Abstract
Pulmonary artery intimal sarcomas are rare and lethal malignant tumors that typically affect larger vessels: the aorta, inferior vena cava, and pulmonary arteries. Since symptoms and imaging of pulmonary arterial intimal sarcomas mimic pulmonary thromboembolism, the differential diagnosis of a patient presenting with chest pain, dyspnea, and filling defect within the pulmonary arteries should include intimal sarcoma. Often right ventricular failure is observed due to pulmonary hypertension caused by the obstructive effect of the tumor and concomitant chronic thromboembolism. We report the case of a 72-year-old African-American male with arterial intimal sarcoma of the left and right pulmonary artery with extension through the right artery into the bronchus and right lung.
Key Words: Pulmonary artery, Intimal sarcoma, Malignant tumor
Introduction
First described in 1923 by Moritz Mandelstamm, intimal sarcomas are malignant tumors with a greater prevalence in the middle aged and females [1, 2]. Between 1923 and 2012, only approximately 200 cases had been described [3]. The incidence of this tumor is 0.001–0.03%, however, it may be underestimated due to misdiagnosis as chronic pulmonary thromboembolism [3]. The prognosis is very poor, so rapid accurate diagnosis is imperative for early effective treatment [4]. Here, we report the case of a 72-year-old African-American retired bus driver with pulmonary artery intimal sarcoma.
Case Report
A 72-year-old African-American male presented to his primary care physician with a 90 lbs weight loss over the past 2 years, chronic dry cough for the past 1 year, and shortness of breath upon exertion for the past 1 month. The patient had a past history of intermediate-type mantle zone lymphoma diagnosed and treated in March 1991, and prostate cancer, which was diagnosed and treated in 2013. He also had a past history of myocardial infarction, osteoarthritis, and exogenous obesity. Over the past 2 years, the patient's weight declined from 320 to 230 lbs. He was referred to an oncologist by his primary care physician.
On physical examination, his lungs were clear to auscultation and his heart had a normal rate and rhythm. No murmurs were audible. The patient stated that he had no history of tobacco, alcohol, or illicit drug use. Due to the weight loss and past history of neoplasms a PET scan and routine labs were obtained.
The PET scan revealed intense uptake in the right lateral cervical, mediastinal, and hilar lymph nodes (fig. 1b). Intense uptake was observed in the right thyroid lobe. Labs revealed a β2-microglobulin of 2.11 μg/ml (1.21–2.70), suggesting it was not a transformation of the lymphoma. The patient was also found to have a PSA of 2.12 ng/ml (0.0–4.0), suggesting that he did not have a recurrence of prostate cancer. CMP was unremarkable except for potassium of 2.8 mmol/l (3.5–5.1) and total bilirubin of 1.70 mg/dl (0.10–1.30). Free T4 and TSH were within normal limits at 1.06 ng/dl (0.61–1.12) and 3.22 μUI/ml (0.34–5.60), respectively. Hemoglobin and hematocrit were 13.5 g/dl (14.0–18.0) and 39.7% (40.0–54.0), respectively. The decreased hemoglobin and hematocrit along with the increased total bilirubin suggest a possible hemolytic process.
Fig. 1.
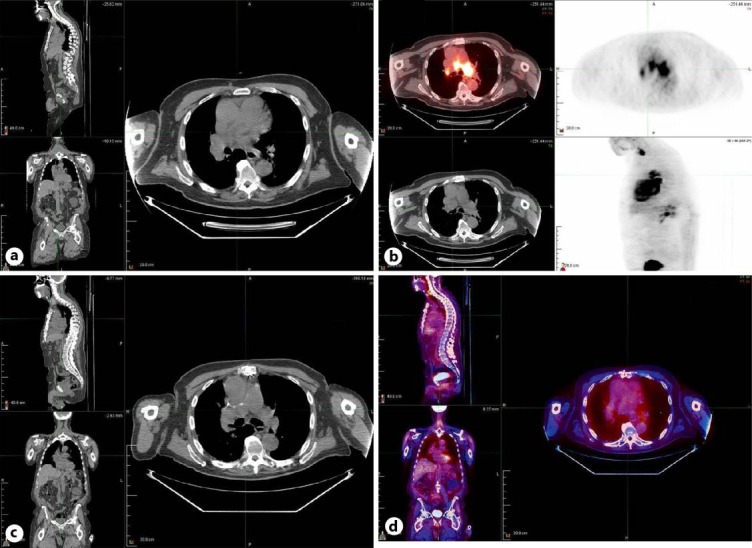
a CT scan with contrast demonstrating a large saddle filling defect. b PET-CT scan of the chest showing intense uptake in the mediastinum, hilar lymph nodes, and right thyroid. c Repeat CT scan of the chest with and without contrast after six courses of chemotherapy. d Repeat PET-CT scan showing resolution of intense FDG uptake after six courses of chemotherapy.
