Abstract
Purpose
We examined the outcome of patients with newly diagnosed acute promyelocytic leukemia (APL) treated with all-trans-retinoic acid (ATRA) and arsenic trioxide (ATO) with or without gemtuzumab ozogamicin (GO) but without traditional cytotoxic chemotherapy.
Patients and Methods
From February 2002 to March 2008, 82 patients with APL were treated with a combination of ATRA plus ATO. The first cohort of 65 patients received ATRA and ATO (beginning on day 10 of ATRA). High-risk patients (WBCs ≥ 10 × 109/L) received GO on the first day. From July 2007, the second cohort of 17 patients received ATRA and ATO concomitantly on day 1. They also received GO on day 1, if high risk, and if their WBC increased to more than 30 × 109/L during induction. Monitoring for PML-RARA fusion gene was conducted after induction and throughout consolidation and follow-up.
Results
Overall, 74 patients achieved complete remission (CR) and one achieved CR without full platelet recovery after the induction, for a response rate of 92%. Seven patients died at a median of 4 days (range, 1 to 24 days) after inclusion in the study from disease-related complications. The median follow-up is 99 weeks (range, 2 to 282 weeks). Among the responding patients, three experienced relapse at 39, 52, and 53 weeks. Three patients died after being in CR for 14, 21, and 71 weeks, all from a second malignancy. The estimated 3-year survival rate is 85%.
Conclusion
The combination of ATRA and ATO (with or without GO) as initial therapy for APL was effective and safe and can substitute chemotherapy-containing regimens.
INTRODUCTION
All-trans-retinoic acid (ATRA) has revolutionized the treatment of acute promyelocytic leukemia (APL). Recent trials have reported complete remission (CR) rates of more than 90% when ATRA was used in combination with chemotherapy.1–4 Early trials established the importance of ATRA in induction and postremission therapy.5–8 Subsequent studies have shown that concomitant administration of ATRA and chemotherapy at induction improved disease-free survival and overall survival.9,10 Standard therapy of APL today consists of induction therapy with ATRA and anthracycline-based chemotherapy. There is controversy regarding whether systemic cytarabine is needed, particularly with respect to the risk of extramedullary relapse.1,2,11,12 Reducing the extent of chemotherapy, or eliminating it from both induction and consolidation, may reduce the morbidity and mortality inherent to intensive chemotherapy, particularly in older patients.13,14 Furthermore, an effective nonchemotherapy regimen may potentially benefit patients unfit to receive intensive chemotherapy (such as those with cardiac dysfunction).14
Arsenic trioxide (ATO) is effective for treatment of patients with APL who have experienced relapse after prior therapy with ATRA-containing regimens.15,16 A recent large randomized study showed that the addition of two courses of ATO consolidation after CR significantly improved DFS and OS.3 ATO is also active as a single agent in front-line therapy of APL, producing high CR rates as well as a 3-year survival rate more than 70%.17,18 ATRA and ATO are synergistic in combination.19,20 Shen et al21 omitted chemotherapy from the induction regimen and randomly assigned untreated patients with APL to ATRA, ATO, or ATRA plus ATO. Although the CR rates in all three groups were high (≥ 90%), the time to achieve CR was significantly shorter with the combination, which also most effectively reduced the disease burden (measured by fold-change of PML-RARA transcripts at CR) and was associated with a lower relapse rate and similar toxicity.21 However, all patients on this study received consolidation with anthracycline-based chemotherapy.21
Gentuzumab ozogamicin (GO), an anti-CD33 monoclonal antibody conjugated to the toxin calicheamicin, has significant activity in APL as a result of the high level expression of the target CD33 antigen on the APL cells.22 GO has been used successfully in combination with ATRA for untreated APL and as a single agent in patients with molecular relapse or in older adult patients with advanced disease who are unable to receive chemotherapy.23–25
Given the positive data with ATO and ATRA in front-line APL therapy, we explored the potential of eliminating traditional chemotherapy and used a combination of ATRA plus ATO, with the addition of GO as the only cytotoxic agent for patients with high risk or molecularly persistent disease.26 Here we report our long-term experience in 82 patients treated with this regimen.
PATIENTS AND METHODS
Patients and Eligibility
Eighty-five consecutive patients with a morphologic diagnosis of APL, confirmed by the presence of t(15;17) by standard cytogenetic analysis or by the presence of the PML-RARA fusion gene by reverse-transcriptase polymerase chain reaction (RT-PCR), were enrolled in two studies. Three patients received anthracyclines in induction or consolidation as a result of the treating physician's preference and were excluded from this analysis. The only exclusion criteria were pregnancy or presence of a pretreatment QTc interval of greater than 480 ms on a 12-lead ECG that could not be corrected with electrolyte replacement. All patients with APL presenting in the study period met the eligibility criteria, and no patients were excluded. The patient characteristics are listed in Table 1. The study was approved by our institutional review board; written informed consents were obtained in accordance with the declaration of Helsinki.
