Abstract
This study focuses on investigation of cryptorchidism induced by flutamide (Flu) and its histopathological damage, and detects retinoic acid concentration in testicle tissue, in order to find a new method for clinical treatment to infertility caused by cryptorchidism. Twenty SD (Sprague Dawley) pregnant rats were randomly divided into Flu cryptorchidism group (n = 10) and normal control group (n = 10). HE stained for observing morphological difference. Transmission electron microscope (TEM) was used for observing the tight junction structure between Sertoli cells. Epididymal caudal sperms were counted and observed in morphology. The expression of stimulated by retinoic acid gene 8 (Stra8) was detected using immunohistochemistry, western blot, and Q-PCR. High performance liquid chromatography (HPLC) analysis was made on retinoic acid content. Sperm count and morphology observation confirmed cryptorchidism group was lower than normal group in sperm quantity and quality. The observation by TEM showed a loose structure of tight junctions between Sertoli cells. Immunohistochemistry, western blot, and Q-PCR showed that cryptorchidism group was significantly lower than normal group in the expression of Stra8. HPLC showed that retinoic acid content was significantly lower in cryptorchid testis than in normal testis. In the cryptorchidism model, retinoic acid content in testicular tissue has a significant reduction; testicles have significant pathological changes; damage exists in the structure of tight junctions between Sertoli cells; Stra8 expression has a significant reduction, perhaps mainly contributing to spermatogenesis disorder.
Keywords: Flutamide, Rat, Cryptorchidism, Testicle, Retinoic acid, Pathological change
1. Introduction
Cryptorchidism is one of the most common congenital malformations in the genitourinary system in boys with an incidence up to 3–4% (Kolon et al., 2014). With social development as well as increased physical and chemical factors in environmental pollution, this incidence has a trend to increase year by year (Guerrero-Bosagna and Skinner, 2014). Studies show that 38% of patients with unilateral cryptorchidism suffer from oligospermia with an infertility rate of 60–100% (La Vignera et al., 2009). Cryptorchidism has become one of important reasons for infertility in males. Currently, the mechanisms causing cryptorchidism and testicular damage are not yet clear, but it is believed that this is a result from genetic and environmental factors (Kurahashi et al., 2005). Studies confirm that Flu can block the effect of androgens in embryonic stage, affect testicular development, and inhibit testicular descent (Okur et al., 2006). In this study, cryptorchidism rats model was used to detect retinoic acid content in testicular tissue and investigate pathology, thereby laying a foundation for the study of male infertility caused by cryptorchidism and providing new ideas for clinical research of treatment programs.
2. Materials and methods
2.1. Reagents
Reagents were analytically pure flutamide (Sigma, F9397, USA), corn oil (Aladdin, Shanghai), rabbit Stra8 antibody (Abcam, ab49602, USA), rabbit Scp3 primary antibody (Abcam, ab85621, USA), goat anti-rabbit secondary antibody detection kit (ZSGB-BIO, PV-6001, Beijing), RNA rapid extraction kit (BioTeke, Beijing), RNA reverse transcription kit (TaKaRa, DRR037S, Japan), total protein extraction kit (KeyGEN, Nanjing), SDS-PAGE gel preparation kit (Beyotime), ECL detection kit (Millpore, Germany), and Stra8 primer sequence (Sangon Biotech, Shanghai).
2.2. Animals and drug
SD rats (purchased from Animal Center of Chongqing Medical University; animal certificate number: SYXK (Chongqing) 2011–0001; weight 280–310 g) were reared in Animal Center, Children’s Hospital of Chongqing Medical University (rearing temperature: 20–25 °C, relative humidity: 40–60%). Once adulthood was reached, males and females were caged together (1:1). Then, observations were made at 08:00 daily on whether the vaginal plug appeared. The female rat having such plug was separately reared (designated as 0 d of gestation, namely, GD0). 20 pregnant rats were randomly divided into 2 groups: (1) Flu group, 10 pregnant rats, GD 11–19 d, injected with anti-androgen drug Flu 25 mg/kg (prepared using corn oil) and (2) control group, 10 pregnant rats, regularly reared, not given any drugs or reagents. According to the criteria by van Haaster and de Rooij (1993), the total number of male offspring and the number of the offspring of cryptorchidism were observed with the incidence of cryptorchidism calculated on postnatal day 20 (PND20). Normal and cryptorchid testicle tissues were extracted from the offspring on PND60, embedded in paraffin, and sliced (4 μm), for HE, immunohistochemical staining. Some of testicular tissues on PND60 were extracted and stored in liquid nitrogen for Western blot and Q-PCR.