Bone marrow aspirate and ultrasound-guided biopsy of the right thyroid nodule were ordered. The bone marrow aspirate was normocellular, with no increase in blasts and no atypical lymphoid aggregates. Absent iron stores were noted. The thyroid nodule was found to be a benign follicular nodule.
A CT scan with contrast revealed a large saddle filling defect extending into the right and left pulmonary arteries as well as the proximal bilateral upper and lower lobe pulmonary artery branches (fig. 1a). At this point, the differential diagnosis included lung cancer, arterial tumor, tumor embolus, and, less likely, a thrombus/embolism. The diagnosis of arterial tumor was considered more likely due to the increased activity observed in the PET scan.
Due to the possibility of thrombus/embolism, the patient was started on Lovenox for anticoagulation with a failed response. A 2D echocardiogram showed a severely enlarged right ventricle with hypokinesis, a severely enlarged right atrium, and multiple abnormalities of the mitral and aortic valves. Venous Doppler duplex imaging revealed normal upper and lower extremity vasculature with no evidence of phlebitis or intra-abdominal deep vein thrombosis.
A tissue sample was taken from the right pulmonary artery by endobronchial ultrasound-guided needle aspiration (EBUS). A second aspirate was collected from the submucosal mass in the right middle lobe bronchus. The samples both showed a malignant spindle cell neoplasm with hypercellularity, moderate nuclear atypicality, pleomorphism, scattered mitoses, and areas of necrosis. Immunohistochemical stains of the samples displayed diffuse and strong staining for vimentin, with moderately intense staining for smooth muscle actin and the CD31 antigen, a marker of vascular endothelium. The samples were negative for CD34, desmin, or cytokeratins (AE1/AE3).
Based on the clinical presentation, imaging findings, and immunohistochemical staining, a diagnosis of pulmonary artery intimal sarcoma was made. The patient was scheduled for tumor debulking.
The patient underwent a median sternotomy with cardiopulmonary bypass. Debulking of the tumor occurred in the main pulmonary artery, as well as the right and left pulmonary arteries. Approximately 86 g were removed. The tumor was densely adherent to the posterior wall of the left pulmonary artery and was unresectable. The right atrium appeared distended with minimal movement because of increased pulmonary blood pressure due to the obstructive effects of the neoplasm.
Samples of the tumor, along with paratracheal lymph nodes were given to pathology for analysis. The lymph nodes were benign, but the pulmonary artery mass was found to contain fragments of malignant spindle cells having abundant eosinophilic cytoplasm and hyperchromatic irregular ovoid nuclei. Increased mitotic activity was present. Immunohistochemical analysis and staining (fig. 2) was positive for smooth muscle actin and positive for CD31 (a marker of endothelial/vascular differentiation).
Fig. 2.