Table 1.
Patient Characteristics at Diagnosis
| Characteristic | No. |
||
|---|---|---|---|
| All Patients | Regimen A | Regimen B | |
| No. of patients | 82 | 65 | 17 |
| Age, years | |||
| Median | 47 | 47 | 49 |
| Range | 14-81 | 14-81 | 22-74 |
| Age ≥ 60 years | |||
| No. | 23 | 20 | 3 |
| % | 28 | 30 | 18 |
| Sex | |||
| Male | 44 | 37 | 7 |
| Female | 38 | 28 | 10 |
| Leukocyte count, × 109/L | |||
| Median | 2.5 | 2.6 | 1.8 |
| Range | 0.4-195.0 | 0.5-195.0 | 0.4-88.7 |
| Platelet count, × 109/L | |||
| Median | 32 | 30 | 37 |
| Range | 7-261 | 7-261 | 8-109 |
| Risk category | |||
| High | 26 | 21 | 5 |
| Low | 56 | 44 | 12 |
| Cytogenetics | |||
| t(15;17) | 14 | 10 | 4 |
| t(15;17) plus other | 58 | 48 | 10 |
| Other, PCR-positive | 3 | 2 | 1 |
| Not done, PCR-positive | 5 | 5 | 0 |
| Insufficient, PCR-positive | 2 | 0 | 2 |
| FAB morphology | |||
| M3 | 70 | 54 | 16 |
| M3v | 12 | 11 | 1 |
| PML-RARA isoforms | |||
| Short | 30 | 26 | 4 |
| Long | 33 | 33 | 0 |
| Positive-undefined | 8 | 2 | 6 |
| Not done/suboptimal | 11 | 4 | 7 |
Abbreviations: PCR, polymerase chain reaction; FAB, French-American-British.
Study Design and Treatment Regimen
Induction therapy.
The details of the regimen have been published previously.26 Briefly, from February 2002 to June 2007, 68 patients were treated as follows. Low-risk patients (defined as those with presenting WBCs < 10 × 109/L) received ATRA 45 mg/m2 in two divided doses daily and, beginning 10 days later, ATO 0.15 mg/kg intravenously daily (regimen A). A bone marrow examination was performed between days 25 and 28 of therapy and repeated weekly, if necessary. Once there were fewer than 5% blasts and no abnormal promyelocytes in the marrow, ATRA and ATO were discontinued, and the patient was monitored until achievement of CR. High-risk patients (defined as having a presentation WBC ≥ 10 × 109/L) were treated in an identical manner, except that they received GO 9 mg/m2 on day 1. All patients were hospitalized at least for the first 12 days of treatment.
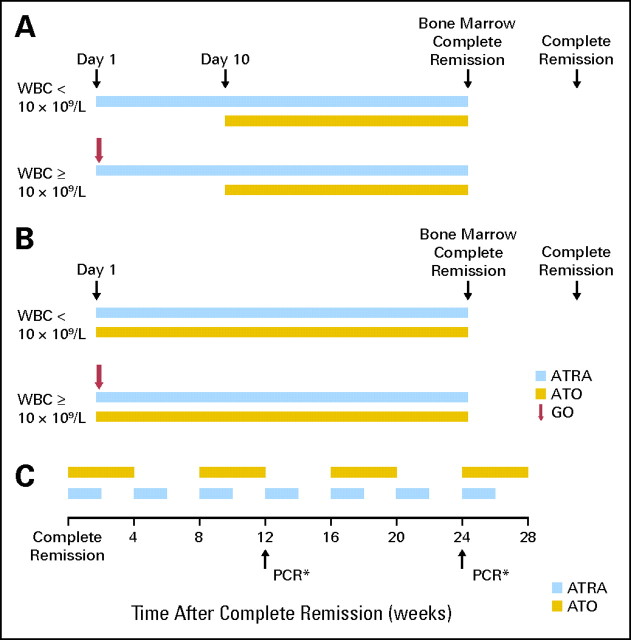
After the development, during induction, of hyperleukocytosis followed by intracranial hemorrhage in one patient, the regimen was modified, and 17 patients have been treated with this modified version (regimen B). Patients with low-risk disease received ATRA 45 mg/m2 daily in two divided doses and ATO 0.15 mg/kg/d, both starting on day 1. GO 9 mg/m2 was given on day 1 to patients with high-risk disease and to other patients if their WBCs increased to more than 30 × 109/L in the first 4 weeks of the treatment. Patients were hospitalized for the first several days of therapy. A bone marrow examination was performed between days 25 to 28 and weekly thereafter, and treatment with ATO and ATRA was continued until there were fewer than 5% blasts and no abnormal promyelocytes in the marrow. The details of the regimens are shown in Figures 1A and 1B.