2.3. HE staining
The testicular tissue from each group was sliced and stained with HE for observing changes in morphology and histology.
2.4. TEM
The rat testis specimens were cut into small pieces (about 1 × 1 × 1 mm3), fixed in 3% glutaraldehyde for 1 h, washed 3 times with PBS, fixed for 1 h in 1% OsO4, dehydrated with ethanol, embedded in epoxy resin, made into ultra-thin slices (50 nm), and stained for observing the tight junction structure in testicle under TEM (Philips TECNAI 10, USA).
2.5. Sperm count
Sperm collection: The bilateral caudal epididymis was placed in two preheated (37 °C) dishes, respectively, and cut longitudinally into pieces with ophthalmic scissors. 1 dish was added 10 mL PBS solution (preheated to 37 °C) where No. 1 sperm suspension was prepared for sperm counts. The other dish was added with 1 mL PBS solution where No. 2 sperm suspension was prepared for observing sperm morphology. Then it was placed in a water bath at 37 °C (constant temperature) and incubated for 10 min, so that sperms could be fully free. Sperm count: The hemocytometer was used to count caudally epididymal sperm density. (1) The hemocytometer pipette was used to transfer 10 μL No. 1 sperm suspension to fill the count pool of the count plate in the said meter; (2) the counting plate was stood still for 15 min on a box covered with a damp cloth, so that all the sperms could settle on the same focal plane of the count plate; (3) the low magnification (10×) microscope was used to find the square of the count plate center, and a count pool where sperms were distributed evenly was selected for counting under the high magnification (40×) microscope. Sperms in 4 small squares in the big square of the center were counted (namely, small squares at four corners). The calculation formula is as follows: total sperm count in four small squares × 50,000 × 100 per mL (Parhizkar et al., 2013). Sperm morphology observation: (1) 10 μL No. 2 sperm suspension was taken and made into a sperm smear, naturally dried at room temperature; (2) this smear was fixed in 99% methanol for 5 min, and naturally dried; (3) this smear was stained with 2% Eosin for 1 h, gently rinsed with distilled water, naturally dried, and mounted; and (4) sperm morphology was observed under an optical microscope.
2.6. Immunohistochemistry
Instructions are followed in the SP immunohistochemistry kit in detecting the paraffin slice of rat testicle tissue from each group. Rabbit anti-rat Stra8 antibody concentration was 1:1000. DAB coloring was made under an optical microscope. PBS instead of primary antibody was as negative control. It was re-stained with hematoxylin. If brown color appeared, then Stra8 would be positive.
2.7. Western blotting
100 mg testicular tissue from each group was taken and cleaved, respectively, according to instructions of KGI total protein extraction kit. The tissue extract was subject to centrifuge for 5 min at 1000 r/min at a low temperature in the centrifugal device. Protein concentration was assayed using BCA. Total protein was added with the loading buffer and boiled for 5 min until fully denatured. Electrophoresis was made at 10% SDS–PAGE, and electroporated into the nitrocellulose membrane. TBST buffer containing 50 g/L skim milk was used to block the nitrocellulose membrane at room temperature for 1 h; added with Stra8 and Scp3 primary antibodies (1:1000), overnight at 4 °C; washed 3 times with TBST, 5 min each time; added with goat anti-rabbit secondary antibody (1:2000); 1 h after washing, rinsed with TBST 5 min × 3 times; and finally, photographed for determination of gray-scale values using ECL technique.
2.8. Q-PCR
Total RNA was extracted from the testicle of male offspring according to instructions of the tissue RNA extraction kit. RNA concentration was determined via an ultraviolet spectrophotometer. RNA purity was identified. β-Actin was as internal control. Application software Primer 5.0 was used to design primer sequences with Stra8 and internal control β-actin. Stra8: forward primer was 5′-AGCACAGCACCTTCTCGG-3′; downstream primer 5′-AAGCTCGCCACATCAAAA-3′; expected size of product fragment was 160 bp. Internal control β-actin: forward primer was 5′-GGAGATTACTGCCCTGGCTCCTA-3′; reverse primer 5′-GACTCATCGTACTCCTGCTTGCTG-3′; expected size of product fragment was 150 bp. Primers were synthesized by Sangon Biotech, Shanghai. Stra8 reaction conditions were as follows: 95 °C denaturation 3 min; 95 °C denaturation 20 s, 54 °C annealing 15 s, extension 60 °C 30 s, a total of 40 cycles. The experiment was repeated three times for each sample. According to Ct values for Stra8 and β-actin in fluorescence quantitative PCR, 2-ΔCt was calculated to express the relative expression level of Stra8 gene.