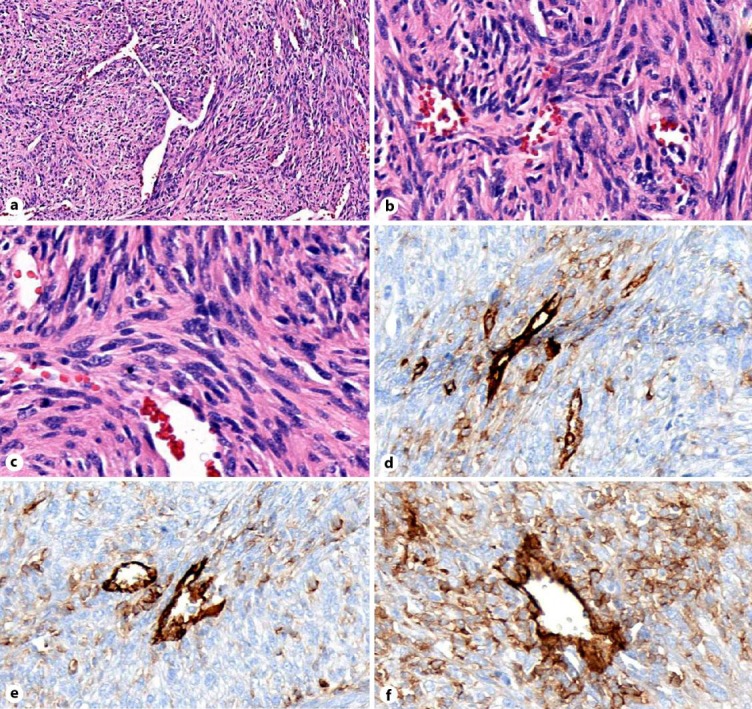
a H&E staining at low magnifiation. b, c H&E staining at higher magnification. d–f CD31 IHC stain showing a blood vessel (strongly CD31 positive) surrounded by weakly staining sarcoma cells indicative of vascular differentiation.
A port-a-cath was inserted in preparation for chemotherapy. Repeat CT scan with contrast showed a mild residual filling defect in the right main pulmonary artery and occlusion of a branch of the right pulmonary artery in the upper lobe. The left upper lobe anterior segmental pulmonary artery was also obstructed. Enlarged hilar lymph nodes were noted along with a mild left pulmonary effusion.
The first round of chemotherapy was initiated with dacarbazine (DTIC) and a doxorubicin pump for 72 h. This regimen was repeated every 21 days.
A follow-up CT of the chest was completed after six rounds of chemotherapy. As seen in figure 1c, the images showed no intraluminal masses in the pulmonary arterial branches. Right paratracheal lymphadenopathy is present, but has improved since the previous imaging study. There is pulmonary fibrosis of the UIP type with subpleural thickening of the septa and honeycombing in the lungs.
A PET-CT was repeated and showed resolution of intense FDG uptake in the region of the pulmonary arteries, compatible with complete response to treatment (fig. 1d). There was no evidence of recurrent or metastatic disease in the chest, abdomen, or pelvis. The patient was then scheduled for consolidation radiotherapy.
Discussion
Multiple methods of imaging and biopsies along with immunohistochemical staining were utilized to more specifically diagnose pulmonary artery intimal sarcoma. One of the methods used was endobronchial ultrasound-guided needle aspiration. With this method, intimal sarcomas will most likely show poorly differentiated malignant mesenchymal tumors [5]. In 2015, Caraway et al. [5] described a case of pulmonary artery intimal sarcoma in which pleomorphic malignant spindled and epithelioid cells were observed. In our case, samples of the tumor showed fragments of malignant spindle cells with broad areas of tumor necrosis and increased mitotic activity as expected. Also, PET scan may be useful in differentiating between pulmonary arterial intimal sarcoma and pulmonary embolism [6].
Immunostaining was positive for smooth muscle actin, which was compatible with sarcoma. Staining was negative for cytokeratins (AE1/AE3, OSCAR, and CK 5/6), as well as p63 and p40. The tumor was weakly positive for CD31. Typically, poorly differentiated tumors such as pulmonary artery intimal sarcomas are positive for vimentin and variably positive for smooth muscle actin [5]. Routine staining of the tumor should be performed for desmin, cytokeratin, vimentin, and smooth muscle actin [7].
Surgical resection followed by chemotherapy remains the mainstay of treatment [8, 9, 10]. Due to the rarity of this disease, data for efficacy of various treatment methods are limited; however, there are several options of chemotherapeutic agents that may be used in the treatment of intimal sarcomas. Targeting the angiogenic factors associated with the endothelial proliferation has been a point of great interest [11]. The rapid progression of the tumor along with the multiple proteins associated with angiogenesis should dictate the types of chemotherapeutic agents used [11].
Despite surgical resection and chemotherapy, the prognosis remains dismal [5, 8]. Prognosis for intimal sarcomas is very low with the median survival time being 11 ± 3 months without resection and 36.5 ± 20.2 months with resection [7]. Patients undergoing multimodal treatments showed a median survival of 24.7 ± 8.5 months [7]. There is limited information on the role of chemotherapy and radiotherapy in the treatment of this disease [12, 13, 14].
