Abstract
Aim
The aim of this study was to report a case of metastatic uveal melanoma in which radioembolized nodular liver metastases decreased in size while infiltrative sinusoidal metastases progressed, leading to jaundice without obstruction of the biliary ducts.
Methods
The relevant clinical features, imaging, and histopathologic findings of this case are reviewed.
Results
A 61-year-old Caucasian male with a history of uveal melanoma of the left eye status post plaque brachytherapy developed numerous liver metastases. After progression on systemic therapies, he underwent palliative radioembolization. Despite some radiographic improvement in the liver metastases, he developed hyperbilirubinemia without biliary tract obstruction or signs of liver failure. A biopsy of radiographically normal liver demonstrated extensive sinusoidal infiltration with melanoma.
Conclusions
Distinct angiographic and histopathologic growth patterns of metastatic uveal melanoma differ in their amenability to radioembolization. Sinusoidal infiltration may lead to hyperbilirubinemia in the absence of overt obstruction or liver failure.
Key Words: Uveal melanoma, Liver metastasis, Radioembolization, Micrometastases
Introduction
Posterior uveal melanoma is the most common intraocular malignancy in adults, with a mean age-adjusted incidence of 5.1 per million [1]. This malignancy typically metastasizes hematogenously, with metastases afflicting approximately half of the affected patients with a primary uveal tumor [2]. The liver is the most common site of distant metastasis, with hepatic metastases detectable in more than 90% of individuals with metastatic disease [3].
Although the reported 5-year relative survival rate from diagnosis of a primary uveal melanoma is approximately 80% [1], the prognosis for patients with uveal melanoma liver metastases is poor, with a median survival of less than 6 months [4]. With no effective systemic chemotherapy available [5], embolization is an important locoregional method of palliation for metastatic disease [6]. Herein, we report a case of worsening hepatic sinusoidal infiltrative metastatic uveal melanoma in the setting of regressing radioembolized nodular liver metastases.
Case Presentation
A 61-year-old Caucasian male patient was diagnosed with choroidal melanoma of the left eye in May 2013 during a routine eye examination. The patient's family history was significant for cutaneous melanoma in his brother. At the time of diagnosis, ultrasound examination showed that the mass measured 5.5 mm in height with basal dimensions of 9 × 15 mm. The tumor was subsequently treated with iodine-125 plaque brachytherapy in June 2013, at a dose of 85 Gy to a depth of 7.55 mm. He was then followed with liver ultrasounds every 3 months.
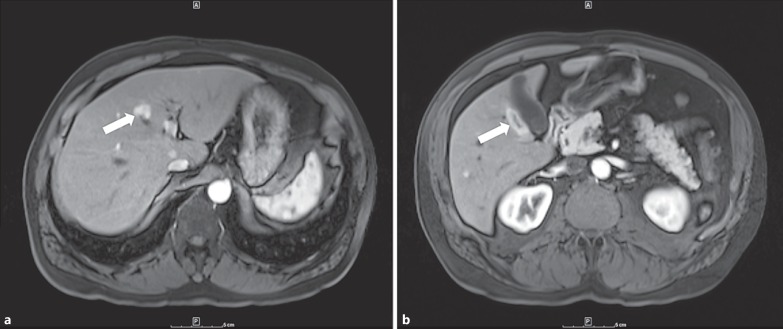
In June 2014, a new solid, hypoechoic lesion measuring 1.7 × 1.3 × 1.8 cm in the right hepatic lobe was first identified by routine surveillance ultrasound. Magnetic resonance imaging (MRI) of the abdomen with and without contrast in July 2014 identified a minimum of 10 new liver lesions which demonstrated arterial-enhancing foci within both hepatic lobes consistent with metastatic melanoma (fig. 1). The largest lesion in segment 5 adjacent to the gallbladder measured 3.1 × 1.2 cm. A computed tomography (CT)-guided cytology specimen demonstrated dispersed malignant melanoma.
Fig. 1.
Largest initially identified metastatic liver lesions. a Metastatic liver lesion measuring approximately 1.8 × 1.5 cm (arrow). b Metastatic liver lesion measuring approximately 3.1 × 1.2 cm adjacent to the gallbladder (arrow).
