Abstract
Objectives: The aim of this study was to determine the uptake mechanism of spinosin (SPI) by the monocarboxylic acid transporters (MCTs) in Caco-2 cells. Methods: The Caco-2 cells were pretreated with various monocarboxylic acids, and the uptake of spinosin from Caco-2 cells was measured by High Performance Liquid Chromatography (HPLC). Key findings: Preloading of various monocarboxylic acids enhanced the uptake of SPI, especially salicylic acid (a substrate of MCTs) had a 23.4 times increase in SPI uptake, indicating that the monocarboxylic acid transporters had an efflux effect on SPI uptake and salicylic acid had a strong inhibition on SPI efflux in Caco-2 cells. At the same time, the uptake of SPI through Caco-2 cells was Na+- and temperature-dependent, pretreatment without Na+ significantly increased the uptake of SPI by 1.85 times and incubated at low temperature (4 °C) SPI uptake increased 20% than that of 37 °C. Furthermore, SPI was transported mainly via a carrier-mediated transport: [Vmax = 5.364 μg/mg protein, Km = 657.0 μg/mL]. Conclusion: The uptake of spinosin (SPI) in Caco-2 cells was mainly regulated by the monocarboxylic acid transporters along with Salicylic acid.
Keywords: Spinosin (SPI), Monocarboxylic acid transporters (MCTs), Salicylic acid, Caco-2 cells
1. Introduction
Spinosin (2″-b-O-glucopyranosyl swertisin, C28H38O15), a C-glycoside flavonoid, is one of the major flavonoids of semen Zizhiphi spinozae (Yuan et al., 1987, Kawashima et al., 1997) Previous studies showed that spinosin played an important role in sedation and hypnosis (Li and Bi, 2006), and exerted anxiolytic-like effects and its mechanism appeared to be modulated by GABAA and 5-HT1A receptors (Liu et al., 2015). Several pharmacokinetic investigations of spinosin revealed that it had a wide brain regional tissue distribution, particularly in corpus striatum and hippocampus (Zhang et al., 2015). However, the absolute bioavailability of spinosin in rat was only 2.2% (Li et al., 2008). It had demonstrated that efflux pump P-glycoprotein (P-gp) affected the absorption of spinosin by vivo microdialysis (Ma et al., 2012) and situ perfusion method (Huang et al., 2014), which may be one of the reasons of its low bioavailability. But remaining less research about the intestinal absorption mechanisms of spinosin was studied.
Transporter-mediated disposition plays an important role in pharmacokinetic changes of many drugs (Feng et al., 2014). Monocarboxylate transporters involve proton dependent monocarboxylate transporters (MCTs; SLC16A) contained 14 members which were identified based on sequence homology (Halestrap and Price, 1999) and sodium coupled monocarboxylate transporters (SMCTs) which contain only two members, SLC5A8 and SLC5A12 (Coady et al., 2004, Gopal et al., 2004, Srinivas et al., 2005). MCT1 is strongly expressed on the basolateral surface of enterocytes, whereas members of the SLC5A8 are expressed primarily on the apical surface (Iwanaga et al., 2006). Monocarboxylate transporters have significant impact on the intestinal absorption of its substrates including some short-fatty acids such as acetic acid, l-lactic acid, butyric acid, salicylic acid, nicotinic acid, succinic acid, citric acid, propionic acid and methanoic acid, and α-cyano-4-hydroxycinnamate (CHC) is a specific competitive inhibitor of MCT1, MCT2, and MCT4 (Halestrap and Wilson, 2012, Halestrap, 2013).
The human colon adenocarcinoma cell line Caco-2 cells are widely used as a valuable transport model system for the small intestinal epithelium when grown on dishes or permeable membranes (Hidalgo et al., 1989). Caco-2 cells express five isoforms of MCTs: MCT1, MCT3, MCT4, MCT5, and MCT6; particularly, MCT1 is most abundant (Hadjiagapiou et al., 2000). Furthermore, Caco-2 cells are often used to test whether a compound is transporter-mediated by MCTs when cultured on dishes or permeable membranes (Martel et al., 2006, Kimura et al., 2014, Kensuke et al., 2014). Shim had reported that the uptake of some flavonoids of naringin, naringenin, morin, silybin and quercetin was affected by MCT1 in Caco-2 cells (Shim et al., 2007). As spinosin which has a relatively low absolute bioavailability is a C-glycoside flavonoid, the aim of the study was to investigate whether the mechanism of SPI uptaked in Caco-2 cells was mediated via MCTs.
