.164(0.14 to 0.189) | 0.192(0.118 to 0.266) |
| Ttrefoil | 0.113(0.101 to 0.125) | 0.13(0.114 to 0.145) | 0.128(0.116 to 0.14) | 0.151(0.128 to 0.173) | 0.189(0.12 to 0.258) |
| order3 | 0.167(0.15 to 0.184) | 0.191(0.173 to 0.209) | 0.185(0.169 to 0.201) | 0.229(0.198 to 0.259) | 0.276(0.179 to 0.372) |
| order4 | 0.094(0.085 to 0.104) | 0.11(0.1 to 0.12) | 0.119(0.109 to 0.129) | 0.135(0.114 to 0.157) | 0.153(0.104 to 0.202) |
| order5 | 0.036(0.032 to 0.04) | 0.039(0.035 to 0.043) | 0.041(0.038 to 0.045) | 0.048(0.042 to 0.055) | 0.05(0.038 to 0.062) |
| RMS HOA | 0.2641(0.2465 to 0.2817) | 0.2872(0.2683 to 0.3061) | 0.3071(0.2869 to 0.3273) | 0.3753(0.3344 to 0.4162) | 0.3949(0.3246 to 0.4651) |
| RMS HOA without Z40 | 0.2436(0.2258 to 0.2614) | 0.265(0.2465 to 0.2835) | 0.2815(0.2624 to 0.3005) | 0.3475(0.3079 to 0.3872) | 0.3658(0.2989 to 0.4327) |
Tcoma: Total Coma (Z31, Z3−1, Z51, Z5−1).
Ttrefoil: Total Trefoil (Z33, Z3−3, Z53, Z5−3).
RMS HOA: Root mean square Higher Order Aberration.
Fig. 2.
The distributions of Zernike coefficients for eyes. Striped boxes with error bars show the mean and standard deviation when all data are pooled.
Fig. 3.
Absolute Zernike coefficients for eyes in this study. Striped boxes with error bar show the mean and standard deviation.
The relationship between HOAs and variables of age, gender, spherical equivalent, axial length, central corneal thickness, corneal power, and intraocular pressure was studied using multiple Generalized Linear Model models. Results of this model are shown in Table 3. As demonstrated in this table, in the studied relationships, spherical aberration (Z40) decreased with age, correlated indirectly with spherical equivalent, mean corneal power, and central corneal thickness, and significantly increased with higher axial length.
Table 3.
Associations between Zernike coefficients and age, Spherical equivalent (SE), axial length (AL), corneal power (CP), intraocular pressure (IOP), and central corneal thickness (CCT) in multiple Generalized Linear Model.
| Z3−1 |
Z31 |
Z3−3 |
Z40 |
Z42 |
Z4−4 |
Z5−1 |
Z51 |
Z5−3 |
Z53 |
Z55 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef (p-value) | Coef (p-value) | Coef (p-value) | Coef (p-value) | Coef (p-value) | Coef (p-value) | Coef (p-value) | Coef (p-value) | Coef (p-value) | Coef (p-value) | Coef (p-value) | |
| Age (year) | −0.004(0.004) | −0.001(0.044) | |||||||||
| Sex | 0.056(0.006) | −0.009(0.046) | 0.005(0.023) | 0.009(0.016) | |||||||
| Se (diopter) | 0.009(0.043) | −0.026(0.004) | 0.013(0.004) | −0.004(0.048) | −0.004(0) | ||||||
| AL (mm) | 0.029(0.058) | ||||||||||
| CP (diopetr) | −0.018(0.021) | 0.008(0.055) | −0.004(0.028) | −0.003(0.012) | 0.003(0.046) | −0.002(0.047) | |||||
| IOP (mmHg) | 0.007(0.003) | ||||||||||
| CCT (micron) | −0.001(0.004) | 0.001(0.023) | −0.001(0.005) | 0(0.041) | 0(0.036) |
In a multiple Generalized Linear model, the relationship of total RMS coma with the above variables showed that total RMS coma significantly increased with age (Coef = 0.003 and p = 0.042). The relationship of total RMS trefoil with the above variables showed that the mean total RMS trefoil was higher in men and had borderline significance (Coef = 0.016 and p = 0.620), and the mean Total RMS trefoil significantly increased with age (Coef = 0.002 and p = 0.013).
The relationship of the third−, fourth−, and fifth-order aberrations with the studied variables showed that in the multiple model, third-order aberration and aging (Coef = 0.004 and p = 0.003), fourth-order aberration and aging (Coef = 0.003 and p < 0.001), male gender (Coef = −0.013 and p = 0.035), and hyperopia (Coef = 0.006 and p = 0.044) significantly correlated, and fifth-order aberration statistically significantly correlated only with age (Coef = 0.0001 and p < 0.001).