In conclusion, due to the aggressive nature of pulmonary artery intimal sarcoma, rapid recognition is needed in order to decrease the time between diagnosis and treatment [4]. CT, PET, and EBUS-guided needle aspiration along with immunohistochemical staining are useful in the rapid diagnosis of pulmonary artery intimal sarcoma. Distinguishing this disease from thromboembolic events is imperative in order to hasten the initialization of surgical resection and chemotherapy.
Statement of Ethics
Informed consent was obtained from the patient for publication of this case report.
Disclosure Statement
The authors of this case report have no conflicts of interest to disclose.
References
- 1.Mandelstamm M. Uber primare Neubildungen des Herzen. Virchows Arch Pathol Anat. 1923;245:43–54. [Google Scholar]
- 2.Hou Y, Shen Z, Gao W, Ye W. Pulmonary artery intimal sarcoma: case report. J Card Surg. 2010;25:29–31. doi: 10.1111/j.1540-8191.2009.00926.x. [DOI] [PubMed] [Google Scholar]
- 3.Mussot S, Ghigna M, Dartevelle P, et al. Retrospective institutional study of 31 patients treated for pulmonary artery sarcoma. Eur J Cardiothorac Surg. 2013;43:787–793. doi: 10.1093/ejcts/ezs387. [DOI] [PubMed] [Google Scholar]
- 4.Medalie N, Vallejo C, Wasserman P. Metastatic pulmonary artery sarcoma. Report of a case with diagnosis by fine needle aspiration. Acta Cytologica. 1998;42:968–972. doi: 10.1159/000331978. [DOI] [PubMed] [Google Scholar]
- 5.Caraway N, Salina D, Deavers M, Morice R, Landon G. Pulmonary artery intimal sarcoma diagnosed using endobronchial ultrasound-guided transbronchial needle aspiration. Cytojournal. 2015;12:3. doi: 10.4103/1742-6413.151667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attinà D, Niro F, Zompatori M, et al. Pulmonary artery intimal sarcoma. Problems in the differential diagnosis. La Radiologia Medica. 2013;118:1259–1268. doi: 10.1007/s11547-013-0943-x. [DOI] [PubMed] [Google Scholar]
- 7.Blackmon S, Rice D, Reardon M, et al. Management of primary pulmonary artery sarcomas. Ann Thorac Surg. 2009;87:977–984. doi: 10.1016/j.athoracsur.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Hoiczyk M, Iliodromitis K, Erbel R, et al. Intimal sarcoma of the pulmonary artery with unusual findings: a case report. Clin Res Cardiol. 2012;101:397–401. doi: 10.1007/s00392-012-0425-5. [DOI] [PubMed] [Google Scholar]
- 9.Wong H, Gounaris I, Hatcher H, et al. Presentation and management of pulmonary artery sarcoma. Clin Sarcoma Res. 2015;5:3. doi: 10.1186/s13569-014-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umezawa K, Takamura K, Yamamoto M, Kikuchi K. Pulmonary artery intimal sarcoma. Am J Resp Crit Care Med. 2014;190:e67–e68. doi: 10.1164/rccm.201407-1311IM. [DOI] [PubMed] [Google Scholar]
- 11.Ravi V, Benjamin R. Systemic therapy for cardiac sarcomas. Methodist Debakey Cardiovasc J. 2010;6:57–60. doi: 10.14797/mdcj-6-3-57. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Ren S, Li A, Zhou C. A case report of chemo-sensitive intimal pulmonary artery sarcoma. Cell Biochem Biophys. 2014;68:153–157. doi: 10.1007/s12013-013-9681-x. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Wang K, Geng Y, Shao Y, Yin Y. A case of intimal sarcoma of the pulmonary artery successfully treated with chemotherapy. Int J Clin Oncol. 2012;17:522–527. doi: 10.1007/s10147-011-0338-8. [DOI] [PubMed] [Google Scholar]
- 14.Uchida A, Tabata M, Tanimoto M, et al. Successful treatment of pulmonary artery sarcoma by a two-drug combination chemotherapy consisting of ifosfamide and epirubicin. Jpn J Clin Oncol. 2005;35:417–419. doi: 10.1093/jjco/hyi106. [DOI] [PubMed] [Google Scholar]