Fig 1.
(A) Details of induction course, regimen A. (B) Details of induction course, regimen B. (C) Details of postremission therapy. (*) If PCR is positive, it is repeated 2 to 4 weeks later and if positive again, start GO once every 4 to 5 weeks. CR, complete remission; ATRA, all-trans-retinoic acid; ATO, arsenic trioxide; GO, gemtuzumab ozogamicin; PCR, polymerase chain reaction.
Postremission therapy.
Once in CR, patients received (1) ATO intravenously at 0.15 mg/kg daily 5 days/wk for 4 weeks every 8 weeks for a total of four cycles, and (2) ATRA 45 mg/m2 daily for 2 weeks every 4 weeks for a total of seven cycles. Total duration of postremission therapy was 28 weeks after the CR date. If either ATRA or ATO were discontinued because of toxicity, GO 9 mg/m2 was administered once every 4 to 5 weeks (depending on the recovery of counts) until 28 weeks had elapsed from the CR date. Details of postremission treatment are shown in Figure 1C.
Supportive care.
All patients received standard supportive care as per institutional guidelines, including prophylactic and therapeutic antibiotics and transfusion of blood products to maintain platelet counts more than 30 × 109/L, fibrinogen more than 150 mg/dL, and the international normalized ratio for prothrombin time less than 1.5. Heparin or tranexamic acid were only used if clinically indicated. Oral solumedrol 20 mg daily for 10 days was administered for regimen A, and oral methylprednisone 50 mg daily for 5 days was administered for regimen B to decrease the risk of differentiation syndrome.
Monitoring and management of minimal residual disease.
Monitoring for PML-RARA was done at CR and every 3 months thereafter for 2 years. If the PCR test was still positive 3 months after the CR date or at any later point, a repeat test was done 2 to 4 weeks later. If the repeat PCR was also positive, a diagnosis of molecular relapse (or molecular failure if the test never became negative) was made, and patients received GO 9 mg/m2 once every 4 to 5 weeks (depending on the recovery of counts) for 3 months while continuing ATO plus ATRA (or resuming it if relapse occurred after discontinuation of therapy). The same approach was to be used in the event of simultaneous molecular and clinical (hematologic or extramedullary) relapse. If the subsequent PCR became negative, 3 more months of GO with ATRA plus ATO were prescribed.
Definitions and Study End Points
The initial WBC count was used to define the disease risk.10,27 Patients with WBCs less than 10.0 × 109/L, which includes low- and intermediate-risk groups in the Sanz's score, were labeled low risk, and patients with WBCs ≥ 10.0 × 109/L were considered as high risk.
CR was defined as presence of fewer than 5% blasts and promyelocytes with normal hematopoiesis in the bone marrow as well as neutrophil count more than 1.0 × 109/L and platelet count more than 100 × 109/L in the peripheral blood. Hematologic relapse was defined as the presence of more than 10% blasts plus abnormal promyelocytes in the marrow or the presence of extramedullary disease. A confirmed molecular relapse detected by the RT-PCR analysis of PML-RARA was also considered as a relapse event.
Survival was calculated from the first day of therapy to death or last visit. Event-free survival was measured from the date of CR until relapse or death from any cause or last visit.
Statistical Analysis
Survival curves were plotted by the Kaplan-Meier method and compared using the log-rank test. Differences in subgroups by different covariates were evaluated using the χ2 test for nominal values and the Mann-Whitney U test for continuous variables.
PCR
PML-RARA transcripts were analyzed by qualitative RT-PCR until April 25, 2005, and thereafter by quantitative RT-PCR. The switch to the quantitative test was because it was faster to perform, cheaper, and provided quantification. RNA was extracted from blood or bone marrow after RBC lysis using the Trizol method, with reverse transcription (RT) using random hexamers and SuperScript RT (Invitrogen, Carlsbad, CA). The qualitative PML-RARA assay used PML exon 3 and RARα exon 3 primers to amplify both short form (SF) and long form (LF) transcripts. Detection of product was done by gel electrophoresis followed by hybridization to a biotin-labeled RARα probe and a colorimetric detection method. The quantitative PCR assay was performed using TaqMan RT-PCR using a kit from Ipsogen (Marseille, France), with separate primers and probes for LF and SF transcripts and normalization to ABL transcript level. The sensitivity of the qualitative and quantitative assays were equivalent, both at least 1 in 10,000 as established by dilution of an LF-expressing cell line (NB4, ATCC) and an SF-expressing line (UF1; gift of Robert Gallagher, Montefiore Medical Center) into HL-60 (details in online-only Appendix).