Mean HOA indices (Zernike coefficients) in myopic, hyperopic, and emmetropic groups are presented in Table 4. Mean spherical aberration (Z40) and Z42 were significantly higher among hyperopic cases, and Z51 was lowest in hyperopes. Total RMS coma was also highest among hyperopes. As demonstrated in Table 4, mean third- and fourth-order aberration were also significantly higher in hyperopic cases.
Table 4.
Mean and 95% confidence interval (CI) for Each Zernicke and Root Mean Square Values of Total−, third−, fourth−, and fifth-order, Higher Order Aberrations, Total Coma (Z31, Z3−1, Z51, Z5−1), and Total Trefoil (Z33, Z3−3, Z53, Z5−3) by refractive errors.
| Emmetropia |
Myopia |
Hyperopia |
p-Value | |
|---|---|---|---|---|
| Mean (95%CI) | Mean (95%CI) | Mean (95%CI) | ||
| Z3−1 | 0.005 (−0.02 to 0.031) | 0.003 (−0.043 to 0.048) | −0.034 (−0.102 to 0.035) | 0.567 |
| Z31 | 0.027 (0.016 to 0.038) | 0.01 (−0.011 to 0.031) | 0.044 (0.015 to 0.073) | 0.153 |
| Z3−3 | 0.124 (0.101 to 0.147) | 0.114 (0.087 to 0.142) | 0.169 (0.113 to 0.225) | 0.214 |
| Z33 | 0.014 (0.002 to 0.026) | 0.023 (0.003 to 0.044) | 0.021 (−0.011 to 0.053) | 0.725 |
| Z40 | −0.158 (−0.174 to −0.142) | −0.104 (−0.133 to −0.074) | −0.242 (−0.277 to −0.208) | 0.000 |
| Z4−2 | −0.001 (−0.005 to 0.003) | 0.005 (−0.003 to 0.013) | 0.003 (−0.007 to 0.013) | 0.405 |
| Z42 | 0.01 (−0.001 to 0.021) | −0.011 (−0.029 to 0.006) | 0.032 (0.007 to 0.057) | 0.014 |
| Z4−4 | −0.003 (−0.01 to 0.003) | −0.006 (−0.02 to 0.009) | −0.001 (−0.016 to 0.015) | 0.901 |
| Z44 | −0.005 (−0.016 to 0.005) | 0.008 (−0.005 to 0.022) | −0.007 (−0.036 to 0.021) | 0.241 |
| Z5−1 | 0.002 (−0.003 to 0.006) | 0.01 (0 to 0.019) | 0 (−0.01 to 0.011) | 0.289 |
| Z51 | 0.007 (0.004 to 0.01) | 0.013 (0.008 to 0.017) | 0 (−0.005 to 0.005) | 0.001 |
| Z5−3 | −0.005 (−0.009 to 0) | −0.006 (−0.012 to 0.001) | −0.007 (−0.017 to 0.002) | 0.851 |
| Z53 | 0.001 (−0.001 to 0.004) | 0.003 (−0.002 to 0.008) | 0.001 (−0.004 to 0.006) | 0.787 |
| Z5−5 | 0.006 (0.001 to 0.011) | 0.003 (−0.005 to 0.01) | 0.01 (0.002 to 0.018) | 0.409 |
| Z55 | −0.001 (−0.005 to 0.003) | 0.002 (−0.007 to 0.01) | 0.007 (−0.003 to 0.018) | 0.337 |
| Tcoma | 0.128(0.118 to 0.137) | 0.139(0.123 to 0.155) | 0.164(0.137 to 0.191) | 0.031 |
| Ttrefoil | 0.127(0.117 to 0.136) | 0.125(0.11 to 0.14) | 0.154(0.128 to 0.179) | 0.140 |
| 3rd Order | 0.183(0.171 to 0.196) | 0.19(0.17 to 0.21) | 0.231(0.197 to 0.266) | 0.038 |
| 4rd Order | 0.108(0.101 to 0.115) | 0.108(0.096 to 0.119) | 0.147(0.129 to 0.166) | 0.000 |
| 5rd Order | 0.039(0.036 to 0.042) | 0.042(0.038 to 0.046) | 0.045(0.04 to 0.05) | 0.102 |
| RMS HOA | 0.286 (0.272 to 0.299) | 0.31 (0.287 to 0.333) | 0.364 (0.331 to 0.398) | 0.000 |
| RMS HOA W/O Z40 | 0.263 (0.25 to 0.276) | 0.289 (0.267 to 0.312) | 0.332 (0.298 to 0.365) | 0.000 |
Tcoma: Total Coma (Z31, Z3−1, Z51, Z5−1).
Ttrefoil: Total Trefoil (Z33, Z3−3, Z53, Z5−3).
