Abstract
The study aimed to explore the protective effects of AS-IV against sepsis-induced ALI. Sepsis was induced by cecal ligation and puncture (CLP) method in Sprague Dawley rats. Rats were randomly assigned into five groups: animals undergoing a sham CLP (sham group); animals undergoing CLP (CLP group); animals undergoing CLP and treated with AS-IV at 2.5 mg/kg bw (low-dose AS-IV [L-AS] group), at 5 mg/kg bw (mid-dose AS-IV [M-AS] group), and at 10 mg/kg bw (high-dose AS-IV [H-AS] group). At 6 h, 12 h and 24 h post-CLP surgery, six rats were respectively sacrificed to collect blood and lung tissue samples. The levels of arterial blood gas index, lung water content, protein level and leukocyte counts (total amount, neutrophils and lymphocytes) in bronchoalveolar lavage fluid (BALF) and cytokines such as TNF-α and IL-6 in BALF were measured at each time point in different groups. HE-staining and optical microscopy were performed to examine the pathological changes in lungs. The 72 h-survival rate of each group was also recorded. PaO2 was decreased significantly, while the lung water content, BALF protein level, cell numbers, BALF cytokine TNF-α and IL-6 levels were increased significantly for CLP group as compared with sham group. Moreover, pathological injury was observed in lung tissue indicating the successful sepsis-induced ALI model. Speaking of the effect of AS-IV, we founded that, compared with the CLP group, the AS-IV treatment groups could significantly alleviate all the above negative changes exited in the CLP group in a dose-dependent manner. What’s more, the pathological injury was also gradually improved by AS-IV treatment compared with the CLP rats. AS-IV exerts its protective effect against sepsis-induced ALI in rats via improving pulmonary ventilation function, decreasing the permeability of alveolar epithelium and capillary as well as repressing lung inflammation.
Keywords: Astragaloside IV, Cecal ligation and puncture, Sepsis, Acute lung injury
1. Introduction
Sepsis, a common complication of burn, trauma, hypoxia, and post-surgery, is a systemic inflammatory response syndrome caused by infection (Tjardes and Neugebauer, 2002, Lever and Mackenzie, 2007). With relatively high morbidity mortality, sepsis is considered to be the leading cause of death of patient in the intensive care unit (ICU) (Tjardes and Neugebauer, 2002, Levy et al., 2001, Angus and Van der Poll, 2013). Multiple organ function impairment may occur at severe sepsis and ultimately develop to multiple organ dysfunction syndrome (Marshould, 2001). Lung is one of the most vulnerable organs at sepsis with acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) which occurs at early stage and is with high morbidity (Hudson et al., 1995, Husak et al., 2010, Angus et al., 2001). The severe pulmonary inflammation, vascular permeability, diffuses infiltration in both lungs, and pulmonary alveoli edema, hypoxemia and lung compliance decrease are the characteristics for ALI (Villar et al., 2011). Both intra pulmonary factors and extrinsic pulmonary factors make contribution to the pathogenesis of ALI/ARDS. The intra factors include aspiration pneumonia, severe diffuse lung infection, pulmonary contusion, and extrinsic factors include sepsis caused by extrinsic pulmonary infection, wound shock and burn (Brun-Buisson et al., 2004, Gattinoni et al., 1998, Sheu et al., 2010). Among these, sepsis is the most common cause of ALI (Suntharalingam et al., 2001, Rocco and Zin, 2005). Currently, there are still no effective drugs and therapies for the treatment of sepsis-associated ALI/ARDS. The main method is supportive treatment such as mechanical ventilation for respiration support. However, increasing evidence has demonstrated that mechanical ventilation brings damage to organs when improving the oxygenation for the patients. Mechanical tension caused by excessive mechanical ventilation, is an important cause for lung injury (Rocco and Zin, 2005, Martin et al., 2003) and could often cause inflammation in lungs. Therefore, development for new method of curing ALI caused by sepsis other than mechanical ventilation is imperative.
Astragaloside IV (AS-IV) is a saponin separated from astragalus with wide biological and pharmacological activity. Pharmacological experiments showed that AS-IV has protective effects, such as anti-inflammation (Zhang et al., 2003, Gui et al., 2013), antioxidant (Gui et al., 2012) anti-brain infarction, neuro-protection (Luo et al., 2004, Cheng et al., 2006), anti-hypertension (Zhang et al., 2006), and myocardial protection (Li and Cao, 2002). However, there are only a few researches about the effect of AS-IV on lung injury. Xiong et al. (2010) found AS-IV is protective for rat pulmonary ischemia–reperfusion lung injuries. Chen et al. (2016) thought AS-IV exerted the protective effect for paraquat induced rat lung injuries by restraining Rho/ROCK/NF-κB signal pathway. This study aimed to seek the effect of AS-IV on ALI through further research on the sepsis ALI rat model.