The patient enrolled in a phase II clinical trial evaluating the MEK inhibitor trametinib alone or in combination with a serine-threonine protein kinase B (AKT) inhibitor. He was randomized to receive trametinib only and began treatment in early September 2014. In late October, a CT scan demonstrated an increased size of the liver lesions, and treatment was discontinued. An ensuing MRI of the abdomen with and without contrast revealed at least 15 heterogeneously arterial-enhancing lesions demonstrating mild T2 hyperintensity and T1 hypointensity. The largest lesion at that time measured 6.1 × 3.3 cm.
The patient underwent yttrium-90 (90Y) resin microsphere radioembolization of the left hepatic lobe with 14.6 mCi of 90Y microspheres for locoregional disease palliation in December 2014. Additionally, treatment with a previously described regimen of ipilimumab and granulocyte colony-stimulating factor (GM-CSF) every 3 weeks was initiated [7]. After receiving the second dose of ipilimumab and GM-CSF, the patient underwent 90Y resin microsphere radioembolization of the right hepatic lobe with 28.2 mCi of 90Y microspheres. The patient received his third and fourth doses of ipilimumab and GM-CSF during the following month.
An MRI in March of 2015 demonstrated changes in the patient's metastatic disease burden. In addition to the expected postembolization changes, an interval increase in both the size and number of metastatic liver lesions was observed in comparison to the most recent prior imaging, with the largest liver lesion measuring 7.1 × 7.9 cm. This change was thought to represent the initial disease flare reaction observed as part of the ipilimumab response, which may take as long as 12 weeks to a year to demonstrate evidence of tumor regression [8]. Approximately 1 month later, the patient presented to the oncology clinic with worsening abdominal discomfort, early satiety, and an increased mass in the center of his abdomen, although he denied complaints of nausea, vomiting, or diarrhea. Laboratory evaluation revealed an abnormal elevation of the liver transaminases (alanine transaminase 80 IU/l and aspartate transaminase 102 IU/l) and alkaline phosphatase (158 IU/l). Additionally, the lactate dehydrogenase value was elevated at 527 IU/l. CT imaging of the abdomen revealed that the liver lesions were stable in size but demonstrated enlarging diaphragmatic and paratracheal lymphadenopathy. After deciding to begin therapy with pembrolizumab, 5 days later, the patient received the initial infusion.