RMS HOA: Root mean square Higher Order Aberration.
W/O Z40: Without Z40.
Higher order Zernike RMS and higher order Zernike RMS w/o Z40
The mean higher-order Zernike RMS in this study was 0.306 (95% CI: 0.295–0.318) micrometer. As demonstrated in Table 2, higher-order RMS significantly increased with age; higher-order RMS in the 40–44 year old group was 0.264 μm, which increased to 0.395 μm in the over 60 age group. Linear regression showed that every increase in age was significantly correlated with 0.007 μm increase in higher-order RMS (p < 0.001). Mean higher-order RMS was not statistically significantly different between men and women (p = 0.705).
After higher-order RMS categorization, it was observed that 61.6% of the studied population had 0.3 or less higher-order RMS, 0.3 to 0.6 in 34.4%, 0.6 to 0.9 in 2.8%, 0.9 to 1.2 in 0.7%, and more than 1.2 μm in 0.5% of the studied population. Fig. 2 shows the distribution of higher-order RMS in the studied population by gender; the inter-gender difference in higher-order RMS was not statistically significant (p = 0.862).
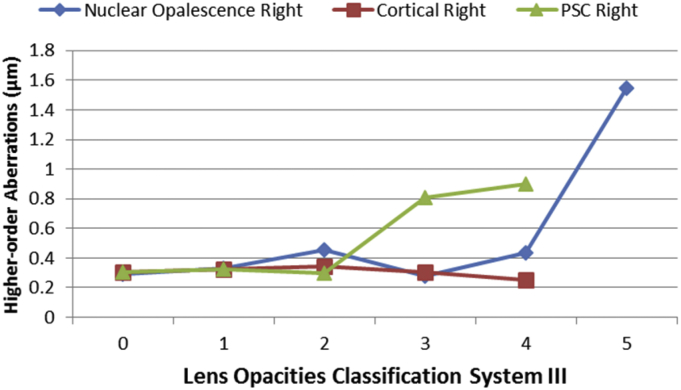
In the linear regression multiple model, entering the variables of age, gender, spherical equivalent, axial length, central corneal thickness, corneal power, and intraocular pressure showed that older age (Coef = 0.007 and p < 0.001) and shorter AL (Coef = −0.019 and p = 0.010) significantly correlated only with higher-order RMS. The highest amounts of higher-order RMS were seen in hyperopes, and the lowest amounts were seen in emmetropes. Generalized Linear Model test revealed that the mean higher-order RMS hyperopes was 0.079 μm higher than in emmetropes (p < 0.001) and 0.054 μm higher than in myopes (p = 0.008). Fig. 4 shows the mean higher-order RMS by different types of lens opacity. Results of the Generalized Linear Model showed that higher-order RMS significantly increased with increased levels of nuclear cataract (p < 0.001), and the higher-order RMS increase with increased levels of posterior sub capsular opacity was of borderline significance (p = 0.427). After adjusting for age and gender, the same results as the simple model were observed. Analysis of Zernike coefficients showed that spherical aberration (Z40) significantly correlated only with nuclear cataract (age-adjusted Coef = 0.370 and p = 0.012), and after adjusting for age, the correlation between total RMS coma and cortical cataract showed borderline significance (Coef = −0.045 and p = 0.098).
Fig. 4.
Mean RMS higher order aberrations by type of lens opacities.
Discussion
We report the distribution of HOAs and Zernike coefficients in an adult population in Iran. There are few similar studies that give a detailed description of these indices by gender, age, and refractive error groups. One of the major problems of a comparison with other studies is the use of different devices for determining aberrations and Zernike indices. Lack of equal pupil size in the measured eyes, different age distributions, various measuring devices, and different visual and refractive statuses of participants in each study necessitates that comparisons be made with caution. Results of 5 studies with different age groups are presented in Table 5 for a comparison with our results. As demonstrated, the mean absolute values of Zernike coefficients is higher in our study.
Table 5.
Absolute Zernike coefficients for eyes in this study and other studies.