2. Materials and methods
2.1. Reagent and animals
Astragaloside IV was purchased from Shrqbio Co. Ltd. (Shanghai, China, purity ⩾98%). BCA Protein Assay Kit, rat TNF-α and IL-6 ELISA kits were purchased from Wuhan Boster Bio-Engineering Co. Ltd. (Wuhan, China).
Healthy male Sprague Dawley rats (200 ± 20 g) were purchased from Guangdong Medical Laboratory Animal Center (Guangdong, China). All animals were raised at SPF laboratory animal room with a 12 h light/dark cycle at 24 ± 2 °C and 40–70% humidity. Animals were allowed to have free access to water and food during experiment period. All animal experiment operations were conducted according to nursing and use guidance for animal experiment operation of National Institutes of Health.
2.2. Animal groups
After a period of adaptation, ninety SD rats were selected and divided into five random groups (n = 20 per group): sham group, CLP control group, low-dose AS-IV (L-AS) group, middle-dose AS-IV (M-AS) group, and high-dose AS-IV (H-AS) group. The AS-IV was diluted into three different concentrations with 1% carboxymethyl cellulose (CMC). The L-AS, M-AS and H-AS groups were administrated with AS-IV by gavage at the dosages of 2.5 mg/kg bw, 5 mg/kg bw and 10 mg/kg bw, respectively, while the sham group and sepsis model group were gavaged with 1% CMC at the same volume. The indicators determined in this study were observed at three time points (6 h, 12 h and 24 h post-CLP), and each time six rats were used. Another fifty SD rats were distributed into the same five groups as above for the observation of survival rate.
2.3. Acute lung injury molding
Sepsis was introduced by CLP technique as described previously (Ritter et al., 2003, Sener et al., 2005). Briefly, after a 12-h deprivation of food but not water, the rats were generally anesthetized with chloral hydrate anesthesia (0.3 ml/100 g bw), and then a midline abdominal incision was made to expose the cecum. After a cecal ligation treatment, an 18-gauge needle was used to puncture through the central segment of ligation, and a small amount of cecal contents was squeezed out through the puncture wound. Then, the cecum was restored into the abdominal cavity and the surgical incision was sutured layer by layer. In sham group rats, the cecum was exposed and the bowel was massaged as described above, but it was not ligated or punctured.
2.4. Aortic blood-gas analyses
Six animals were sacrificed 6 h, 12 h and 24 h after CLP, respectively, by intraperitoneal injection of chloral hydrate (0.3 ml/100 g bw) for general anesthesia. Blood samples of 2 ml were obtained from aorta abdominals for arterial blood gas analyses by automatic blood gas analyzer (America Nova Company, Nova-K type).
2.5. Dry/wet ratio of lung
Right upper pulmonary lobe was excised, blotted dry and weighed, and then placed in an oven at 70 °C for 48 h to obtain the dry weight. The ratio of the wet lung to the dry lung was calculated to assess tissue edema.
2.6. Histopathology observation
Right lower lobe was taken from the animals which had been executed after 6 h, 12 h and 24 h of CLP respectively. After being washed wash with saline solution, the organ was fixed by formalin to make paraffin section. The pathological changes of lung tissue should be observed by optical microscope after hematoxylin and eosin staining.
Four fields at 200× magnification were randomly selected from each lung tissue section and six sections were randomly chosen from each mouse. Two investigators blinded to group assignments analyzed the samples and determined levels of lung injury according to the semiquantitative scoring outlined below. The degree of lung injury was evaluated using a five-grade method described by Franco as follows: 1. Normal; 2. Interstitial inflammatory cell infiltration <50%; 3. Interstitial inflammatory cell infiltration >50%; 4. Consolidation of lung and inflammatory cell infiltration <50%; 5. Consolidation of lung and inflammatory cell infiltration >50%. The mean score was used for comparison between groups.