RESULTS
Overall Response
Overall, 74 patients achieved CR and one achieved CR without full platelet recovery (CRi) after the induction course for an overall response rate of 92%. Seven patients died after a median of 4 days (range, 1 to 24 days) after inclusion in the study from disease-related complications, including hemorrhage, disseminated intravascular coagulation, and multiorgan failure (Appendix Table A1, online only). Twenty-five patients with high-risk disease received GO with induction; one patient with a WBC of 11.3 × 109/L did not receive GO despite having high-risk disease. By design, no patients on regimen A and four patients on regimen B received GO for increasing WBCs. The peak WBC of 30 × 109/L was reached 7 to 10 days after the start of treatment in these four patients, and the WBC count rapidly decreased after the administration of GO.
The CR rates for low-risk and high-risk patients were 95% and 81%, respectively. Nineteen (83%) of the 23 patients aged 60 years and older compared with 56 (95%) of 59 patients younger than 60 years achieved CR/CRi (P = .17). Among 75 patients who achieved a response, three patients with high-risk disease experienced relapse at 39, 52, and 53 weeks (median follow-up, 99 weeks; range, 2 to 282+ weeks). One patient, an 81-year old man with a presenting WBC count of 47.4 × 109/L, had cytogenetic relapse which was treated with GO and achieved a cytogenetic but not a molecular remission; he then had an isolated CNS relapse treated unsuccessfully with intrathecal chemotherapy. The second patient, a 42-year-old man, was treated for molecular and then hematologic relapse with a combination of ATRA and an anthracycline and achieved a second CR, but eventually died from the complications of an unrelated donor stem-cell transplantation performed in third CR. The third patient, a 70-year-old woman, was treated for hematologic relapse occurring after 53 weeks with a regimen identical to her induction and achieved a second CR, which is ongoing at 74+ weeks. The overall incidence of relapse was 4%.
Hematologic and Molecular Responses After Induction and Consolidation
The median time to achieving CR was similar for the two regimens (29 and 30 days, respectively). Overall, 60 patients had a confirmed molecular remission after the completion of consolidation therapy; no patients required the addition of GO for molecularly persistent disease. Among the other 15 patients achieving CR/CRi, two patients with CR and the patient with CRi did not have molecular testing, and 12 patients, all treated with regimen B, are too early for assessment. The median time to CR was 28 days (range, 19 to 70 days) and the median time to molecular CR was 116 days (range, 20 to 323 days; Appendix Table A2, online only). The mean time to molecular remission was 118 days (95% CI, ± 13 days) for regimen A versus 107 days (95% CI, ± 185 days) for regimen B. However, fewer patients on regimen B were available for this assessment, and this difference did not reach statistical significance. With continued follow-up, with the exception of patients experiencing relapse described above, all other patients have remained in molecular remission (Table 2). In addition to the three patients who experienced relapse who had a positive PCR at 7 to 12 months after CR, four other patients had a positive PCR test at 7 to 9 months after CR (two patients), 19 months after CR (one patient), and 28 months after CR (one patient). In all four patients, the PCR was weakly positive, the confirmatory repeat test performed within a month was negative, and all follow-up PCR tests have remained negative without evidence of relapse (Table 2).
Table 2.
Molecular Status With Follow-Up
| Month | No. of Patients | PCR-Negative |
|
|---|---|---|---|
| No. | % | ||
| At CR | 13 | 5 | 38 |
| 1-3 | 50 | 47 | 94 |
| 4-6 | 49 | 49 | 100 |
| 7-9 | 49 | 46 | 94* |
| 10-12 | 43 | 41 | 95* |
| 13-18 | 53 | 53 | 100 |
| 19-24 | 37 | 36 | 97* |
| 25-36 | 36 | 35 | 97* |
| 37-48 | 17 | 17 | 100 |
| 49-63 | 5 | 5 | 100 |
Abbreviations: PCR, polymerase chain reaction; CR, complete remission.
Late PCR positive tests were seen in three patients with relapse as well as in four patients who had a transient weak-positive PCR result.
Toxicity
Most severe adverse events occurred at induction and were related to the disease and its complications. Treatment-related grade 3 and 4 adverse events are shown in Table 3. In five patients, GO was substituted for ATO in consolidation because of adverse cardiac events.28 Differentiation syndrome occurred in 13 patients (11 of 65 patients on regimen A and two of 17 patients on regimen B; P = not significant) and was successfully managed in all patients by withholding ATRA and administering corticosteroids according to standard practice; ATO was not discontinued. Presenting WBCs did not predict the development of differentiation syndrome (eight [14%] of 56 low-risk patients v five [19%] of 26 high-risk patients; P = .57).