| Author | Salmon18 | This study | Kirwan(19) | Prakash(35) | Wang(38) | Wei(14) |
|---|---|---|---|---|---|---|
| Pupil size | 6 | 5 | 6 | 6 | 6 | 6 |
| Age | 21–65 | 40–64 | 4–14 | 18–34 | 20–71 | 21.5–52.8 |
| Zernike notification | ||||||
| Z3−3 | 0.069 ± 0.056 | 0.195 ± 0.194 | 0.091 ± 0.102 | 0.146 ± 0.14 | 0.096 ± 0.076 | 0.154 ± 0.121 |
| Z3−1 | 0.082 ± 0.069 | 0.189 ± 0.214 | 0.215 ± 0.245 | 0.102 ± 0.09 | 0.121 ± 0.093 | 0.217 ± 0.157 |
| Z31 | 0.056 ± 0.047 | 0.142 ± 0.131 | 0.146 ± 0.175 | 0.075 ± 0.08 | 0.082 ± 0.067 | 0.117 ± 0.086 |
| Z33 | 0.052 ± 0.043 | 0.125 ± 0.141 | 0.141 ± 0.20 | 0.050 ± 0.04 | 0.077 ± 0.059 | 0.136 ± 0.104 |
| Z4−4 | 0.023 ± 0.020 | 0.063 ± 0.068 | 0.05 ± 0.060 | 0.076 ± 0.08 | 0.037 ± 0.034 | 0.055 ± 0.040 |
| Z4−2 | 0.017 ± 0.015 | 0.039 ± 0.038 | 0.045 ± 0.056 | 0.092 ± 0.09 | 0.027 ± 0.024 | 0.056 ± 0.043 |
| Z40 | 0.064 ± 0.049 | 0.185 ± 0.148 | 0.171 ± 0.187 | 0.057 ± 0.05 | 0.122 ± 0.077 | 0.237 ± 0.122 |
| Z42 | 0.026 ± 0.023 | 0.078 ± 0.090 | 0.068 ± 0.078 | 0.042 ± 0.04 | 0.052 ± 0.038 | 0.076 ± 0.058 |
| Z44 | 0.025 ± 0.022 | 0.076 ± 0.094 | 0.065 ± 0.093 | 0.032 ± 0.03 | 0.046 ± 0.036 | 0.059 ± 0.059 |
| Z5−5 | 0.011 ± 0.010 | 0.041 ± 0.041 | 0.039 ± 0.066 | 0.035 ± 0.03 | 0.027 ± 0.021 | 0.031 ± 0.030 |
| Z5−3 | 0.01 ± 0.0090 | 0.034 ± 0.034 | 0.026 ± 0.036 | 0.030 ± 0.03 | 0.026 ± 0.021 | 0.034 ± 0.030 |
| Z5−1 | 0.012 ± 0.011 | 0.038 ± 0.042 | 0.042 ± 0.052 | 0.024 ± 0.02 | 0.028 ± 0.023 | 0.038 ± 0.031 |
| Z51 | 0.009 ± 0.008 | 0.026 ± 0.027 | 0.027 ± 0.032 | 0.025 ± 0.03 | 0.026 ± 0.021 | 0.020 ± 0.016 |
| Z53 | 0.008 ± 0.007 | 0.026 ± 0.027 | 0.024 ± 0.033 | 0.026 ± 0.03 | 0.021 ± 0.017 | 0.021 ± 0.018 |
| Z55 | 0.01 ± 0.0090 | 0.039 ± 0.043 | 0.03 ± 0.038 | 0.028 ± 0.03 | 0.027 ± 0.021 | 0.026 ± 0.020 |
On the other hand, mean Zernike coefficients are expected to be lower in 2.5–5 mm pupil diameters. Since the pupil diameter in our study was 5 mm, this difference cannot be attributed to pupil diameter. A better explanation for this difference would be the age distribution of the studied sample. As presented in Table 5, the mean age in our study was the highest, and older age is associated with increasing amounts of HOAs. Overall, comparison of third-to fifth-order order aberrations in this study indicated that they were higher than that of other studies, though they were still within normal ranges.
However, In terms of RMS higher-order aberration, the average of this index in this study was 0.306 μm. Results of other studies are presented in Table 6. Mean total HOA widely ranges from 0.554 μm in the Malaysian population to 0.23 μm in the Caucasian race (Table 6).
Table 6.
Comparison of higher-order aberration in present study with other studies.
| Location | Age | Device | Pupil size | Mean ± SD (micrometer) |
|---|---|---|---|---|
| USA38 | 20–71 | Hartmann-Shack, WaveScan (Visx) | 6 | 0.305 ± 0.095 |
| USA,spain, japan18 | 21–65 | Hartmann Shack zywave (Baush & Lomb) | 6 | 0.327 ± 0.130 |
| Malaysia17 | 22–52 | Hartmann Shack zywave (Baush & Lomb) | 6 | 0.554 ± 0.428 |
| Singapore48 | 18–27 | Hartmann Shack zywave (Baush & Lomb) | 6 | 0.385 ± 0.118 |
| Chine14 | 21.5–52.8 | Hartmann Shack zywave (Baush & Lomb) | 6 | 0.49 ± 0.16 |
| India35 | 18–34 | Hartmann Shack zywave (Baush & Lomb) | 6 | 0.36 ± 0.26 |
| USA (caucasian)49 | 20–67 | Hartmann-Shack,WaveScan (Visx) | 6 | 0.23 ± 0.11 |
| Current study (Iran) | 40–64 | Hartmann Shack zywave (Baush & Lomb) | 5 | 0.306 ± 0.16 |
| Brazil (asian)50 | 32.78 ± 7.69 | OPD Scan (Nidek) | 6 | 0.514 ± 0.711 |
| Brazil (non-asian)50 | 30.93 ± 7.98 | OPD Scan (Nidek) | 6 | 0.553 ± 0.705 |
Since every millimeter increase in pupil size can overestimate total HOA by 0.12 μm, it seems that our sample falls in the mid-range in terms of total higher-order RMS. The highest amount of total higher-order RMS is seen in the eastern Asian population, and the lowest amount in Americans.