2.7. Measurement of protein content, cytokine and cell counting in the BALF
Left lung was obtained and lavaged by 0.5 ml saline from the bronchus alveolar for 4 times. And then, the BALF was collected and centrifuged for 10 min with the speed of 2500 r/min at room temperature. Supernatant was collected and stored at −80 °C for the measurement of protein content and inflammatory mediators such as TNF-a and IL-6, while the sediment was used for the leukocyte differential counting. The protein content was determined by the method of BCA and level of TNF-α and IL-6 was tested with corresponding ELISA kit. The BALF cell sediment was re-suspended in 0.1 ml of saline, centrifuged onto slides and stained for 8 min with Wright–Giemsa staining. Differential cell counting for neutrophil and lymphocyte was conducted through quantification of the slides by counting a total of 200 cells/slide at 40∗ magnification.
2.8. Survival study
The rat’s survival rates were recorded at 72 h in another experiment (n = 10 per group) as mentioned above to confirm whether AS-IV treatment would confer protection against sepsis-induced ALI. And pathological autopsy was done for the dead rats to observe the definite symptoms.
2.9. Statistical analysis
Statistical analysis of data was performed by Graph Pad Prism5.00 and all data were expressed as means ± SD. Changes of different groups were compared by the method of one-way ANOVA. The survival rate curve should be analyzed by log-rank. A P value < 0.05 was considered statistically significant.
3. Results
3.1. Effect of AS-IV on the aorta blood oxygen pressure of CLP-induced ALI rat
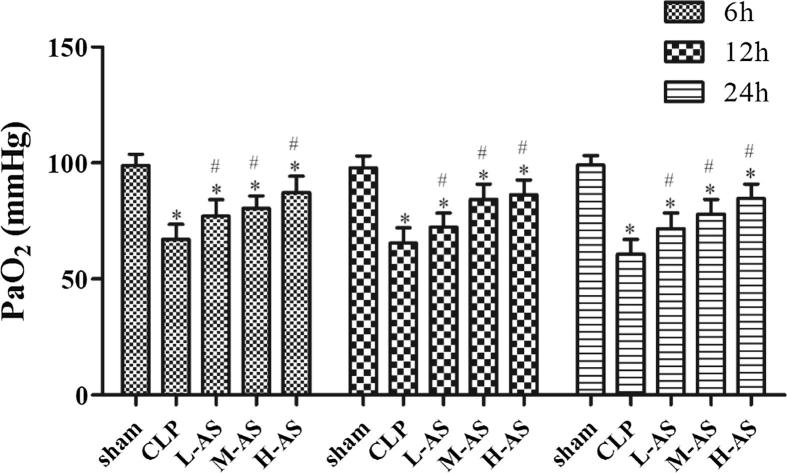
As demonstrated in Fig. 1, the PaO2 of CLP group at every time point was significantly lower than the sham group (P < 0.05), indicating the pulmonary gas exchange function of rats in CLP group was significantly decreased. And compared with CLP group, PaO2 of the three AS-IV treatment groups was significantly increased (P < 0.05) in a dose-dependent manner. The result demonstrated that AS-IV had an obvious improving effect on the gas exchange function of lung.
Figure 1.
AS-IV increased PaO2 in CLP-induced ALI rats. Data are presented as mean ± SD, n = 6. *P < 0.05 compared with sham rats and #P < 0.05, compared with CLP rats.
3.2. Effect of AS-IV on the lung water content of CLP-induced ALI rat
As shown in Fig. 2, the lung water content in CLP group was significantly increased (P < 0.05) at every time point compared with sham group, indicating the severe pulmonary edema in CLP group. When it comes with the AS-IV treatment groups, at 6 and 12 h post-CLP, lung water content of M-AS and H-AS group was significantly lower (P < 0.05) than CLP group, while there was no obvious difference for the lung water content (P > 0.05) between the L-AS group and CLP group. When at 24 h post-CLP, lung water content of the three AS-IV treatment groups was all significantly lower (P < 0.05) than that of CLP group.
Figure 2.
AS-IV reduced lung W/D ratio in CLP-induced ALI rats. Data are presented as mean ± SD, n = 6. *P < 0.05 compared with sham rats and #P < 0.05 compared with CLP rats.