2.9. HPLC
Column: Shimadzu C18 column (4.6 × 250 mm); mobile phase; V (acetonitrile): V (0.1% acetic acid) = 77:23; detection wavelength of flow rate 1.0 mL/min: 350 nm; column temperature: 25 °C; Configuring standard solution: Weigh accurately 1 mg ATRA standard product to a brown simpler vial; dissolve with acetonitrile as solvent by ultrasonic wave and dilute to obtain 1 mg/mL standard product, which is in dark and refrigerated for HPLC analysis. Processing tissue samples: Take 600 μL 60d testicular tissue homogenate in a clean brown flask, add 2 mL 0.025 mol/L NaOH solution in methanol, shake well 1 min, add 6 mL n-hexane, shake well 1 min, centrifuge (8000 r/min) 5 min, discard n-hexane phase, add 120 μL 4 mol/L HCl in aqueous phase, shake well 1 min, add 6 mL n-hexane, shake well 1 min, centrifuge (8000 r/min) 5 min, transfer upper phase to a clean and dry glass bottom-pointed test tube. Dry with a nitrogen-drying apparatus. Dissolve the residue with 200 μL acetonitrile, shake well 1 min, and transferred to a brown sampler for HPLC analysis. Perform HPLC analysis on all-trans retinoic acid content from each group.
2.10. Statistical methods
All experimental data were expressed in mean ± standard deviation (x ± s) or rate (%). Statistical analysis of each group was made using SPSS 17.0 software. Groups were compared using t-test and χ2 test. P < 0.05 was considered statistically significant.
3. Results
3.1. Incidence of cryptorchidism
The total number of rat pups from Flu cryptorchidism and normal control group was 98 and 93, respectively, and the number of male rat pups was 48 and 42, respectively. Flu group successfully induced cryptorchidism model with the incidence of cryptorchidism being 42.86% (18/42), unilateral cryptorchidism 33.33% (14/42), and bilateral cryptorchidism 9.52% (4/42). In the normal control group, the incidence of cryptorchidism was 4.17% (2/48), all unilateral.
3.2. Morphological change of testes
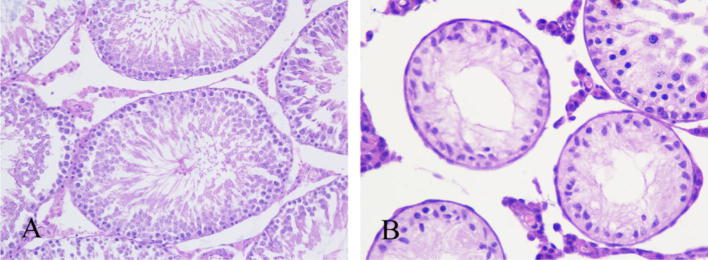
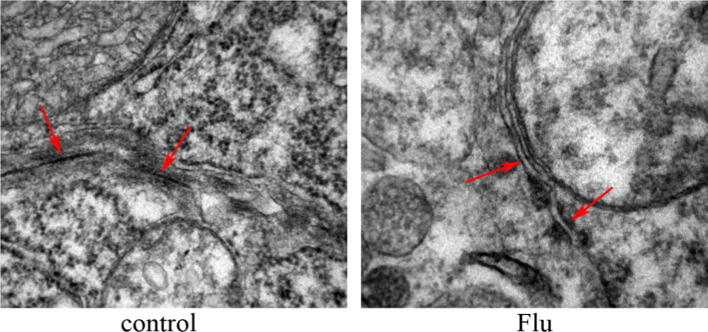
HE results showed that in the Flu cryptorchidism group, the seminiferous tubule had a significant constriction with the tubule gap becoming larger; spermatogenic cells were disorder, reduced in the number, and found with growth retardation; no fluff sperm formation was found in the tubule center (Fig. 1). TEM showed that the tight junction structure was absent and had a gap between two Sertoli cells in the cryptorchidism group (Fig. 2).