2. Materials and methods
2.1. Materials
Caco-2 cells were obtained from Institute of Basic Medical Cell Resource Center of Chinese Academy of Medical Sciences. Spinosin and phlorizin were purchased from Si Chuan Weikeqi Medical Technology Co., Ltd. (Chengdu, China), purity ⩾98%. Acetic acid, l-lactic acid, butyric acid, salicylic acid, nicotinic acid, succinic acid, citric acid, propionic acid, methanoic acid, α-cyano-4-hydroxycinnamate (CHC) and NaN3 were obtained from Dengke Chemical Industries, Ltd. (Tianjin, China), purity ⩾98%. Thymidine, inosine, and uracil were purchased from Solarbio Science & Technology, Ltd. (Beijing, China), purity ⩾98%. Dulbecco’s Modified Eagle’s Medium F-12 (DMEM F-12) and fetal bovine serum (FBS) were purchased from Hyclone Life Technologies (Beijing, China). Acetonitrile of HPLC grade was from Tedia Company, Inc. (Fairfield, OH, USA). Dimethyl sulfoxide (DMSO) was purchased from MP Biomedicals, LLC (Illkirch, France). The highest grade reagents were purchased in this experiment.
2.2. Cell culture
The Caco-2 cells were grown in the 100 ∗ 20 mm culture dishes at 37 °C in a 5% CO2–95% air atmosphere between passages 35 and 45. The culture medium consisted of DMEM F-12, 10% FBS, 100 μg/mL streptomycin and 70 μg/mL penicillin G. The confluent Caco-2 cells were cultured for 7–9 days for uptake experiments, and the culture medium was fed with fresh incubation medium three times every week.
2.3. Uptake experiments
Caco-2 cells were seeded in 100 ∗ 20 mm culture dishes by 30 × 104 cell/mL for uptake experiments. HBSS balanced salt solution (8 g/L NaCl, 0.4 g/L KCl, 0.14 g/L CaCl2, 0.06 g/L MgSO4·7H2O, 0.06 g/L KH2PO4, 0.12 g/L mM Na2HPO4·12H2O), 1.0 g/L d-glucose, pH 5.0, 6.8, 7.0 or 7.4, and the same concentration of KCl instead of Na+ was used as Na+ free HBSS balanced salt solution (pH 7.4). In time-, extracellular pH- and concentration-dependence on SPI uptake experiments, cells were washed twice with 5.0 ml of HBSS balanced salt solution (pH 7.4) then preincubated with 10 ml of fresh HBSS for 20 min at 37 °C to decrease interference. After preincubation, the supernatant was removed, and 10 ml of HBSS containing different concentration of SPI was added to each dish for certain times at 37 °C. SPI and other medications were dissolved in DMSO and the final concentration of DMSO in HBSS was lower than 1% for the uptake experiments.
In effect of low temperature, metabolic inhibitor and Na+-dependence on SPI uptake experiments, Caco-2 cells were preincubated with 10 mM NaN3, Na+ free or 4 °C in the incubation medium (pH 7.4) at 37 °C or 4 °C for 20 min before incubating with SPI 10 min at the same temperature with preincubation. To investigate the effect of various monocarboxylic acids and other transporters inhibitors on SPI uptake, the cells were preincubated with 10 mM CHC, acetic acid, l-lactic acid, butyric acid, salicylic acid, nicotinic acid, succinic acid, citric acid, propionic acid, methanoic acid or 1 mM thymidine, inosine, uracil, 0.5 mM phlorizin (pH 7.4) for 20 min at 37 °C then incubated with SPI 10 min at the same temperature with preincubation.
After treatment with SPI, the supernatant was removed, and the cell surface was washed thirdly with 5 mL ice-cold HBSS. Then 2.5 mL of solution (water/methanol = 1:1) was added to each dish incubated for 60 min to extract SPI at room temperature, then using a cell scraper the cells were scraped off and collected in EP tubes. The suspension was centrifuged at 15,000g for 20 min, and a 30-μL aliquot of the supernatant was injected into the High Performance Liquid Chromatography (HPLC) system. The Bradford method was used to assay the concentration of protein in each dish (Bradford, 1976).