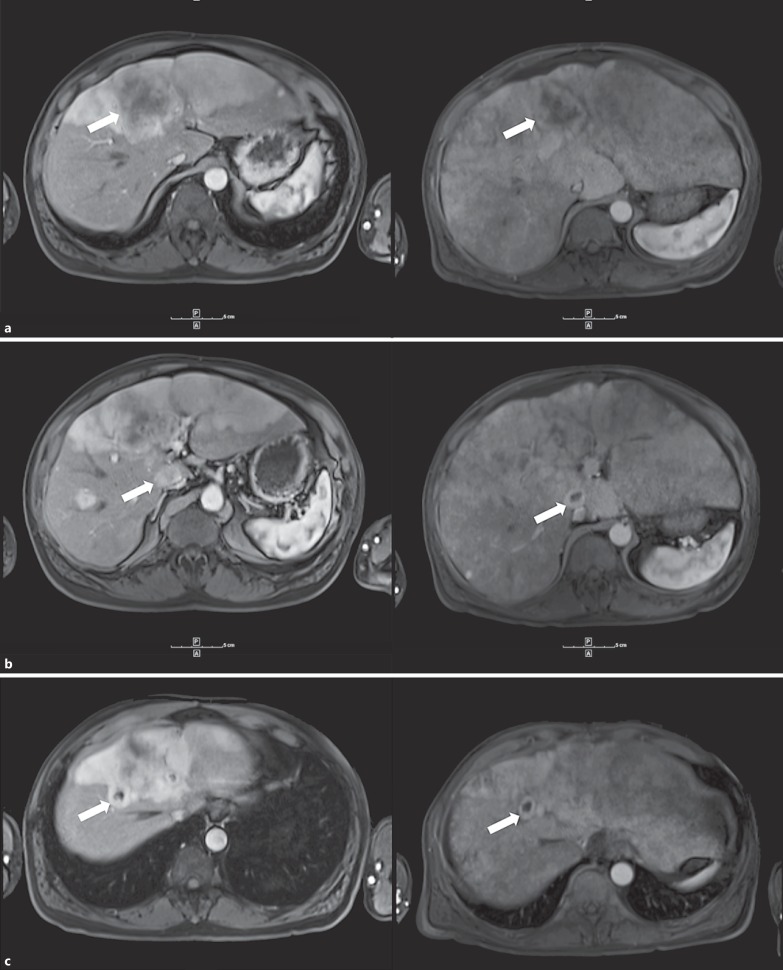
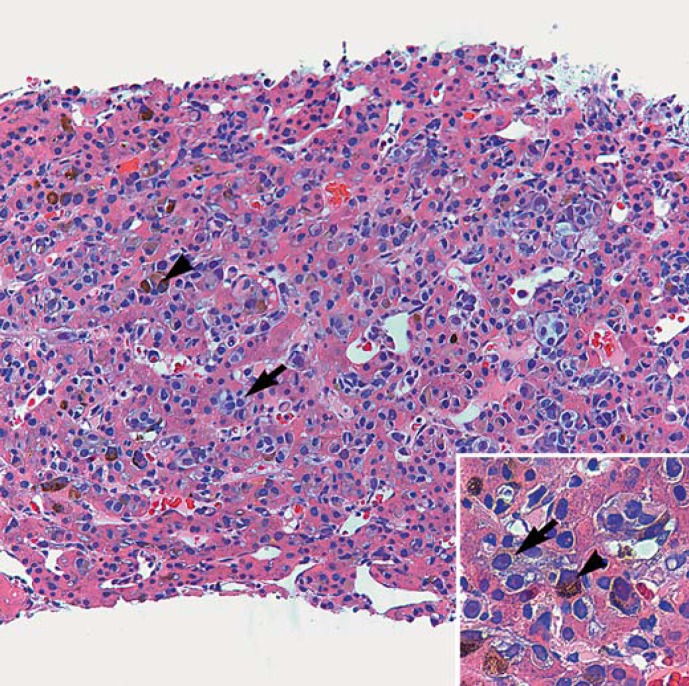
Two weeks later, the patient presented to the oncology clinic for worsening fatigue. He additionally reported noticing a recent darkening in color of his urine over the past several days. On exam, the patient demonstrated hepatomegaly, abdominal distension, and possible ascites. Laboratory evaluation at that time revealed the following: total bilirubin of 8.1 mg/dl, albumin of 3.3 mg/dl, alanine transaminase of 60 IU/l, aspartate transaminase of 126 IU/l, prothrombin time of 11.9 s, and lactate dehydrogenase of 780 IU/l. The patient was admitted for failure to thrive. Right upper quadrant ultrasonography imaging revealed an enlarged, heterogeneous liver and trace ascites but was negative for intrahepatic and extrahepatic biliary dilatation. MRI of the abdomen and pelvis with and without contrast was also negative for intrahepatic or extrahepatic biliary dilatation but demonstrated extensive posttreatment changes status post radioembolization, extensive heterogeneous enhancement thought to represent treatment-induced hepatitis or anomalous perfusion, stable-decreased metastatic liver lesions, and multiple bilateral subcentimeter lung nodules (fig. 2). The lesion in segment 4A measured 5.8 × 5.0 cm (previously 7.4 × 6.4 cm), the lesion in segments 5 and 1 measured 2.2 × 1.9 cm (previously 2.6 cm × 1.9 cm), and the lesion in segment 8 measured 1.7 × 2.2 cm (previously 2.3 × 2.2 cm) (fig. 2). The patient underwent ultrasound-guided core biopsy of radiographically normal liver to further evaluate the changes seen on MRI, which demonstrated extensive infiltration of malignant melanoma cells within the sinusoidal spaces (fig. 3). The patient was eventually referred for inpatient hospice care.
Fig. 2.
Regression of metastatic liver lesions after 90Y microsphere radioembolization (left: before treatment; right: after treatment). a Metastatic lesion in segment 4A demonstrates reduction in size from 7.4 × 6.4 to 5.8 × 5.0 cm (arrows). b Metastatic lesion in segment 5/1 demonstrates reduction in size from 2.6 × 1.9 to 2.2 × 1.9 cm (arrows). c Metastatic lesion in segment 8 demonstrates reduction in size from 2.3 × 2.2 to 1.7 × 2.2 cm (arrows).
Fig. 3.