Figure 1.
Testicular histology of rat (PND 60 d, HE, 400×). (A) Normal control group (spermatogenic cells in normal development; lots of sperm cells and sperms in tubule center); (B) Flu cryptorchidism group (tubule showed obvious atrophy; spermatogenic cells were significant reduction and no sperm was observed in tubule center).
Figure 2.
Tight junctions in testicle in normal control group and Flu cryptorchid group (PND 60 d, TEM, 50,000x).
3.3. Sperm count and morphology
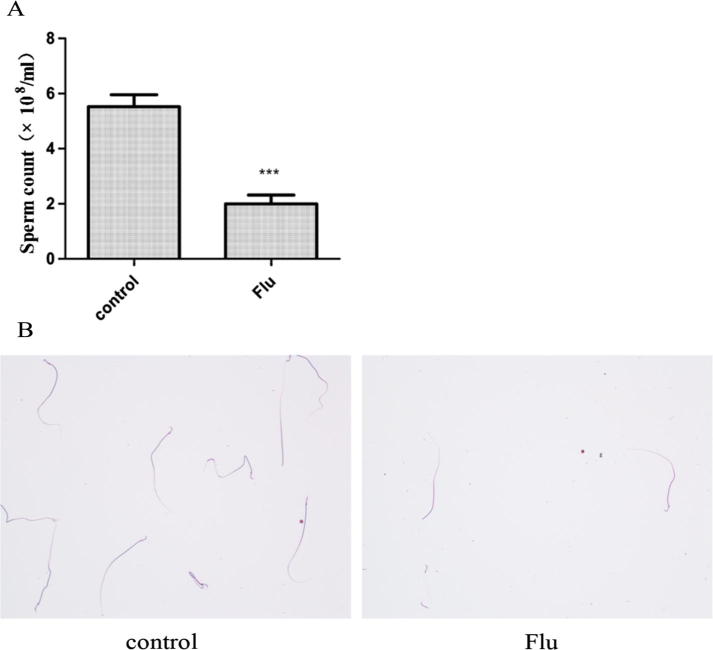
Sperm count was (5.53 ± 0.17) × 108/mL in normal control group and (1.99 ± 0.13) × 108 in cryptorchidism group (Fig. 3A). Morphological observation showed that sperms in cryptorchidism group had a significant reduction in density, and that deformity was much higher (including broken heads, short tails, and 2-head malformations) (Fig. 3B); therefore, the sperm quantity and quality, cryptorchidism group were lower than normal control group.
Figure 3.
Sperm count and morphological observation (200×). (A) Cryptorchidism group was less than normal control group (P < 0.05); (B) sperms were found with malformations (including broken heads) in cryptorchidism group.
3.4. Stra8 expression
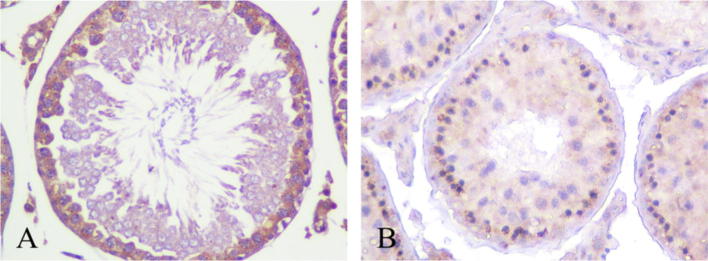
Immunohistochemistry was used to detect the expression of Stra8 in each group. Results showed a lot of Stra8 protein expression in normal control group, but in Flu cryptorchidism group, Stra8 expression was significantly down-regulated (Fig. 4).
Figure 4.
Stra8 expression in testis (PND 60 d, SP, 400×). (A) Normal control group, Stra8 resides in cytoplasm, positive staining is brown. (B) Flu cryptorchidism group, intensity of Stra8 is lower than control group.