2.4. Instrumentation and chromatographic conditions
The extract SPI was determined by an HPLC system with a Waters 2695 pump and Waters 2489 UV detector, using an EMPORE-2000 workstation for data acquisition. And a XBP C18 (L) (5 μm, 4.6 mm × 250 mm) analytical column from Venusil Co. was used. The mobile phase was a binary mixture of acetonitrile–water (20:80, v/v) and at a flow rate of 1.0 mL/min, the wavelength of SPI detection was set at 335 nm, and all measurements were performed at room temperature.
2.5. Statistical analyses
The parameters of the Michaelis–Menten equation to analyze the saturation curve of SPI uptake, were performed by GraphPad Prism 5.0.
The results are reported as the mean ± standard deviation (SD), and were performed by one-way analysis of variance or unpaired Student’s t-test, using the SPSS Statistics 17.0. The results were considered significant if P < 0.05.
3. Results
3.1. Time-, extracellular pH- and concentration-dependence on SPI uptake
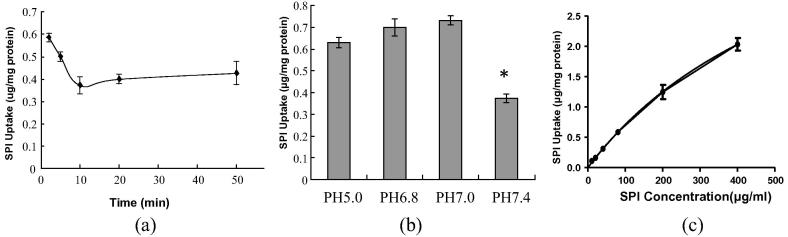
When Caco-2 cells were incubated with SPI (80 μg/mL) up to 50 min at 37 °C, there are no morphologic changes for 50 min for the cells under microscope observation (4 × 10 magnification). As shown in Fig. 1(a), the uptake of SPI that transported into the cells decreased slowly, it reached a stable level at 10 min, and the 10-min incubation time was chosen to determine the uptake mechanism of the following studies. To investigate extracellular pH effect on SPI uptake, Caco-2 cells were incubated with SPI (80 μg/mL) in Caco-2 cells at 10 min at 37 °C at different pH of 5.0, 6.8, 7.0, and 7.4. As the extracellular pH increased the content of SPI distinctly increased (Fig. 1(b)), whereas the uptake of SPI at pH 7.4 had a 40% reduction than that of pH 7.0, that indicating a pH-stimulated on SPI uptake.
Fig. 1.
Time-, extracellular pH- and concentration-dependence on SPI uptake. a) Time course of the uptake of 80 μg/mL SPI by Caco-2 cells. (b) Effect of extracellular pH on the uptake of SPI by Caco-2 Cells were incubated in a medium at each pH containing SPI (80 μg/mL). (c) Concentration dependence of the uptake of SPI by Caco-2 cells. Each point represents the mean ± SE of 4–6 determinations. *P < 0.05 significantly different from the control.
As shown in Fig. 1(c), concentration dependence of SPI uptake was investigated at 37 °C at pH 7.4. For kinetic studies, the concentration of SPI in the medium up 10–400 μg/mL was nonlinear, and the evaluated kinetic parameters Vmax and km were 5.364 μg/mg protein and 657.0 μg/mL according to the Michaelis–Menten equation. The results indicated that SPI uptake was an unsaturable process and mainly via a carrier-mediated transport at the range of 10–400 μg/mL in Caco-2 cells.
3.2. Effect of low temperature, metabolic inhibitor and Na+-dependence on SPI uptake
To examine whether the uptake of SPI was related to temperature, Na+ and energy expenditure, Caco-2 cells were incubated with SPI 80 μg/mL for 10 min at low temperature (4 °C), or preincubated with NaN3 (a spiratory chain inhibitor) or without Na+ free HBSS balanced salt solution (pH 7.4) for 20 min. The results (Table 1) showed that SPI uptake at low temperature (4 °C) increased 20% than that of 37 °C, and preincubation with NaN3 had no significant effect on SPI uptake but decreased 6.4% lower than control. However, pretreatment without Na+ significantly increased the uptake of SPI by 1.85 times, suggesting that the uptake of SPI was dependence of Na+ and temperature.