Overall, in addition to age and measurement method, genetic and ethnic differences seems to be one of the important reasons for differences in aberrations. Lim and Fam17 attributed this difference in eastern Asians to less corneal prolateness compared to other races. Prakash et al35 demonstrated that the amount of aberrations in normal eyes of the Indian ethnicity was close to that in Caucasians but different from that in the Chinese. Overall, these ethnicity-related differences in the amount of aberrations must be considered in each population separately when developing nomograms for refractive surgery.
In this study, we observed that different orders of Zernike coefficients are affected by different factors. As demonstrated, age was the most important variable that correlated with some Zernike indices in the multiple model with RMS higher-order aberration. Several studies have documented the relationship between aberrations and age.18, 36, 37, 38, 39 Amano et al36 observed that coma, especially corneal coma, increased with age. This relationship and an increase in trefoil with age were also shown in our study. It seems that developing an imbalance between the aberrations of the anterior corneal surface and the interior eye may be responsible for the increase in ocular aberrations with age.14 However, it must be noted that the important changes in the crystalline lens fibers and its thickening with aging cause the aberrations of the crystalline lens to increase, and even in eye diseases such as retinal diseases and cataract, they are important causes of increased age-related aberrations. Overall, other studies have shown increasing aberrations with age.18, 36, 37, 38, 39 However, some studies also show a decrease in age-related aberrations up to the third decade due to the optical structure and emmetropization, and an increase in ocular aberrations after the third decade of life due to lens changes.20, 39
As we observed, hyperopes had the highest levels of aberrations. Although most studies state that myopes have more aberrations,15, 20 overall, there are contrasting results in this regard. Kirwan et al19 demonstrated that third-order aberrations were higher in myopia, and other studies showed higher levels of coma in myopes.19, 40 Nonetheless, in some studies, there were no significant differences among different refractive groups in terms of aberrations.41 Marcos observed a significant increase in corneal spherical aberrations at higher degrees of myopia in young people, but the intraocular spherical aberrations became more negative in these people due to the crystalline lens.42 Llorente et al43 reported that third-order aberrations (coma and trefoil) and total ocular spherical aberration were higher in young hyperopic eyes than young myopic eyes, while intraocular spherical aberration was not significantly different between these two groups. Studying 5 mm pupils in 675 adolescents, Philip et al44 demonstrated more positive total spherical aberration in hyperopic eyes compared to myopic and emmetropic eyes, but there was no difference among the three groups in terms of corneal spherical aberrations. Results of this study are indicative of crystalline lens changes in hyperopes compared to emmetropes and myopes, and higher aberrations in these people are attributed to their lenses. Observing higher aberrations in our study appears to be in relation to the lens. The age group in our study was 40–64 years old, and hyperopia in this population is usually due to changes in the lens; therefore, these changes are associated with an increase in aberrations. Furthermore, total RMS coma constituted a larger proportion of HOAs in hyperopes. As we know, the angle kappa is larger in hyperopes compared to myopes and emmetropes, and a larger displacement of the pupilary axis from the visual axis is responsible for higher levels of coma among hyperopes.44 Overall, since aberrations, especially higher orders of aberrations can negatively impact the quality of the retinal image, they can be associated with progression of refractive errors.45 Based on our findings after adjusting for age and gender, increased nuclear opacity significantly correlated with increased RMS higher-order aberration. PSC opacity also correlated with RMS higher-order aberration with borderline level of significance. The relationships between different types of cataract and aberrations have previously been shown by Rocha et al16 and Sachdev et al.46 As demonstrated, spherical aberration and nuclear cataract significantly correlated in our study, which was reported by previous studies as well.16, 46, 47 Other studies also showed a significant correlation between coma and cortical cataract while this correlation in our study showed borderline significance. Since the opacity appears in the center of the lens in nuclear cataract and does not allow simultaneous focus of peripheral and central rays, the appearance of spherical aberrations is not unexpected.
There were some limitations in this study. The most important limitation was that aberrations were assessed in 5 mm pupil diameter only. The assessment of aberration were done in a subsample of study.