3.3. The effect of AS-IV on the BALF protein content of CLP-induced ALI rat
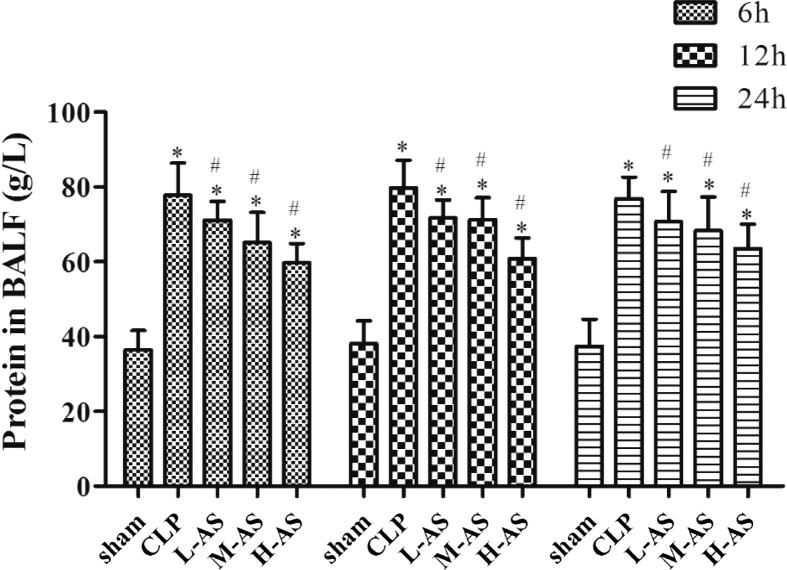
As demonstrated in Fig. 3, the BALF protein content in CLP group at each time point was markedly higher (P < 0.05) than that of sham group, which indicated that, after lung injury, the obvious capillary permeability of alveolar led to leakage of protein. Speaking of the effect of AS-IV, all of the three AS-IV treatment groups showed varying degrees’ decrease (P < 0.05) on the level of BALF protein compared with CLP group.
Figure 3.
AS-IV decreased the amount of protein in the BALF of CLP-induced ALI rats. Data are presented as mean ± SD, n = 6. *P < 0.05 compared with sham rats and #P < 0.05 compared with CLP rats.
3.4. Effect of AS-IV on the BALF cell cytokine of CLP-induced ALI rat
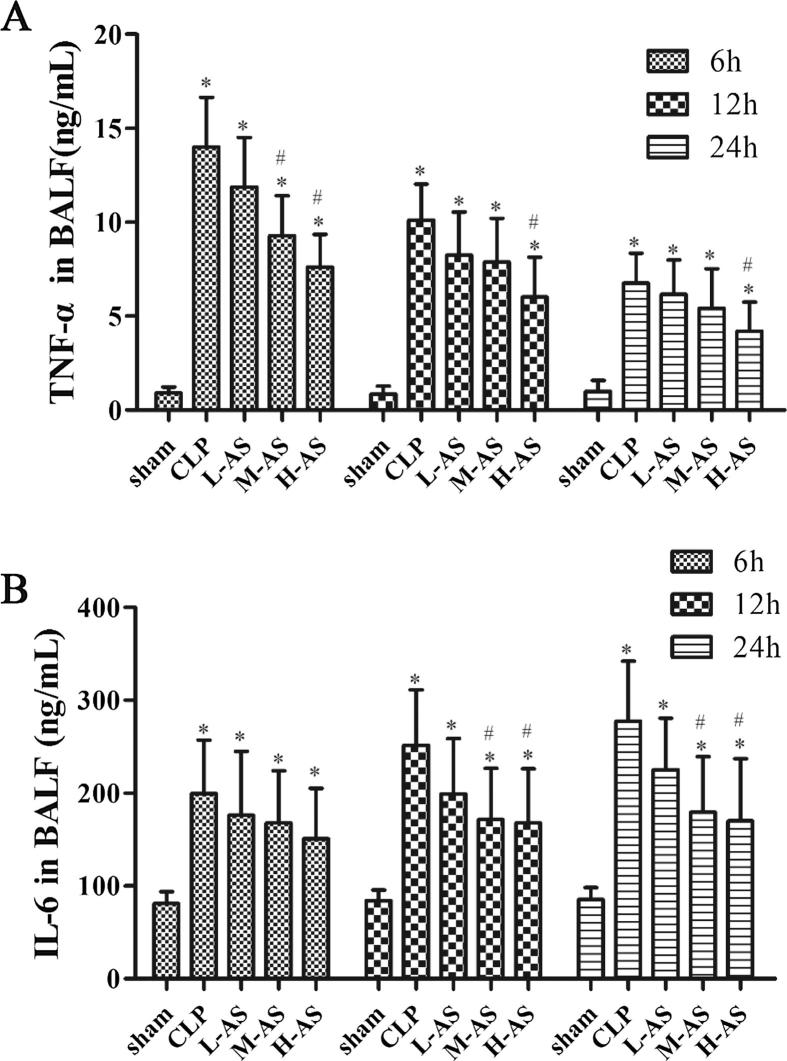
BALF cell cytokine levels of TNF-α and IL-6 were detected to investigate the effect of AS-IV on the BALF Cell Cytokine secretion. As shown in Fig.4(A), TNF-α level of CLP group rats at each time point increased significantly (P < 0.05) compared with the sham group, reaching its peak 6 h after CLP surgery and then declining gradually over time. Speaking of the effect of AS-IV treatment, only the TNF-α level in H-AS group was significantly decreased (P < 0.05) at all of the three time points. From Fig.4(B) we found that, IL-6 level of rats in CLP group at each time point was notably higher than that in sham group (P < 0.05), and grew with the time, finally reaching its peak at 24 h after CLP surgery. At 6 h post-CLP, there was no significant difference (P > 0.05) between all of the three dose AS-IV groups and CLP group. When it comes to the time points of 12 and 24 h after operation, the IL-6 level in H-AS group and M-AS group was significantly decreased (P < 0.05) compared with CLP group, while there was no significant difference for IL-6 level between L-As group and CLP group (P > 0.05).