Table 1.
Effects of low temperature, Na+ and metabolic inhibitor on the initial uptake of SPI in Caco-2 cells.
| Compound | Concentration (mM) | n | SPI uptake (% of control) |
|---|---|---|---|
| Control 37 °C | 5 | 100 ± 2.95 | |
| 4 °C | 5 | 120.11 ± 8.79⁎ | |
| Na+ free | 5 | 285.52 ± 10.19⁎ | |
| NaN3(10 mM) | 10 | 5 | 94.64 ± 5.95 |
Each point represents the mean ± SD for 5 determinations.
P < 0.05 significantly different from the control.
3.3. Effect of various monocarboxylic acids on SPI uptake
To determine whether the various monocarboxylic acids affect SPI uptake, Caco-2 cells were preincubated with 10 mM salicylic acid, CHC, l-lactic acid, butyric acid, nicotinic acid, succinic acid, citric acid, methanoic acid, propionic acid and acetic acid at 37 °C for 20 min before incubated with SPI for 10 min at same temperature with preincubation. The results (Table 2) showed that preincubating salicylic acid had a 23.4 times increase in SPI uptake, and CHC had a 4.3 times increase in SPI uptake. Moreover, preincubated with some short-chain fatty acids l-lactic acid, butyric acid, nicotinic acid, succinic acid, citric acid, and methanoic acid also profoundly increased SPI uptake by 23–150%. In contrast, propionic acid and acetic acid had no significantly impact on SPI uptake. These results suggested that monocarboxylic acid transporters had an efflux effect on SPI uptake in Caco-2 cells.
Table 2.
Effects of various compounds on the uptake of SPI in Caco-2 cells.
| Compound | Concentration (mM) | n | SPI uptake (% of control) |
|---|---|---|---|
| Control | 10 | 5 | 100.1 ± 1.17 |
| CHC | 10 | 5 | 530.58 ± 18.19 ⁎ |
| Acetic acid | 10 | 5 | 119.44 ± 6.91 |
| l-Lactic acid | 10 | 5 | 123.33 ± 3.74 ⁎ |
| Butyric acid | 10 | 5 | 151.52 ± 5.64 ⁎ |
| Salicylic acid | 10 | 5 | 2444.02 ± 38.31 ⁎ |
| Nicotinic acid | 10 | 5 | 202.4 ± 12.61 ⁎ |
| Succinic acid | 10 | 5 | 124.17 ± 4.84 ⁎ |
| Citric acid | 10 | 5 | 250.95 ± 13.19 ⁎ |
| Propionic acid | 10 | 5 | 120.35 ± 6.25 |
| Methanoic acid | 10 | 5 | 201.28 ± 8.42 ⁎ |
| Nucleosides | |||
| Thymidine | 1 | 5 | 118.76 ± 19.43 |
| Inosine | 1 | 5 | 122.26 ± 7.11 |
| Uracil | 1 | 5 | 109.33 ± 0.55 |
| SGLT2-inhibitor | |||
| Phlorizin | 0.5 | 5 | 164.13 ± 4.3 ⁎ |
Each point represents the mean ± SD for 5 determinations.
P < 0.05 significantly different from the control.
3.4. Concentration dependence of CHC and salicylic acid on SPI uptake
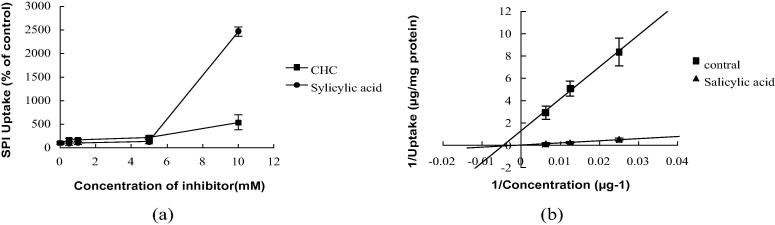
Concentration dependence of CHC and salicylic acid on SPI uptake was investigated (Fig. 2(a)). During preincubation with CHC and salicylic acid at the range of 0.1–10 mM at 37 °C for 20 min, the uptake of SPI increased as the concentration of CHC and salicylic acid increased. But salicylic acid was more effective than CHC when increasing SPI uptake.
Fig. 2.