Table 3.
Grade 3 and 4 Adverse Events
| Adverse Event | Grade 3 | Grade 4 |
|---|---|---|
| Esophagitis | 1 | |
| Rash | 1 | |
| Headache | 5 | |
| Atrial arrhythmia | 3 | |
| Elevated liver enzymes | 2 | |
| Back pain | 1 | |
| MI | 1 | 1 |
| SAH | 1 | |
| Constipation | 1 | |
| Renal failure | 4 | |
| Respiratory failure | 1 | |
| GI bleed | 1 | |
| Muscle weakness | 1 | |
| Cerebral infarct | 2 | 1 |
| Bone pain | 1 | |
| Nausea | 1 |
Abbreviations: MI, myocardial infarction; SAH, subarachnoid hemorrhage.
Outcome and Survival
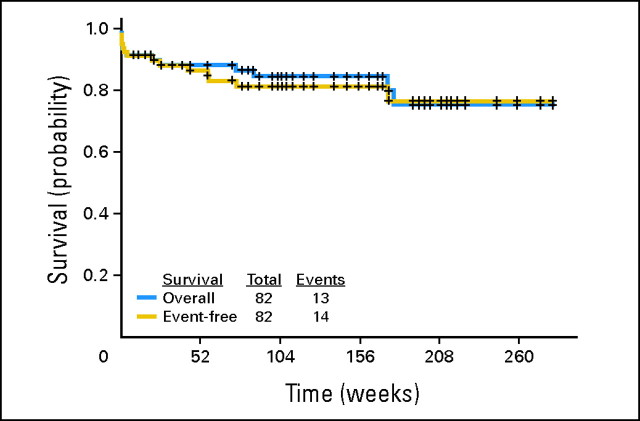
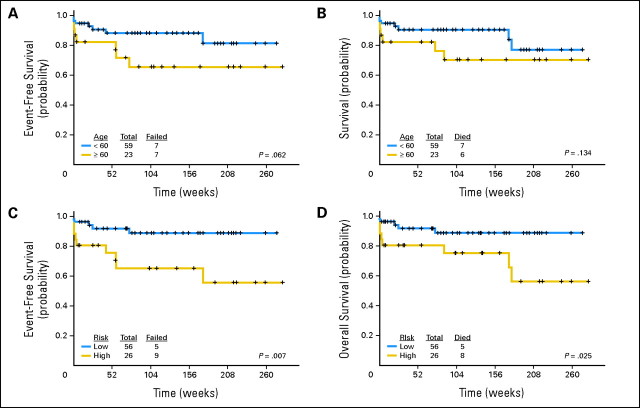
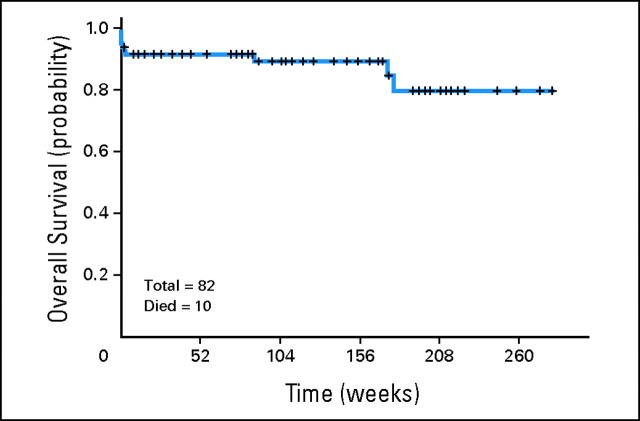
The median follow-up is 99 weeks (range, 2 to 282+). As mentioned, three responding patients have experienced relapse 39 to 53 weeks after achieving CR/CRi. Four patients (all treated with regimen A) died in CR/CRi, three as a result of a second, preexisting malignancy (one patient from metastatic breast cancer at 14 weeks, one patient from metastatic prostate cancer at 21 weeks, and one patient from metastatic melanoma at 71 weeks) and one from an unknown cause (170 weeks after CR). The median survival has not been reached (89+ weeks; range, 0 to 282+); 13 patients have died (seven during induction, three from unrelated causes, one from an unknown cause, and two after relapse). One patient who had experienced relapse remains alive in continuous second CR for 74+ weeks. Event-free survival and survival for the entire group are shown in Figure 2. Survival censoring for the three patients who died of other cancers is shown in Appendix Figure A1 (online only). Event-free survival and survival by age are shown in Figures 3A and 3B. Event-free survival and survival by risk group are shown in Figures 3C and 3D. Overall, two, nine, and 11 patients have remained in CR for more than 5, 4, and 3 years, respectively, indicating that the responses are durable. Among the patients aged ≥ 60 years, 74% remain alive and disease-free at a median follow-up of 108+ weeks (range, 2 to 282+; Fig 3).