Metastatic melanoma infiltrates in the sinusoids of the liver. Residual hepatocytes with pink cytoplasm appear atrophic in-between the abundant melanoma cells (HE, ×200). Inset The neoplastic cells are plasmacytoid with light grey-purple cytoplasm and prominent nucleoli. Scattered melanoma cells exhibit cytoplasmic granular melanin pigment (HE, ×400). Arrows point to two (of numerous) nonpigmented melanoma cells, and arrowheads point to two pigmented melanoma cells.
Discussion
The liver, a large vital organ with a dual blood supply, is the primary location of metastasis for posterior uveal melanoma [3]. Uveal hepatic metastases are common, developing in approximately 40% of patients within 10 years of diagnosis of the primary tumor [9], and portend a poor prognosis [1]. Overall, the 5-year survival rate associated with the diagnosis of uveal melanoma has remained unchanged for decades [1]. Through determination of the tumor doubling time, investigators have hypothesized that the seeding of liver micrometastases likely precedes recognition of the primary uveal tumor [10]. Recently, histological work demonstrating quiescent, avascular micrometastases in liver specimens has offered credence to this theory; however, at this early stage, these tiny metastases are undetectable on MRI using conventional contrast agents [11,12].
Although to date systemic chemotherapies for metastatic uveal melanoma have been relatively unsuccessful [5], a variety of new treatment approaches are currently under exploration. Recent investigations of targeted molecular therapies including BRAF and MEK inhibitors appear to demonstrate a survival or progression-free survival benefit among patients with metastatic melanoma harboring certain genetic mutations [13,14,15]. Immunomodulation utilizing antibodies that antagonize cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed death receptor 1 (PD1), and cluster of differential 40 (CD40) as well as various cytokines and cancer vaccines represents another emerging category of therapeutic agents [16]. Our patient's disease progressed despite receiving the MEK inhibitor trametinib and two different immunomodulatory agents (ipilimumab and pembrolizumab), all of which are agents with a demonstrated overall survival benefit for patients with metastatic melanoma [14,17,18]. This lack of response to systemic therapies motivated the decision to alternatively pursue palliation.
Locoregional treatment of metastatic uveal melanoma is indicated when there is lack of response to systemic chemotherapy, the disease burden is limited to the liver, or there is urgent need for local control [6]. Transarterial embolization of the liver utilizing a variety of embolic materials, including cytotoxic and chemotherapeutic drugs, drug-eluting beads, immunologic stimulants, and radioactive microspheres, is an established and effective therapy for some patients with unresectable hepatic metastases [6]. Chemoembolization utilizing cisplatin-based therapies was among the earliest techniques and has been in use for nearly 3 decades, with reported response rates approaching 40% in some studies [19,20]. A newer locoregional technique is percutaneous hepatic perfusion (Hepatic CHEMOSAT® Delivery System; Delcath Systems, Inc., New York, N.Y., USA), which allows for high-dose chemosaturation of the liver through isolation of the liver blood supply and the use of a veno-veno bypass with extracorporeal filtration of the toxin-containing blood [6]. Our patient underwent 90Y brachytherapy radioembolization of both hepatic lobes. He was treated with SIR-Spheres (Sirtex, Sydney, N.S.W., Australia), which differ from the other commercially available 90Y microsphere product, TheraSpheres (MDS Nordion, Ottawa, Ont., Canada), through the following: they are made of resin rather than glass, they measure 20-40 µm rather than 20-30 µm, and the activity per microsphere is much lower (50 vs. 2,500 Bq), thus a larger number of particles are typically delivered to achieve the desired effect [6].
Radioembolization has proven to be a promising treatment for uveal melanoma metastasis restricted to the liver. In the first study of 11 patients investigating the use of 90Y SIR-Spheres to treat uveal melanoma, a response rate of 100% and a 1-year survival rate of 80% were reported for the 10 subjects who could be contacted for follow-up, with 1 patient experiencing a complete response at 3 months as assessed by PET/CT [21]. Since the original investigation [21], more recent studies have reported median disease progression-free and overall survivals ranging from 4.7 to 5.9 and from 7 to 12.3 months, respectively, among patients with uveal melanoma liver metastases treated with 90Y microsphere radioembolization [22,23,24].