Concentration dependent of CHC and salicylic acid on SPI uptake. (a) Concentration-dependent of CHC, Salicylic acid on SPI uptake by Caco-2 cells. (b) Lineweaver–Burk plots for the uptake of SPI by Caco-2 cells. Each point represents the mean ± SD of 4–6 determinations.
To identify whether SPI uptake in Caco-2 cells was transported by monocarboxylic transporter along with Salicylic acid, cells were preloaded with or without 10 mM Salicylic acid before incubation with SPI at the range of 40–160 μg/mL. As shown in Fig. 2(b), a Lineweaver–Burk plot suggested that uptake of SPI was significantly stimulated by treatment with salicylic acid, and the inhibition constant value (Ki) was −20.75 ± 5.38 μg/mL (mean ± SD) that indicated salicylic acid had a strong inhibition on SPI efflux in Caco-2 cells.
4. Discussion
In our study, we investigated whether SPI was taken up by monocarboxylate transporters in Caco-2 cells. The uptake of SPI that transported into the Caco-2 cells decreased slowly, and it reached a stable level at 10 min. Moreover, the SPI uptake was Na+, temperature-dependent (Table 1) in Caco-2 cells, pretreatment without Na+ significantly increased the uptake of SPI by 1.85 times and SPI uptake incubated at low temperature (4 °C) increased 20% than that of 37 °C. Preincubation with NaN3 (a spiratory chain inhibitor) had no significant impact but decreased 6.4% lower than in control cells. The concentration of SPI in the medium up to 10–400 μg/mL was nonlinear, suggesting that SPI uptake was an unsaturable process and mainly via a carrier-mediated transport in Caco-2 cells [Vmax = 5.364 μg/mg protein, km = 657.0 μg/mL] according to the Michaelis–Menten equation. Preloading with various monocarboxylic acids could increase the uptake of SPI; especially, salicylic acid had a 23.4 times increase in SPI uptake (Table 2), indicating that monocarboxylic acid transporters had an efflux effect on SPI uptake. Furthermore, the effect of salicylic acid on SPI uptake was concentration-dependent and had a strong inhibition on SPI efflux in Caco-2 cells. These results indicated that SPI was transported by monocarboxylate transporters along with salicylic acid.
MCTs may be involved in the efflux or trans-stimulation effect of certain drugs thereby playing an important role in drug disposition (Vijay and Morris, 2014). For instance, the restricted brain distribution of probenecid (Deguchi et al., 1997) and 6-mercaptopurine (6-MP) (Deguchi et al., 2000) was due to MCT mediated efflux from the brain. Moreover, elangovan gopal HRPE cells were transfected with mouse SMCT cDNA and in the presence of the inhibitor CHC, uptakes of L-[14C] lactate and [14C] pyruvate increased 170 ± 7% and 165 ± 15% higher than in control cells. Acetylsalicylic acid inhibited uptake of a low concentration of 14C-butyrate while increasing uptake of a high concentration in caco-2 cell (Gonçalves et al., 2009), and the uptake of telmisartan was enhanced by preloading of acetic acid (Goto et al., 2005). In this study, we found that monocarboxylic acid transporters had an efflux effect on SPI uptake and salicylic acid had a strong inhibition on SPI efflux in Caco-2 cells, which may be an effective way to improve its pharmacodynamic activity and intestinal absorption.
Nucleoside transporters (NTs) expressed in human gastrointestinal tract (Ritzel et al., 2001, Meier et al., 2007) are important determinants for the transport of nucleoside-derived drugs across cell membranes, and nucleosides such as thymidine, inosine and uracil which are its substrates (Damaraju et al., 2003, Jordheim and Dumontet, 2007, Cano-Soldado et al., 2008). However preincubation with thymidine, inosine, and uracil did not significantly affect SPI uptake (Table 2), and these results suggested that SPI was not taken up by NTs across Caco-2 cells. Sodium-dependent glucose transporter 1 (SGLT1) is highly expressed on the brush border membrane of the small intestine, and phloridzin is an inhibitor of SGLT1 (Ikeda et al., 1989, Schulze et al., 2014). Preincubation with phloridzin significantly increased the uptake of SPI by 64% (Table 2) suggesting that SPI was taken up partly via SGLT1 across Caco-2 cells. In summary, the uptake of SPI was mainly transported by MCTs and partly by SGLT1, but not mediated by NTs in Caco-2 cells.