This report is the first to describe the distribution of HOAs in an Iranian population. HOAs in this study were on average higher than those reported in previous studies, and this issue must be considered in diagnostic and therapeutic procedures. HOAs increase with aging and differ by refractive group.
Financial support
This project was supported by Noor Ophthalmology Research Center and Shahroud University of Medical Sciences.
Conflict of interest
No conflicting relationship exists for any author.
Footnotes
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Carvalho L.A., Castro J.C., Carvalho L.A. Measuring higher order optical aberrations of the human eye: techniques and applications. Braz J Med Biol Res. 2002;35:1395–1406. doi: 10.1590/s0100-879x2002001100019. [DOI] [PubMed] [Google Scholar]
- 2.Salmon T.O., van de Pol C. Evaluation of a clinical aberrometer for lower-order accuracy and repeatability, higher-order repeatability, and instrument myopia. Optometry. 2005;76:461–472. doi: 10.1016/j.optm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Hofer H., Chen L., Yoon G.Y., Singer B., Yamauchi Y., Williams D.R. Improvement in retinal image quality with dynamic correction of the eye's aberrations. Opt Express. 2001;8:631–643. doi: 10.1364/oe.8.000631. [DOI] [PubMed] [Google Scholar]
- 4.Thibos L.N. Retinal image quality for virtual eyes generated by a statistical model of ocular wavefront aberrations. Ophthalmic Physiol Opt. 2009;29:288–291. doi: 10.1111/j.1475-1313.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi T., Negishi K., Ohnuma K., Tsubota K. Correlation between contrast sensitivity and higher-order aberration based on pupil diameter after cataract surgery. Clin Ophthalmol. 2011;5:1701–1707. doi: 10.2147/OPTH.S21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buhren J., Martin T., Kuhne A., Kohnen T. Correlation of aberrometry, contrast sensitivity, and subjective symptoms with quality of vision after LASIK. J Refract Surg. 2009;25:559–568. doi: 10.3928/1081597X-20090610-01. [DOI] [PubMed] [Google Scholar]
- 7.Oshika T., Okamoto C., Samejima T., Tokunaga T., Miyata K. Contrast sensitivity function and ocular higher-order wavefront aberrations in normal human eyes. Ophthalmology. 2006;113:1807–1812. doi: 10.1016/j.ophtha.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 8.Padmanabhan P., Basuthkar S.S., Joseph R. Ocular aberrations after wavefront optimized LASIK for myopia. Indian J Ophthalmol. 2010;58:307–312. doi: 10.4103/0301-4738.64139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAlinden C., Moore J.E. Comparison of higher order aberrations after LASIK and LASEK for myopia. J Refract Surg. 2010;26:45–51. doi: 10.3928/1081597X-20101215-07. [DOI] [PubMed] [Google Scholar]
- 10.Gatinel D., Adam P.A., Chaabouni S. Comparison of corneal and total ocular aberrations before and after myopic LASIK. J Refract Surg. 2010;26:333–340. doi: 10.3928/1081597X-20090617-01. [DOI] [PubMed] [Google Scholar]
- 11.Nanavaty M.A., Spalton D.J., Marshall J. Effect of intraocular lens asphericity on vertical coma aberration. J Cataract Refract Surg. 2010;36:215–221. doi: 10.1016/j.jcrs.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Kim S.W., Ahn H., Kim E.K., Kim T.I. Comparison of higher order aberrations in eyes with aspherical or spherical intraocular lenses. Eye Lond. 2008;22:1493–1498. doi: 10.1038/eye.2008.302. [DOI] [PubMed] [Google Scholar]
- 13.Tzelikis P.F., Akaishi L., Trindade F.C., Boteon J.E. Ocular aberrations and contrast sensitivity after cataract surgery with AcrySof IQ intraocular lens implantation clinical comparative study. J Cataract Refract Surg. 2007;33:1918–1924. doi: 10.1016/j.jcrs.2007.06.053. [DOI] [PubMed] [Google Scholar]
- 14.Wei R.H., Lim L., Chan W.K., Tan D.T. Higher order ocular aberrations in eyes with myopia in a Chinese population. J Refract Surg. 2006;22:695–702. doi: 10.3928/1081-597X-20060901-11. [DOI] [PubMed] [Google Scholar]