Figure 4.
AS-IV inhibited TNF-α and IL-6 expression in the BALF of CLP-induced ALI rats. Data are presented as mean ± SD, n = 6. *P < 0.05 compared with sham rats and #P < 0.05 compared with CLP rats.
3.5. Effect of AS-IV on the BALF inflammation cell counting
As shown in Fig. 5, the total BALF cell amount, neutrophil granulocyte and lymph cell counting of CLP at each time point were significantly higher than those of sham group (P < 0.05). And compared with CLP group, the BALF total cells, neutrophils and lymphocytes in AS-IV treated groups decreased remarkably at each time point and the differences were of statistical significance (P < 0.05).
Figure 5.
AS-IV reduced the number of total cells, neutrophils and lymphocytes in the BALF of CLP-induced ALI rats. Data are presented as mean ± SD, n = 6. *P < 0.05 compared with sham rats and #P < 0.05 compared with CLP rats.
3.6. Effect of AS-IV on pulmonary morphology
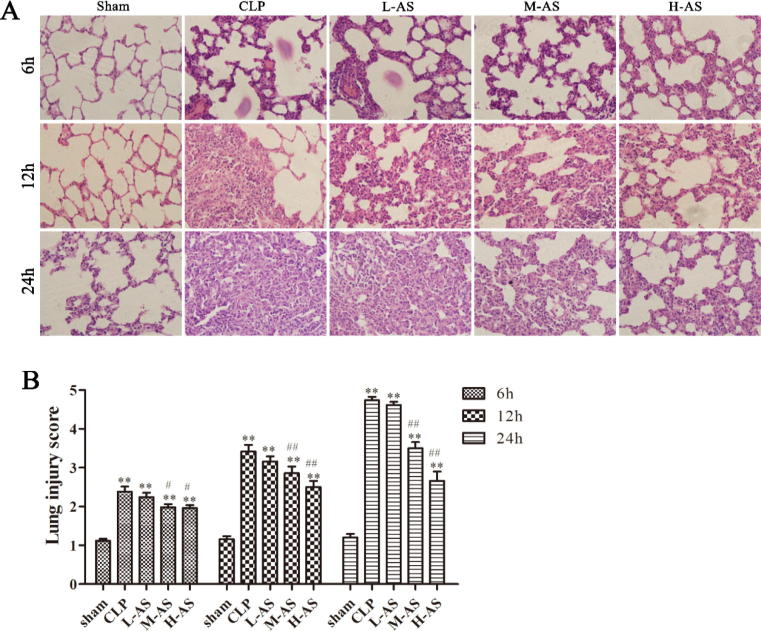
Histopathological examination (Fig. 6) showed that, the lung tissue of rats in sham group was morphologically normal at 6, 12 and 24 h post-CLP, without broadening of alveolar septum, inflammation cell in alveolar cavity and leakage of interstitial fluid. But compared with the sham groups, severe damage on the pulmonary alveoli structure was observed for all of the CLP and AS-IV-treated groups, with the appearance of histopathological changes such as complete destruction of pulmonary alveoli structure, pulmonary edema, inflammation cell infiltration, pulmonary vascular congestion and hemorrhage. And the harm was more and more serious over time. AS-IV-treated groups gradually alleviated the pulmonary alveoli damage by increase in dosage administration. In terms of pulmonary morphology at each time point, the grading result of lung tissue pathology indicated that the lung tissue pathology grade for CLP was always remarkably higher than the sham group (all is P < 0.01). The lung tissue pathology grading of H-As and M-As group fell significantly as compared with CLP group (P < 0.05; P < 0.01; P < 0.01). However, there was no significant difference between the H-As group and CLP group (all is P > 0.05). The pulmonary morphology results indicated that the AS-IV could inhibit the pathological damage of lung tissue for the sepsis rat in a dose-dependent.