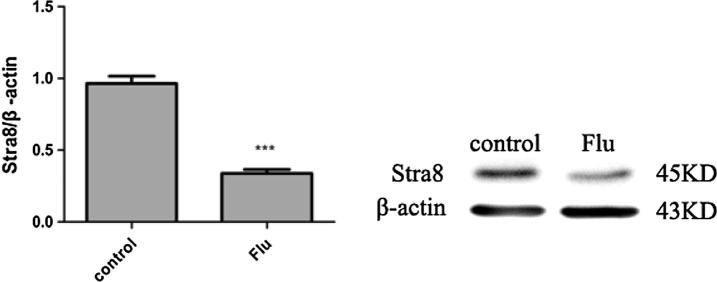
Western Blot results showed that Stra8 expression had a significant down-regulation in Flu cryptorchidism group (Fig. 5). Semi-quantitative analysis was made on gray values of the strip by ImageJ.
Figure 5.
Stra8 expression in testis, Flu cryptorchidism group (0.34 ± 0.05) was significantly lower than normal control group (0.96 ± 0.09) (P < 0.05).
Q-PCR results were well consistent with the results of immunohistochemistry and Western Blot (Fig. 6).
Figure 6.
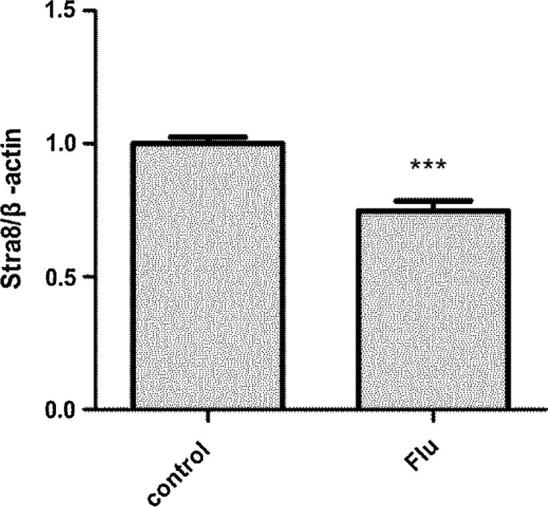
Stra8 mRNA expression in testicle of rats on PND 60 d, Flu group (0.765 ± 0.015) was lower than control group (1 ± 0.01) (P < 0.05).
3.5. Retinoic acid content
To confirm the relationship between cryptorchidism and RA, we detected RA concentration in testes of the two groups, and HPLC results showed that RA concentration in Flu cryptorchidism group was significantly lower than normal control group (P < 0.05) (Fig. 7).
Figure 7.
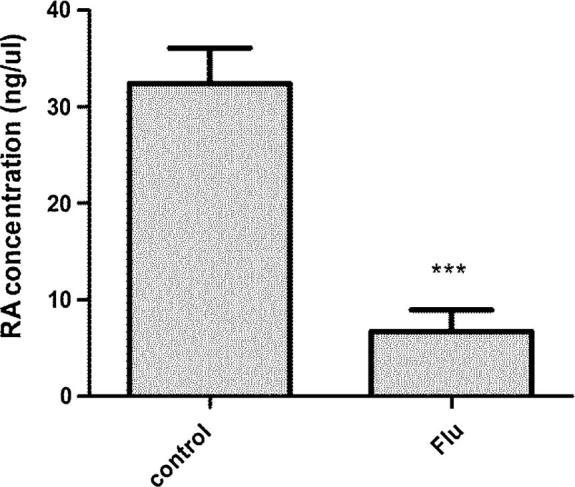
RA concentration in testis tissue, this concentration in normal control group was 19.28 ± 1.03 ng/μL, and cryptorchidism group had 7.25 ± 0.16 ng/μL.
4. Discussions
Male infertility is a common complication of cryptorchidism. Cryptorchidism has become one of important reasons for male infertility. To study histopathological changes of cryptorchidism and detect retinoic acid concentration in testicles are very meaningful for the treatment of male infertility.
The pathological changes of testis with cryptorchidism were observed in the past mostly from clinical biopsy (Moretti et al., 2007). In this study, the rats with cryptorchidism were successfully induced by giving Flu during pregnancy (time of administering Flu was selected at key time for male reproductive system development which was also sensitive time for the Flu reproductive toxicity). The cryptorchidism model showed that testis had obvious pathological changes, thereby being consistent with previous reports of biopsy results (Bergh and Soder, 2007).