5. Conclusion
The mechanism of spinosin uptake in Caco-2 cells was mainly regulated by MCTs along with Salicylic acid, suggesting that monocarboxylate transporters (MCTs) may directly affect the therapeutic safety and efficacy of spinosin.
Acknowledgements
This work was supported by Natural Science Foundation of China (Grant Ref. No. U1304824) and the Foundation of Henan Traditional Chinese Medicine (MP 2013-15; MP 2014-89).
Footnotes
Peer review under responsibility of King Saud University.
References
- Bradford M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. J. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cano-Soldado P. Compensatory effects of the human nucleoside transporters on the response to nucleoside-derived drugs in breast cancer MCF7 cells. J. Biochem. Pharmacol. 2008;75:639–648. doi: 10.1016/j.bcp.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Coady M.J. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J. Physiol. 2004;557:719–731. doi: 10.1113/jphysiol.2004.063859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju V.L. Nucleoside anticancer drugs: the role of nucleoside transporters in resistance to cancer chemotherapy. J. Oncogene. 2003;22:7524–7536. doi: 10.1038/sj.onc.1206952. [DOI] [PubMed] [Google Scholar]
- Deguchi Y. Quantitative evaluation of brain distribution and blood-brain barrier efflux transport of probenecid in rats by microdialysis: possible involvement of the monocarboxylic acid transport system. J. Pharmacol. Exp. Ther. 1997;280:551–560. [PubMed] [Google Scholar]
- Deguchi Y. Brain distribution of 6-mercaptopurine is regulated by the efflux transport system in the blood-brain barrier. J. Life Sci. 2000;66:649–662. doi: 10.1016/s0024-3205(99)00637-2. [DOI] [PubMed] [Google Scholar]
- Feng B. In vitro and in vivo approaches to characterize transporter-mediated disposition in drug discovery. J. Expert Opin. Drug Discov. 2014;9:873–890. doi: 10.1517/17460441.2014.922540. [DOI] [PubMed] [Google Scholar]
- Gonçalves Pedro. Modulation of butyrate transport in Caco-2 cells. Naunyn-Schmied Arch. Pharmacol. 2009;379:325–336. doi: 10.1007/s00210-008-0372-x. [DOI] [PubMed] [Google Scholar]
- Gopal E. Expression of slc5a8 in kidney and its role in Na(+)-coupled transport of lactate. J. Biol. Chem. 2004;279:44522–44532. doi: 10.1074/jbc.M405365200. [DOI] [PubMed] [Google Scholar]
- Goto Yoshikazu. Transepithelial transport of telmisartan in Caco-2 monolayers. Biol. Pharm. Bull. 2005;28:2235–2239. doi: 10.1248/bpb.28.2235. [DOI] [PubMed] [Google Scholar]
- Hadjiagapiou C. Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. J. Am. Physiol. Gastrointest. Liver Physiol. 2000;279:775–780. doi: 10.1152/ajpgi.2000.279.4.G775. [DOI] [PubMed] [Google Scholar]
- Halestrap Andrew P. Monocarboxylic acid transport. J. Compr. Physio. 2013;3:1611–1643. doi: 10.1002/cphy.c130008. [DOI] [PubMed] [Google Scholar]
- Halestrap Andrew P., Price N.T. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. J. Biochem. 1999;15:281–299. [PMC free article] [PubMed] [Google Scholar]
- Halestrap Andrew P., Wilson Marieangela C. The monocarboxylate transporter family—role and regulation. J. IUBMB Life. 2012;64:109–119. doi: 10.1002/iub.572. [DOI] [PubMed] [Google Scholar]
- Hidalgo I.J. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. J. Gastroenterol. 1989;96:736–749. [PubMed] [Google Scholar]
- Huang H.Y. Comparison alone or combined with P-gp inhibitors on spinosin absorption using single-pass intestinal perfusion. J. Biotechnol.: India J. 2014;10:36–40. [Google Scholar]
- Ikeda T.S. Characterization of a Na+/glucose cotransporter cloned from rabbit small intestine. J. Membr. Biol. 1989;110:87–95. doi: 10.1007/BF01870995. [DOI] [PubMed] [Google Scholar]
- Iwanaga T. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, humans, with special reference to slc5a8. J. Biomed. Res. 2006;27:243–254. doi: 10.2220/biomedres.27.243. [DOI] [PubMed] [Google Scholar]