Figure 6.
AS-IV ameliorated histopathological changes in lung tissues of CLP-induced ALI rats. (A) Representative images of hematoxylin and eosin (H&E) staining from each experimental group at 6 h, 12 h and 24 h after CLP. Original magnification, (B) mean histopathological lung injury scores 6 h, 12 h and 24 h after CLP. Data are presented as mean ± SD, n = 6. *P < 0.05, **P < 0.01 compared with sham rats and #P < 0.05, ##P < 0.01 compared with CLP rats.
3.7. Effect of AS-IV on survival rate
As demonstrated in Fig. 7, the survival rates of CLP group and L-As group were 40% and 60%, respectively, which were significantly different (P < 0.01; P < 0.05) compared with the sham group. Although the 72 h survival rate of L-As group was higher than CLP group there was no statistical significance (P > 0.05). The 72 h survival rates for M-As and H-As group were 80% and 90%, respectively and 40% and 50% (P < 0.05; P < 0.05) higher than the CLP group. Moreover, there were no significant differences for the 72 h survival rates between M-As, H-As group and sham group (P > 0.05; P > 0.05). The result indicated that both the AS-IV median/high dose could effectively raise the 72 h survival rate of rat with acute lung injury.
Figure 7.
AS-IV increased the 72 h survival curves of CLP-induced ALI rats, n = 10 for each group. *P < 0.05, **P < 0.01 compared with sham rats and #P < 0.05 compared with CLP rats.
Meanwhile, hemorrhagic odor exudation in the abdominal cavity, flatulence in small intestine, swelling, darkening, necrosis, adhesion at cecal of ligation end, and obvious lung congestion were found through pathological autopsy for the dead rat. And the symptoms of animals in the three AS-IV treatment groups were lighter than CLP group which indicated AS-IV had a protective effect on sepsis acute lung injury rat.
4. Discussion
Sepsis, the clinic syndrome of extreme high morbidity rate and mortality, is regarded as the first cause of death for ICU patient. The lung is one of the most vulnerable target organs and ALI induced by sepsis is one of the major causes for death (Brun-Buisson et al., 2004, Gattinoni et al., 1998). Natural products have long been used to prevent and treat diseases, including inflammatory and immune-related disease. Recent research has found that the AS-IV, which is the natural compound extracted from a traditional Chinese herb Astragalus membranaceus, has protective effect for rat pulmonary ischemia–reperfusion lung injuries (Xiong et al., 2010, Chen et al., 2016). So, the present study aimed to investigate whether AS-IV has the ability of protecting rats of ALI associated with sepsis and improving pathophysiologic changes in sepsis-induced ALI.
As we know, early responses for CLP (sluggish response, activity decrease, excretion at oral cavity and eye, watery stool, shiver and body temperature decrease) occurred in rat after 6 h of operation, and the obvious inflammatory responses (extrinsic allergic alveolitis, alveolar hemorrhage and pulmonary edema) came at 12 h after operation, and then lethality occurred around 18 h after operation, reaching peak after 24 h. And considering that, restorative change happened after 72 h by rat resumed intake of food and water, increase in activity, gradual alleviation of pathological change, this research chose three time points at 6 h, 12 h, and 24 h after CLP operation for sampling and observed 72 h survival rate combined with general condition change, pulmonary inflammation cell infiltration, pulmonary alveoli blood capillary membrane permeability increase and pulmonary congestion, hemorrhage change and time related change pattern.