RA plays a very important role in the process of spermatogenesis. Wolbach found that vitamin A deficiency could delay spermatogenesis in 1925. Further studies showed that this was mainly due to RA as an active metabolite of vitamin A, and can regulate some processes in spermatogenesis, including spermatogonium differentiation, meiosis, sperm formation. RA has some isomers, of which all-trans RA (atRA) mainly affects cell proliferation, differentiation and apoptosis (Huang et al., 2001).
Therefore, in this study, the change of RA content was detected in testis to reveal the relationship between RA and infertility caused cryptorchidism. HPLC results showed that RA content had a significant reduction in testis with cryptorchidism, which may be related with the damage of Sertoli cells in cryptorchid testes. Studies have shown that most effects of retinoic acid on testes of fetal and newborn rats are mediated by retinoic acid receptor α (RARα) (Duong and Rochette-Egly, 2011). The study by Doyle et al. showed that RARα was mainly in Sertoli cells, cellular retinol binding protein I (CRBPI) and CRBPII were in spermatogenic cells and Sertoli cells, and Sertoli cells are also the main place for storage of retinol. Therefore, the cryptorchidism-induced serious damage to spermatogenic cells and Sertoli cells will directly affect RA intake and storage in testicular tissue (Doyle et al., 2007).
At the same time, through regulating functions of Sertoli cells, the RA signaling pathway can be involved in maintaining the integrity of the blood-testis barrier (BTB). The lack of retinoic acid in the testis of rats with cryptorchidism may be one of reasons for the damage to BTB. The internal environment of the testicles is very important on germ cell differentiation and sperm formation. Maintenance of this environment depends on the presence of the blood-testis barrier. The integrity of blood-testis barrier structure and function is crucial for sperm generation and maturation. The tight junction structure is an important part of the blood-testis barrier (Perez et al., 2013). TEM results of this study showed that in testis with cryptorchidism, the tight junctions were destroyed, suggesting that blood-testis barrier was incomplete, which may cause an escape of sperms and autoimmune reactions. In addition, certain macromolecules entered the seminiferous epithelium through blood vessels and lymph and resulting in the spermatogenic environment and leading to spermatogenic disorders.
Studies have shown that spermatogonia cannot through the meiosis stage, which is also a major cause of infertility induced by cryptorchidism (Zhang et al., 2002). While finding the pathological changes, this study detected Stra8 expression in testicular tissue to investigate the reason of germ cells cannot through meiosis in the undescended testicles, and establish an experiment basis for the further study on the preventive treatment of infertility induced by cryptorchidism.
Stra8 is recognized as a promoter gene in the meiosis. Stra8 is a target gene for RA and its upregulation is required in the changing process of germ cells from mitosis to meiosis (Mark et al., 2008, Childs et al., 2011). Immunohistochemical analysis showed Stra8 protein was mainly localized in the cytoplasm (Giuili et al., 2002). Studies showed that Stra8 expression was induced by retinoic acid, which was closely related to meiosis of the germ cells (Ohta et al., 2010). Also, decreased retinoic acid content in the tissue of undescended testicles could directly down-regulate the expression of Stra8, so causing an obstacle in meiosis.
5. Conclusion
In this study, the cryptorchidism model is successfully induced by Flu. The study confirms that the undescended testicles have significant pathological changes, the content of retinoic acid is decreased, tight junction structure is damaged between Sertoli cells, and quantity and quality of sperms decline, which may be an important cause for reproductive and developmental disorders. If some mean can be taken (for example, supplementing retinoic acid to the tissue of undescended testicles) to repair and restore the damaged spermatogenic microenvironment, up-regulating the expression of Stra8, then it will bring new hope for treatment of the dyszoospermia in undescended testicles.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant 81370701), Program for Innovation Team Building at Institutions of Higher Education in Chongqing, Project of Chongqing Municipal Education Commission ([2013]No. 12, KJ130308), the National Natural Science Foundation of China (Grant 31200853), the National Natural Science Foundation of China (No. 81100415), Chongqing Natural Science Foundation of Committee of Science and Technology (No. CSTC, 2010BB5377; CSTC2012jjA1512), the authors are grateful to all study participants.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Deying Zhang, Email: dzdy199@126.com.
Guanghui Wei, Email: ghwei1963@126.com.