Fig 2.
Kaplan-Meier curve of event-free survival and survival for the entire group.
Fig 3.
(A) Event-free survival by age. (B) Survival by age. (C) Event-free survival by risk group. (D) Survival by risk group.
DISCUSSION
Progress in the treatment of APL has changed its course from a highly fatal to a highly curable disease.1,3,4,8,29 With ATRA-based regimens the, 5-year disease-free survival rate is 75%.8 Furthermore, the ability to monitor the molecular marker of the disease, the PML-RARA fusion transcripts, has further enhanced our ability to detect early relapse.16,30,31
Minimizing the toxicities of cytotoxic chemotherapy may further improve the outcome in APL, particularly for older adult patients, who account for 15% to 20% of patients with APL.8,9 In general, the CR rate for patients older than 60 years is significantly lower than that of younger patients.14 This is typically due to a higher incidence of early death owing to infection and bleeding. The outcome for this population can be improved by taking advantage of high sensitivity of the disease to anthracyclines and omitting cytarabine.32–34 A recent study showed that compared with the Medical Research Council (MRC) regimen, the less intensive Programa de Estudio y Tratamiento de las Hemopatías Malignas (PETHMA) regimen yielded similar CR and relapse rates and equivalent 4-year survival but a lower incidence of death in CR and a better quality of life.13 The use of anthracyclines has a significant risk of cardiotoxicity and contributes to induction mortality.35 Therefore, an effective regimen with reduced risk of myelosuppression and limited or no anthracycline exposure would be particularly desirable. Such a regimen would also be preferable in younger patients who were historically not included in clinical trials because they were “unfit for chemotherapy.”
Omission of anthracyclines and cytarabine may also improve on long-term consequences of therapy. Although the risk of secondary myelodysplastic syndrome and acute myeloid leukemia is low in the published series, omission of cytotoxic chemotherapy will also reduce the incidence of this complication.36–38 The risk of development of second cancers resulting from ATO exposure is not known.
An argument in favor of the more intensive approaches, in particular those containing cytarabine, is to reduce the likelihood of extramedullary relapse, particularly in the sanctuary sites like the CNS.2,11 However, the incidence of extramedullary relapse in patients with APL who have achieved a CR has been extremely low.11,12 Only one patient in our series had a CNS relapse.
We have demonstrated that a regimen of ATRA plus ATO, with or without GO, is safe and effective in patients with newly diagnosed APL. In contrast to the study by Shen et al,15 we showed that chemotherapy can be eliminated in consolidation, with responses that seem to be durable and with few late relapses. The main advantage of this regimen is in patients who are unlikely to tolerate cytotoxic chemotherapy (eg, older patients or patients with cardiac dysfunction or multiple comorbidities). Furthermore, the high CR rate, the tolerability of the regimen, and its efficacy provide an argument for its use in all patients with newly diagnosed APL. This strategy is currently under investigation in large trials in the United States and Europe.
Appendix
The Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
Both assays could detect 1 in 10,000 RNA equivalents from the two cell lines and had inconsistent low-level detection of PML-RARA in the 1 in 100,000 dilutions. These cell line sensitivity controls were run on each run in both assays, and the assay was repeated if the 1:10,000 control was not detected. Therefore, any detectable PML-RARA transcript in the new quantitative assay was regarded as equivalent to a positive in the qualitative assay.
Using the qualitative assay, a few transient, weak-positive results were seen in follow-up. For both assays, RNA was converted to cDNA and then split into two polymerase chain reaction replicates. Positives in either assay were only reported if both polymerase chain reaction replicates were positive, which in the quantitative assays were those that showed exponential amplification curves of 42 or fewer cycles; in the qualitative assay, weak-positive indicated very lightly detected bands of the appropriate size in both replicates.
Fig A1.
Kaplan-Meier curve of survival (censoring for deaths from other cancers).
Table A1.