- Jordheim L.P., Dumontet C. Review of recent studies on resistance to cytotoxic deoxynucleoside analogues. J. Biochim. Biophys. J. Acta. 2007;1776:138–159. doi: 10.1016/j.bbcan.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Kawashima K. Pharmacological properties of traditional medicines. XXIII. Searching for active compounds in the blood and bile of rats after oral administrations of extracts of sansohnin. J. Biol. Pharm. Bull. 1997;20:1171–1174. doi: 10.1248/bpb.20.1171. [DOI] [PubMed] [Google Scholar]
- Kensuke T. Steric hindrance of 2,6-disubstituted benzoic acid derivatives on the uptake via monocarboxylic acid transporters from the apical membranes of Caco-2 cells. J. Pestic. Biochem. Physiol. 2014;111:38–42. doi: 10.1016/j.pestbp.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Kimura Osamu. Uptake of aristolochic acid I into Caco-2 cells by monocarboxylic acid transporters. J. Biol. Pharm. Bull. 2014;37:1475–1479. doi: 10.1248/bpb.b14-00219. [DOI] [PubMed] [Google Scholar]
- Li Y.J., Bi K.S. Study on the therapeutic material basis of traditional Chinese medicinal preparation Suanzaoren Decoction. J. Chem. Pharm. Bull. (Tokyo) 2006;54:847–851. doi: 10.1248/cpb.54.847. [DOI] [PubMed] [Google Scholar]
- Li Y.J. Quantitative determination of spinosin in rat plasma by liquid chromatography-tandem mass spectrometry method. J. Pharm. Biomed. Anal. 2008;48:1169–1173. doi: 10.1016/j.jpba.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Liu J. GABA and 5-HT systems are implicated in the anxiolytic-like effect of spinosin in mice. J. Pharmacol. Biochem. Behav. 2015;128:41–49. doi: 10.1016/j.pbb.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Ma R.H. In vivo microdialysis with LC–MS for analysis of spinosin and its interaction with cyclosporin in rat brain, blood and bile. J. Pharm. Biomed. Anal. 2012;61:22–29. doi: 10.1016/j.jpba.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Martel F. Absorption of folate by Caco-2 cells is not affected by high glucose concentration. J. Eur. Pharmacol. 2006;551:19–26. doi: 10.1016/j.ejphar.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Meier Y. Regional distribution of solute carrier RNA expression along the human intestinal tract. J. Drug Metab. Dispos. 2007;35:590–594. doi: 10.1124/dmd.106.013342. [DOI] [PubMed] [Google Scholar]
- Ritzel M.W. Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib) J. Biol. Chem. 2001;26:2914–2927. doi: 10.1074/jbc.M007746200. [DOI] [PubMed] [Google Scholar]
- Schulze C. Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. J. Mol. Nutr. Food Res. 2014;58:1795–1808. doi: 10.1002/mnfr.201400016. [DOI] [PubMed] [Google Scholar]
- Shim C.K. Inhibition effect of flavonoids on monocarboxylate transporter 1 (MCT1) in Caco-2 cells. J. Pharm. Pharmacol. 2007;59:1515–1519. doi: 10.1211/jpp.59.11.0008. [DOI] [PubMed] [Google Scholar]
- Srinivas S.R. Cloning and functional identification of slc5a12 as a sodium-coupled low-affinity transporter for monocarboxylates (SMCT2) J. Biochem. 2005;392:655–664. doi: 10.1042/BJ20050927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay Nisha, Morris Marilyn E. Role of monocarboxylate transporters in drug delivery to the brain. J. Curr. Pharm. Des. 2014;20:1487–1498. doi: 10.2174/13816128113199990462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C.L. Sedative and hypnotic constituents of flavonoids in the seeds of Ziziphus spinosae. Zhong Yao Tong Bao. 1987;12:34–36. [PubMed] [Google Scholar]
- Zhang Y.Q. Brain tissue distribution of spinosin in rats determined by a new high-performance liquid chromatography-electrospray ionization–mass/mass spectrometry. Meth. J. Chromatogr. Sci. 2015;53:97–103. doi: 10.1093/chromsci/bmu025. [DOI] [PubMed] [Google Scholar]