References
- Bergh A., Soder O. Studies of cryptorchidism in experimental animal models. Acta Paediatr. 2007;96:617–621. doi: 10.1111/j.1651-2227.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- Childs A.J., Cowan G., Kinnell H.L. Retinoic acid signalling and the control of meiotic entry in the human fetal gonad. PLoS ONE. 2011;6:e20249. doi: 10.1371/journal.pone.0020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle T.J., Braun K.W., McLean D.J. Potential functions of retinoic acid receptor A in sertoli cells and germ cells during spermatogenesis. Ann. NY. Acad. Sci. 2007;1120:114–130. doi: 10.1196/annals.1411.008. [DOI] [PubMed] [Google Scholar]
- Duong V., Rochette-Egly C. The molecular physiology of nuclear retinoic acid receptors. From health to disease. Biochim. Biophys. Acta. 2011;1812:23–31. doi: 10.1016/j.bbadis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Giuili G., Tomljenovic A., Labrecque N. Murine spermatogonial stem cells: targeted transgene expression and purification in an active state. EMBO Rep. 2002;3:753–759. doi: 10.1093/embo-reports/kvf149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Bosagna C., Skinner M.K. Environmentally induced epigenetic transgenerational inheritance of male infertility. Curr. Opin. Genet. Dev. 2014;26:79–88. doi: 10.1016/j.gde.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F.J., Wu T.C., Tsai M.Y. Effect of retinoic acid on implantation and post-implantation development of mouse embryos in vitro. Hum. Reprod. 2001;16:2171–2176. doi: 10.1093/humrep/16.10.2171. [DOI] [PubMed] [Google Scholar]
- Kolon T.F., Herndon C.D., Baker L.A. Evaluation and treatment of cryptorchidism: AUA guideline. J. Urol. 2014;192:337–345. doi: 10.1016/j.juro.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Kurahashi N., Kasai S., Saijo Y. Exposure to endocrine disrupting chemicals and human health: a review of epidemiological studies focused on hypospadias and cryptorchidism. Nihon Eiseigaku Zasshi. 2005;60:15–22. doi: 10.1265/jjh.60.15. [DOI] [PubMed] [Google Scholar]
- La Vignera S., Calogero A.E., Condorelli R. Cryptorchidism and its long-term complications. Eur. Rev. Med. Pharmacol. Sci. 2009;13:351–356. [PubMed] [Google Scholar]
- Mark M., Jacobs H., Oulad-Abdelghani M. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J. Cell. Sci. 2008;121:3233–3242. doi: 10.1242/jcs.035071. [DOI] [PubMed] [Google Scholar]
- Moretti E., Di C.G., Capitani S. Cryptorchidism and semen quality: a TEM and molecular study. J. Androl. 2007;28:194–199. doi: 10.2164/jandrol.106.000828. [DOI] [PubMed] [Google Scholar]
- Ohta K., Lin Y., Hogg N., Yamamoto M., Yamazaki Y. Direct effects of retinoic acid on entry of fetal male germ cells into meiosis in mice. Biol. Reprod. 2010;83:1056–1063. doi: 10.1095/biolreprod.110.085787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okur H., Muhtaroglu S., Bozkurt A. Effects of prenatal flutamide on testicular development, androgen production and fertility in rats. Urol. Int. 2006;76:130–133. doi: 10.1159/000090875. [DOI] [PubMed] [Google Scholar]
- Parhizkar S., Yusoff M.J., Dollah M.A. Effect of Phaleria macrocarpa on sperm characteristics in adult rats. Adv. Pharm. Bull. 2013;3:345–352. doi: 10.5681/apb.2013.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez C.V., Theas M.S., Jacobo P.V. Dual role of immune cells in the testis: protective or pathogenic for germ cells. Spermatogenesis. 2013;3:e23870. doi: 10.4161/spmg.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haaster L.H., de Rooij D.G. Spermatogenesis is accelerated in the immature Djungarian and Chinese hamster and rat. Biol. Reprod. 1993;49:1229–1235. doi: 10.1095/biolreprod49.6.1229. [DOI] [PubMed] [Google Scholar]
- Zhang R.D., Wen X.H., Kong L.S. A quantitative (stereological) study of the effects of experimental unilateral cryptorchidism and subsequent orchiopexy on spermatogenesis in adult rabbit testis. Reproduction. 2002;124:95–105. doi: 10.1530/rep.0.1240095. [DOI] [PubMed] [Google Scholar]