Causes of Early Failure
| Patient | Regimen | Presenting WBCs × 109/L | Died on Study Day | DIC, intracerebral hemorrhage |
|---|---|---|---|---|
| Patient | Regimen | Presenting WBCs × 109/L | Died on Study Day | Cause of Death |
| 1 | A | 1.3 | 9 | Respiratory failure |
| 2 | A | 44.7 | 1 | DIC, intracerebral hemorrhage |
| 3 | A | 22.2 | 3 | DIC, DAH, respiratory failure |
| 4 | A | 2.3 | 4 | MOF: DIC, CA, hepatic shock |
| 5 | A | 87.6 | 17 | Cerebral infarct, pneumonia |
| 6 | A | 11.5 | 1 | ARDS |
| 7 | B | 162.5 | 24 | MOF: ARDS, CA, ARF, sepsis |
Abbreviations: DIC, disseminated intravascular coagulation; DAH, diffuse alveolar hemorrhage; MOF, multi-organ failure; CA, cerebrovascular accident; ARDS, adult respiratory distress syndrome; ARF, acute renal failure.
Table A2.
Time to Response
| Days to Response | Days to CR | Days to Molecular CR* |
|---|---|---|
| Days to Response | Days to CR | Days to Molecular CR* |
| Median | 28 | 116 |
| Minimum | 19 | 20 |
| Maximum | 70 | 323 |
| No. of patients | 82 | 60 |
Abbreviation: CR, complete remission.
Molecular testing was not performed at exactly the same time points in all patients.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Farhad Ravandi, Cephalon (C) Stock Ownership: None Honoraria: Farhad Ravandi, Cephalon Research Funding: Eli Estey, Cephalon, Cell Therapeutics Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Farhad Ravandi, Eli Estey, Hagop Kantarjian
Provision of study materials or patients: Farhad Ravandi, Eli Estey, Dan Jones, Stefan Faderl, Susan O'Brien, Deborah Blamble, Zeev Estrov, William Wierda, Alessandra Ferrajoli, Srdan Verstovsek, Guillermo Garcia-Manero, Jorge Cortes, Hagop Kantarjian
Collection and assembly of data: Farhad Ravandi, Eli Estey, Dan Jones, Jackie Fiorentino, Sherry Pierce
Data analysis and interpretation: Farhad Ravandi, Eli Estey, Sherry Pierce, Hagop Kantarjian
Manuscript writing: Farhad Ravandi, Hagop Kantarjian
Final approval of manuscript: Farhad Ravandi, Eli Estey, Hagop Kantarjian
REFERENCES
- 1.Sanz MA, Martin G, Gonzalez M, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: A multicenter study by the PETHEMA group. Blood. 2004;103:1237–1243. doi: 10.1182/blood-2003-07-2462. [DOI] [PubMed] [Google Scholar]
- 2.Adès L, Chevret S, Raffoux E, et al. Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol. 2006;24:5703–5710. doi: 10.1200/JCO.2006.08.1596. [DOI] [PubMed] [Google Scholar]
- 3.Powell BL, Moser B, Stock W, et al. Effect of consolidation with arsenic trioxide (As2O3) on event-free survival (EFS) and overall survival (OS) among patients with newly diagnosed acute promyelocytic leukemia (APL): North American Intergroup Protocol C9710. J Clin Oncol. 2007;25(suppl):1s. abstr 2. [Google Scholar]
- 4.Wang ZY, Chen Z. Acute promyelocytic leukemia: From highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 5.Huang ME, Ye YC, Chen SR, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. doi: 10.1182/blood-2016-11-750182. [DOI] [PubMed] [Google Scholar]
- 6.Fenaux P, Le Deley MC, Castaigne S, et al. Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial: European APL 91 Group. Blood. 1993;82:3241–3249. [PubMed] [Google Scholar]
- 7.Fenaux P, Chevret S, Guerci A, et al. Long-term follow-up confirms the benefit of all-trans retinoic acid in acute promyelocytic leukemia: European APL group. Leukemia. 2000;14:1371–1377. doi: 10.1038/sj.leu.2401859. [DOI] [PubMed] [Google Scholar]
- 8.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 9.Fenaux P, Chastang C, Chevret S, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia: The European APL Group. Blood. 1999;94:1192–1200. [PubMed] [Google Scholar]
- 10.Burnett AK, Grimwade D, Solomon E, et al. Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: Result of the Randomized MRC Trial. Blood. 1999;93:4131–4143. [PubMed] [Google Scholar]
- 11.Ravandi F. Prophylactic intrathecal chemotherapy in acute promyelocytic leukemia (APL) Leukemia. 2004;18:879–880. doi: 10.1038/sj.leu.2403306. [DOI] [PubMed] [Google Scholar]
- 12.de Botton S, Sanz MA, Chevret S, et al. Extramedullary relapse in acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Leukemia. 2006;20:35–41. doi: 10.1038/sj.leu.2404006. [DOI] [PubMed] [Google Scholar]