Although our patient's abdominal MRI suggested a response to radioembolization as demonstrated by reductions in the size of nodular liver metastases, the patient has nonetheless progressed to hyperbilirubinemia, presumably due to the extensive tumor sinusoidal infiltration which was visible on liver biopsy. Previous studies have identified two different patterns of uveal melanoma liver metastasis angiographically during transarterial chemoembolization which are predictive of survival: nodular and infiltrative [25,26]. The nodular metastatic appearance on angiography, which is characterized by distinct vascular foci, has been associated with a significantly longer survival after chemoembolization than the infiltrative pattern [25,26]. The investigators in these studies have speculated that these metastatic patterns are indicative of inherent differences in tumor genetics and suggested that a deletion in chromosome 8p may possibly be associated with the infiltrative pattern [25,26].
Paralleling the recognition of these discrete angiographic metastatic patterns, two patterns of uveal melanoma metastatic growth in histologic liver specimens have been previously described. The ‘lobular’ or ‘infiltrative’ pattern refers to infiltrative growth within the hepatic lobules with accompanying perilobular septal fibrosis, while the ‘portal’ or ‘nodular’ pattern describes growth in which clusters of tumor cells near the portal venules push aside adjacent liver parenchyma [12]. In our case, the infiltrative growth pattern was likely present from the beginning and progressed over time. These histologic growth patterns are congruent with those identified for colorectal carcinoma [27,28].
In our patient, hepatic angiography revealed multiple vascular foci consistent with a nodular metastatic disease pattern. The radioembolization was apparently successful in treating the large metastatic nodules. These appear to represent the macroscopic extension of the vascularized portal histologic growth pattern in which the nodule of the tumor is vascularized, thus making it amenable to embolization. However, coexisting infiltrative growth, which we speculate corresponds to the avascular lobular histologic growth pattern, is inaccessible by embolization and was ultimately responsible for our patient's disease progression, as evidenced by sinusoidal tumor infiltration. Further research is needed to determine if newer locoregional techniques such as percutaneous hepatic perfusion may better treat infiltrative growth.
Statement of Ethics
This study is IRB exempt at our institution.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgement
This study was in part supported by NIH P30EY06360 and R01CA176001, and Research to Prevent Blindness, Inc.
References
- 1.Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Spagnolo F, Caltabiano G, Queirolo P. Uveal melanoma. Cancer Treat Rev. 2012;38:549–553. doi: 10.1016/j.ctrv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Collaborative Ocular Melanoma Study Group Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report No 15. Arch Ophthalmol. 2001;119:670–676. doi: 10.1001/archopht.119.5.670. [DOI] [PubMed] [Google Scholar]
- 4.Singh AD, Borden EC. Metastatic uveal melanoma. Ophthalmol Clin North Am. 2005;18:143–150. doi: 10.1016/j.ohc.2004.07.003. ix. [DOI] [PubMed] [Google Scholar]
- 5.Buder K, Gesierich A, Gelbrich G, Goebeler M. Systemic treatment of metastatic uveal melanoma: review of literature and future perspectives. Cancer Med. 2013;2:674–686. doi: 10.1002/cam4.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eschelman DJ, Gonsalves CF, Sato T. Transhepatic therapies for metastatic uveal melanoma. Semin Intervent Radiol. 2013;30:39–48. doi: 10.1055/s-0033-1333652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luke JJ, Donahue H, Nishino M, Giobbie-Hurder A, Davis M, Bailey N, et al. Single institution experience of ipilimumab 3 mg/kg with sargramostim (GM-CSF) in metastatic melanoma. Cancer Immunol Res. 2015;3:986–991. doi: 10.1158/2326-6066.CIR-15-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 9.Singh AD, Shields CL, Shields JA. Prognostic factors in uveal melanoma. Melanoma Res. 2001;11:255–263. doi: 10.1097/00008390-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Eskelin S, Pyrhönen S, Summanen P, Hahka-Kemppinen M, Kivelä T. Tumor doubling times in metastatic malignant melanoma of the uvea: tumor progression before and after treatment. Ophthalmology. 2000;107:1443–1449. doi: 10.1016/s0161-6420(00)00182-2. [DOI] [PubMed] [Google Scholar]