- 15.Li T., Zhou X., Chen Z., Chu R., Hoffman M.R. Relationship between ocular wavefront aberrations and refractive error in Chinese school children. Clin Exp Optom. 2012;95:399–403. doi: 10.1111/j.1444-0938.2012.00739.x. [DOI] [PubMed] [Google Scholar]
- 16.Rocha K.M., Nose W., Bottos K., Bottos J., Morimoto L., Soriano E. Higher-order aberrations of age-related cataract. J Cataract Refract Surg. 2007;33:1442–1446. doi: 10.1016/j.jcrs.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 17.Lim K.L., Fam H.B. Ethnic differences in higher-order aberrations: spherical aberration in the South East Asian Chinese eye. J Cataract Refract Surg. 2009;35:2144–2148. doi: 10.1016/j.jcrs.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Salmon T.O., van de Pol C. Normal-eye Zernike coefficients and root-mean-square wavefront errors. J Cataract Refract Surg. 2006;32:2064–2074. doi: 10.1016/j.jcrs.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Kirwan C., O'Keefe M., Soeldner H. Higher-order aberrations in children. Am J Ophthalmol. 2006;141:67–70. doi: 10.1016/j.ajo.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 20.He J.C., Sun P., Held R., Thorn F., Sun X., Gwiazda J.E. Wavefront aberrations in eyes of emmetropic and moderately myopic school children and young adults. Vis Res. 2002;42:1063–1070. doi: 10.1016/s0042-6989(02)00035-4. [DOI] [PubMed] [Google Scholar]
- 21.Cervino A., Hosking S.L., Ferrer-Blasco T., Montes-Mico R., Gonzalez-Meijome J.M. A pilot study on the differences in wavefront aberrations between two ethnic groups of young generally myopic subjects. Ophthalmic Physiol Opt. 2008;28:532–537. doi: 10.1111/j.1475-1313.2008.00592.x. [DOI] [PubMed] [Google Scholar]
- 22.KhabazKhoob M., Hashemi H., Yazdani K., Mehravaran S., Yekta A., Fotouhi A. Keratometry measurements, corneal astigmatism and irregularity in a normal population: the Tehran Eye Study. Ophthalmic Physiol Opt. 2010;30:800–805. doi: 10.1111/j.1475-1313.2010.00732.x. [DOI] [PubMed] [Google Scholar]
- 23.Hashemi H., Khabazkhoob M., Yazdani K., Mehravaran S., Mohammad K., Fotouhi A. White-to-white corneal diameter in the tehran eye study. Cornea. 2010;29:9–12. doi: 10.1097/ICO.0b013e3181a9d0a9. [DOI] [PubMed] [Google Scholar]
- 24.Hashemi H., KhabazKhoob M., Mehravaran S., Yazdani K., Mohammad K., Fotouhi A. The distribution of anterior chamber depth in a Tehran population: the Tehran eye study. Ophthalmic Physiol Opt. 2009;29:436–442. doi: 10.1111/j.1475-1313.2009.00647.x. [DOI] [PubMed] [Google Scholar]
- 25.Hashemi H., Yazdani K., Mehravaran S. Corneal thickness in a population-based, cross-sectional study: the tehran eye study. Cornea. 2009;28:395–400. doi: 10.1097/ICO.0b013e31818c4d62. [DOI] [PubMed] [Google Scholar]
- 26.Hashemi H., Yazdani K., Khabazkhoob M., Mehravaran S., Mohammad K., Fotouhi A. Distribution of photopic pupil diameter in the Tehran eye study. Curr Eye Res. 2009;34:378–385. doi: 10.1080/02713680902853327. [DOI] [PubMed] [Google Scholar]
- 27.Hashemi H., Khabazkhoob M., Jafarzadehpur E. High prevalence of myopia in an adult population, Shahroud. Iran Optom Vis Sci. 2012;89:993–999. doi: 10.1097/OPX.0b013e31825e6554. [DOI] [PubMed] [Google Scholar]
- 28.Rezvan F., Khabazkhoob M., Fotouhi A. Prevalence of refractive errors among school children in Northeastern Iran. Ophthalmic Physiol Opt. 2012;32:25–30. doi: 10.1111/j.1475-1313.2011.00879.x. [DOI] [PubMed] [Google Scholar]
- 29.Yekta A., Fotouhi A., Hashemi H. The prevalence of anisometropia, amblyopia and strabismus in schoolchildren of Shiraz, Iran. Strabismus. 2010;18:104–110. doi: 10.3109/09273972.2010.502957. [DOI] [PubMed] [Google Scholar]
- 30.Yekta A., Fotouhi A., Hashemi H. Prevalence of refractive errors among schoolchildren in Shiraz. Iran Clin Exp Ophthalmol. 2010;38:242–248. doi: 10.1111/j.1442-9071.2010.02247.x. [DOI] [PubMed] [Google Scholar]
- 31.Fotouhi A., Hashemi H., Khabazkhoob M., Mohammad K. The prevalence of refractive errors among schoolchildren in Dezful, Iran. Br J Ophthalmol. 2007;91:287–292. doi: 10.1136/bjo.2006.099937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fotouhi A., Hashemi H., Shariati M. Cohort profile: shahroud eye cohort study. Int J Epidemiol. 2012;42:1300–1308. doi: 10.1093/ije/dys161. [DOI] [PubMed] [Google Scholar]