Firstly, we examined the function of AS-IV on lung air exchange in sepsis-induced ALI. The air exchange is the most important function for the lung. The change of pulmonary ventilation function is the important basis for evaluation of ALI progress. ALI will lead to the drop of pulmonary volume, compliance, imbalance of ventilation/perfusion ratio, and oxygenation damage, which characterized clinically symptoms as refractory hypoxemia. PaO2 is a simple and effective objective evidence for the evaluation of lung air exchange function (El-Khatib and Jamaleddine, 2004). Therefore, this study adopted PaO2 to evaluate the change of lung air exchange of each group. The result showed that the PaO2 was ameliorated by all of the three AS-IV treatment groups, indicating that the AS-IV could improve the lung air exchange function.
Then, the effect of AS-IV on lung epithelial permeability and microvascular permeability in sepsis-induced ALI rats was also evaluated. A major feature of ALI is the change of permeability of lung epithelial and capillaries micrangium caused by lung epithelial and capillaries micrangium cell damage. The failure of normal fluid exudation and resorption could cause pulmonary edema. Abnormal increase in vascular permeability would result in permeation of macromolecule protein beyond the blood vessel, which led to increase of protein in alveoli and pulmonary interstitium (Perl et al., 2011). In this research, pulmonary water and protein content of BALF were both adopted to evaluate the change of permeability of lung epithelial and capillaries micrangium. The result showed that the AS-IV could significantly reduce the pulmonary water and BALF protein contents of sepsis-induced ALI rats, indicating that the AS-IV is capable of alleviating the permeability of lung epithelial and capillaries micrangium.
Also, indicators of inflammation reaction were detected in this study, since ALI is considered as the manifestation of systematic inflammation at lung. The occurrence of pro-inflammatory cell factor in the early stage of inflammation reaction is important for lung injury at late stage (Lotze and Tracey, 2005, Marques-Rocha et al., 2015). This research detected the level of TNF-α and IL-6 which are the mediators of inflammation reaction (Marques-Rocha et al., 2015, Siebert et al., 2015).
The result showed that AS-IV could remarkably reduce the level of inflammation factor TNF-α and IL-6 of CLP-induced ALI rats. In addition, the ALI would lead to activation and accumulation of lung inflammation cell to generate the enhanced infection cell factor and expedite the permeability of alveolar blood capillary. This research has found that AS-IV could reduce the total amount of leukocyte, the amount of neutrophile granulocyte and lymph cell. The pathological stain of pulmonary morphology indicated that AS-IV could effectively restrain the pathological changes of pulmonary edema, inflammation reaction, pulmonary vascular congestion and hemorrhage. These results were consistent with the previous reports in literatures, indicating that AS-IV could effectively alleviate the lung inflammation reaction of CLP-induced ALI rats and more effective with higher dosage (Xiong et al., 2010, Chen et al., 2016). However, the specific signal pathway that is affected by AS-IV to restrain the lung inflammation reaction is yet to be further elaborated.
5. Conclusion
In summary, this research revealed that AS-IV could protect sepsis-induced ALI rats from lung injury by improving lung air exchange, restraining the change of lung epithelial permeability and pulmonary microvascular permeability, lowering lung inflammation reaction and alleviating lung pathological damage injury, which eventually raised the survival rate of sepsis-induced ALI rats.
Footnotes
Peer review under responsibility of King Saud University.
References
- Angus D.C. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Angus D.C., Van der Poll T. Severe sepsis and septic shock. N. Engl. J. Med. 2013;369:2063. doi: 10.1056/NEJMc1312359. [DOI] [PubMed] [Google Scholar]
- Brun-Buisson C. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intens. Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- Chen T. Protective effect of AS-IV against paraquat-induced lung injury in mice by suppressing Rho signaling. Inflammation. 2016;39:483–492. doi: 10.1007/s10753-015-0272-4. [DOI] [PubMed] [Google Scholar]
- Cheng C.Y. The role of astragaloside in regeneration of the peripheral nerve system. J. Biomed. Mater. Res. Part A. 2006;76:463–469. doi: 10.1002/jbm.a.30249. [DOI] [PubMed] [Google Scholar]
- El-Khatib M.F., Jamaleddine G.W. A new oxygenation index for reflecting intrapulmonary shunting in patients undergoing open-heart surgery. Chest. 2004;125:592–596. doi: 10.1378/chest.125.2.592. [DOI] [PubMed] [Google Scholar]