- 11.Xue S, Yang H, Qiao J, Pu F, Jiang J, Hubbard K, et al. Protein MRI contrast agent with unprecedented metal selectivity and sensitivity for liver cancer imaging. Proc Natl Acad Sci USA. 2015;112:6607–6612. doi: 10.1073/pnas.1423021112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grossniklaus HE. Progression of ocular melanoma metastasis to the liver: the 2012 Zimmerman Lecture. JAMA Ophthalmol. 2013;131:462–469. doi: 10.1001/jamaophthalmol.2013.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 15.Carvajal RD, Sosman JA, Quevedo JF, Milhem MM, Joshua AM, Kudchadkar RR, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014;311:2397–2405. doi: 10.1001/jama.2014.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sznol M. Advances in the treatment of metastatic melanoma: new immunomodulatory agents. Semin Oncol. 2012;39:192–203. doi: 10.1053/j.seminoncol.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Kumar SS, McNeil CM. Pembrolizumab for the treatment of melanoma. Expert Rev Clin Pharmacol. 2015;8:515–527. doi: 10.1586/17512433.2015.1061430. [DOI] [PubMed] [Google Scholar]
- 18.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrasco CH, Wallace S, Charnsangavej C, Papadopoulos NE, Patt YZ, Mavligit GM. Treatment of hepatic metastases in ocular melanoma. Embolization of the hepatic artery with polyvinyl sponge and cisplatin. JAMA. 1986;255:3152–3154. [PubMed] [Google Scholar]
- 20.Bedikian AY, Legha SS, Mavligit G, Carrasco CH, Khorana S, Plager C, et al. Treatment of uveal melanoma metastatic to the liver. A review of the MD Anderson Cancer Center experience and prognostic factors. Cancer. 1995;76:1665–1670. doi: 10.1002/1097-0142(19951101)76:9<1665::aid-cncr2820760925>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy AS, Nutting C, Jakobs T, Cianni R, Notarianni E, Ofer A, et al. A first report of radioembolization for hepatic metastases from ocular melanoma. Cancer Invest. 2009;27:682–690. doi: 10.1080/07357900802620893. [DOI] [PubMed] [Google Scholar]
- 22.Klingenstein A, Haug AR, Zech CJ, Schaller UC. Radioembolization as locoregional therapy of hepatic metastases in uveal melanoma patients. Cardiovasc Intervent Radiol. 2013;36:158–165. doi: 10.1007/s00270-012-0373-5. [DOI] [PubMed] [Google Scholar]
- 23.Gonsalves CF, Eschelman DJ, Sullivan KL, Anne PR, Doyle L, Sato T. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience. AJR Am J Roentgenol. 2011;196:468–473. doi: 10.2214/AJR.10.4881. [DOI] [PubMed] [Google Scholar]
- 24.Eldredge-Hindy H, Ohri N, Anne PR, Eschelman D, Gonsalves C, Intenzo C, et al. Yttrium-90 microsphere brachytherapy for liver metastases from uveal melanoma: clinical outcomes and the predictive value of fluorodeoxyglucose positron emission tomography. Am J Clin Oncol. 2014 doi: 10.1097/COC.0000000000000033. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma KV, Gould JE, Harbour JW, Linette GP, Pilgram TK, Dayani PN, et al. Hepatic arterial chemoembolization for management of metastatic melanoma. AJR Am J Roentgenol. 2008;190:99–104. doi: 10.2214/AJR.07.2675. [DOI] [PubMed] [Google Scholar]
- 26.Dayani PN, Gould JE, Brown DB, Sharma KV, Linette GP, Harbour JW. Hepatic metastasis from uveal melanoma: angiographic pattern predictive of survival after hepatic arterial chemoembolization. Arch Ophthalmol. 2009;127:628–632. doi: 10.1001/archophthalmol.2009.45. [DOI] [PubMed] [Google Scholar]
- 27.Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Høyer-Hansen G, et al. The multifaceted role of the microenvironment in liver metastasis: biology and clinical implications. Cancer Res. 2013;73:2031–2043. doi: 10.1158/0008-5472.CAN-12-3931. [DOI] [PubMed] [Google Scholar]
- 28.Eefsen RL, Van den Eynden GG, Høyer-Hansen G, Brodt P, Laerum OD, Vermeulen PB, et al. Histopathological growth pattern, proteolysis and angiogenesis in chemonaive patients resected for multiple colorectal liver metastases. J Oncol. 2012;2012:907971. doi: 10.1155/2012/907971. [DOI] [PMC free article] [PubMed] [Google Scholar]