- 33.Hament W.J., Nabar V.A., Nuijts R.M. Repeatability and validity of Zywave aberrometer measurements. J Cataract Refract Surg. 2002;28:2135–2141. doi: 10.1016/s0886-3350(02)01333-0. [DOI] [PubMed] [Google Scholar]
- 34.Livingstone S.J., Looker H.C., Hothersall E.J. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012;9:e1001321. doi: 10.1371/journal.pmed.1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prakash G., Sharma N., Choudhary V., Titiyal J.S. Higher-order aberrations in young refractive surgery candidates in India: establishment of normal values and comparison with white and Chinese Asian populations. J Cataract Refract Surg. 2008;34:1306–1311. doi: 10.1016/j.jcrs.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 36.Amano S., Amano Y., Yamagami S. Age-related changes in corneal and ocular higher-order wavefront aberrations. Am J Ophthalmol. 2004;137:988–992. doi: 10.1016/j.ajo.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Calver R.I., Cox M.J., Elliott D.B. Effect of aging on the monochromatic aberrations of the human eye. J Opt Soc Am A Opt Image Sci Vis. 1999;16:2069–2078. doi: 10.1364/josaa.16.002069. [DOI] [PubMed] [Google Scholar]
- 38.Wang L., Koch D.D. Ocular higher-order aberrations in individuals screened for refractive surgery. J Cataract Refract Surg. 2003;29:1896–1903. doi: 10.1016/s0886-3350(03)00643-6. [DOI] [PubMed] [Google Scholar]
- 39.Brunette I., Bueno J.M., Parent M., Hamam H., Simonet P. Monochromatic aberrations as a function of age, from childhood to advanced age. Invest Ophthalmol Vis Sci. 2003;44:5438–5446. doi: 10.1167/iovs.02-1042. [DOI] [PubMed] [Google Scholar]
- 40.Buehren T., Collins M.J., Carney L. Corneal aberrations and reading. Optom Vis Sci. 2003;80:159–166. doi: 10.1097/00006324-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Atchison D.A., Schmid K.L., Pritchard N. Neural and optical limits to visual performance in myopia. Vis Res. 2006;46:3707–3722. doi: 10.1016/j.visres.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Radermecker R.P., Philips J.C., Scheen A.J. How I treat...severe hypoglycemia in a diabetic patient. Rev Med Liege. 2003;58:119–122. [PubMed] [Google Scholar]
- 43.Llorente L., Barbero S., Cano D., Dorronsoro C., Marcos S. Myopic versus hyperopic eyes: axial length, corneal shape and optical aberrations. J Vis. 2004;4:288–298. doi: 10.1167/4.4.5. [DOI] [PubMed] [Google Scholar]
- 44.Guilleminault C., Yuen K.M., Gulevich M.G., Karadeniz D., Leger D., Philip P. Hypersomnia after head-neck trauma: a medicolegal dilemma. Neurology. 2000;54:653–659. doi: 10.1212/wnl.54.3.653. [DOI] [PubMed] [Google Scholar]
- 45.Charman W.N. Aberrations and myopia. Ophthalmic Physiol Opt. 2005;25:285–301. doi: 10.1111/j.1475-1313.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 46.Sachdev N., Ormonde S.E., Sherwin T., McGhee C.N. Higher-order aberrations of lenticular opacities. J Cataract Refract Surg. 2004;30:1642–1648. doi: 10.1016/j.jcrs.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 47.Wali U.K., Bialasiewicz A.A., Al-Kharousi N., Rizvi S.G., Baloushi H. Subjective and quantitative measurement of wavefront aberrations in nuclear cataracts – a retrospective case controlled study. Middle East Afr J Ophthalmol. 2009;16:9–14. doi: 10.4103/0974-9233.48858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carkeet A., Velaedan S., Tan Y.K., Lee D.Y., Tan D.T. Higher order ocular aberrations after cycloplegic and non-cycloplegic pupil dilation. J Refract Surg. 2003;19:316–322. doi: 10.3928/1081-597X-20030501-08. [DOI] [PubMed] [Google Scholar]
- 49.Netto M.V., Ambrosio R., Jr., Shen T.T., Wilson S.E. Wavefront analysis in normal refractive surgery candidates. J Refract Surg. 2005;21:332–338. doi: 10.3928/1081-597X-20050701-06. [DOI] [PubMed] [Google Scholar]
- 50.Nakano E.M., Bains H., Nakano K. Wavefront analysis in Asian-Brazilians. J Refract Surg. 2006;22:S1024–S1026. doi: 10.3928/1081-597X-20061102-03. [DOI] [PubMed] [Google Scholar]