- Gattinoni L. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am. J. Respir. Crit. Care Med. 1998;158:3–11. doi: 10.1164/ajrccm.158.1.9708031. [DOI] [PubMed] [Google Scholar]
- Gui D. Astragaloside IV ameliorates renal injury in streptozotocin-induced diabetic rats through inhibiting NF-kappaB-mediated inflammatory genes expression. Cytokine. 2013;61:970–977. doi: 10.1016/j.cyto.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Gui D. AS-IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS ONE. 2012;7:e39824. doi: 10.1371/journal.pone.0039824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L.D., Milberg J.A., Anardi D., Maunder R.J. Clinical risks for development of the acute respiratory distress syndrome. Am. J. Res. Crit. Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- Husak L. National analysis of sepsis hospitalizations and factors contributing to sepsis in-hospital mortality in Canada. Healthc. Quart. 2010;13:35–41. doi: 10.12927/hcq.2010.21963. [DOI] [PubMed] [Google Scholar]
- Lever A., Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ. 2007;335:879–883. doi: 10.1136/bmj.39346.495880.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M.M. SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit. Care. Med. 2001;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- Li Z.P., Cao Q. Effects of AS-IV on myocardial calcium transport and cardiac function in ischemic rats. Acta Pharmacol. Sinica. 2002;23:898–904. [PubMed] [Google Scholar]
- Lotze M.T., Tracey K.J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Luo Y. AS-IV protects against ischemic brain injury in a murine model of transient focal ischemia. Neurosci. Lett. 2004;363:218–223. doi: 10.1016/j.neulet.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Marques-Rocha J.L. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J.: Off. Pub. Fed. Am. Soc. Exp. Biol. 2015;29:3595–3611. doi: 10.1096/fj.14-260323. [DOI] [PubMed] [Google Scholar]
- Martin G.S., Mannino D.M., Eaton S., Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Marshould J.C. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit. Care Med. 2001;29:S99–106. doi: 10.1097/00003246-200107001-00032. [DOI] [PubMed] [Google Scholar]
- Perl M., Lomas-Neira J., Venet F., Chung C.S., Ayala A. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev. Resp. Med. 2011;5:115–126. doi: 10.1586/ers.10.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter C. Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intens. Care Med. 2003;29:1782–1789. doi: 10.1007/s00134-003-1789-9. [DOI] [PubMed] [Google Scholar]
- Rocco P.R., Zin W.A. Pulmonary and extrapulmonary acute respiratory distress syndrome: are they different? Curr. Opin. Crit. Care. 2005;11:10–17. doi: 10.1097/00075198-200502000-00003. [DOI] [PubMed] [Google Scholar]
- Sener G., Toklu H., Ercan F., Erkanli G. Protective effect of beta-glucan against oxidative organ injury in a rat model of sepsis. Int. Immunopharmacol. 2005;5:1387–1396. doi: 10.1016/j.intimp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Sheu C.C. Clinical characteristics and outcomes of sepsis-related vs non-sepsis-related ARDS. Chest. 2010;138:559–567. doi: 10.1378/chest.09-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert S., Tsoukas A., Robertson J., McInnes I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol. Rev. 2015;67:280–309. doi: 10.1124/pr.114.009639. [DOI] [PubMed] [Google Scholar]
- Suntharalingam G., Regan K., Keogh B.F., Morgan C.J., Evans T.W. Influence of direct and indirect etiology on acute outcome and 6-month functional recovery in acute respiratory distress syndrome. Crit. Care Med. 2001;29:562–566. doi: 10.1097/00003246-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Tjardes T., Neugebauer E. Sepsis research in the next millennium: concentrate on the software rather than the hardware. Shock. 2002;17:1–8. doi: 10.1097/00024382-200201000-00001. [DOI] [PubMed] [Google Scholar]
- Villar J. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intens. Care Med. 2011;37:1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- Xiong P., Jiang L.Z., Liao X.Q. Morphological investigation of the protective effect of astragaloside preconditioning against ischemia-reperfusion lung injury in rats. Nan fang yi ke da xue xue bao – J. South. Med. Uni. 2010;30:1864–1867. [PubMed] [Google Scholar]
- Zhang W.J., Hufnagl P., Binder B.R., Wojta J. Antiinflammatory activity of AS-IV is mediated by inhibition of NF-kappaB activation and adhesion molecule expression. Thromb. Haemos. 2003;90:904–914. doi: 10.1160/TH03-03-0136. [DOI] [PubMed] [Google Scholar]
- Zhang W.D. AS-IV dilates aortic vessels from normal and spontaneously hypertensive rats through endothelium-dependent and endothelium-independent ways. Planta Med. 2006;72:621–626. doi: 10.1055/s-2006-931572. [DOI] [PubMed] [Google Scholar]