- 13.Burnett AK, Hills RK, Grimwade D, et al. Idarubicin and ATRA Is as effective as MRC chemotherapy in patients with Acute Promyelocytic Leukaemia with lower toxicity and resource usage: Preliminary results of the MRC AML15 Trial. Blood. 2007;110:181a. abstr 589. [Google Scholar]
- 14.Fenaux P, Chevret S, de Botton S. Treatment of older adults with acute promyelocytic leukaemia. Best Pract Res Clin Haematol. 2003;16:495–501. doi: 10.1016/s1521-6926(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 15.Shen ZX, Chen GQ, Ni JH, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 16.Soignet SL, Frankel SR, Douer D, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–3860. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 17.Mathews V, George B, Lakshmi KM, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: Durable remissions with minimal toxicity. Blood. 2006;107:2627–2632. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 18.Alimoghaddam K, Shariftabrizi A, Tavangar SM, et al. Anti-leukemic and anti-angiogenesis efficacy of arsenic trioxide in new cases of acute promyelocytic leukemia. Leuk Lymphoma. 2006;47:81–88. doi: 10.1080/10428190500300373. [DOI] [PubMed] [Google Scholar]
- 19.Lallemand-Breitenbach V, Guillemin MC, Janin A, et al. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J Exp Med. 1999;189:1043–1052. doi: 10.1084/jem.189.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jing Y, Wang L, Xia L, et al. Combined effect of all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia cells in vitro and in vivo. Blood. 2001;97:264–269. doi: 10.1182/blood.v97.1.264. [DOI] [PubMed] [Google Scholar]
- 21.Shen ZX, Shi ZZ, Fang J, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2004;101:5328–5335. doi: 10.1073/pnas.0400053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeshita A, Shinjo K, Naito K, et al. Efficacy of gemtuzumab ozogamicin on ATRA- and arsenic-resistant acute promyelocytic leukemia (APL) cells. Leukemia. 2005;19:1306–1311. doi: 10.1038/sj.leu.2403807. [DOI] [PubMed] [Google Scholar]
- 23.Estey EH, Giles FJ, Beran M, et al. Experience with gemtuzumab ozogamycin (“mylotarg”) and all-trans retinoic acid in untreated acute promyelocytic leukemia. Blood. 2002;99:4222–4224. doi: 10.1182/blood-2001-12-0174. [DOI] [PubMed] [Google Scholar]
- 24.Lo-Coco F, Cimino G, Breccia M, et al. Gemtuzumab ozogamicin (Mylotarg) as a single agent for molecularly relapsed acute promyelocytic leukemia. Blood. 2004;104:1995–1999. doi: 10.1182/blood-2004-04-1550. [DOI] [PubMed] [Google Scholar]
- 25.Breccia M, Cimino G, Diverio D, et al. Sustained molecular remission after low dose gemtuzumab-ozogamicin in elderly patients with advanced acute promyelocytic leukemia. Haematologica. 2007;92:1273–1274. doi: 10.3324/haematol.11329. [DOI] [PubMed] [Google Scholar]
- 26.Estey E, Garcia-Manero G, Ferrajoli A, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107:3469–3473. doi: 10.1182/blood-2005-10-4006. [DOI] [PubMed] [Google Scholar]
- 27.Sanz MA, Lo Coco F, Martin G, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: A joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96:1247–1253. [PubMed] [Google Scholar]
- 28.Patel SP, Garcia-Manero G, Ferrajoli A, et al. Cardiotoxicity in African-American patients treated with arsenic trioxide for acute promyelocytic leukemia. Leuk Res. 2006;30:362–363. doi: 10.1016/j.leukres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Asou N, Kishimoto Y, Kiyoi H, et al. A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia who have become negative for PML-RARalpha transcript after consolidation therapy: The Japan Adult Leukemia Study Group (JALSG) APL97 study. Blood. 2007;110:59–66. doi: 10.1182/blood-2006-08-043992. [DOI] [PubMed] [Google Scholar]
- 30.Diverio D, Rossi V, Avvisati G, et al. Early detection of relapse by prospective reverse transcriptase-polymerase chain reaction analysis of the PML/RARalpha fusion gene in patients with acute promyelocytic leukemia enrolled in the GIMEMA-AIEOP multicenter “AIDA” trial: GIMEMA-AIEOP Multicenter “AIDA” Trial. Blood. 1998;92:784–789. [PubMed] [Google Scholar]
- 31.Gallagher RE, Yeap BY, Bi W, et al. Quantitative real-time RT-PCR analysis of PML-RAR alpha mRNA in acute promyelocytic leukemia: Assessment of prognostic significance in adult patients from intergroup protocol 0129. Blood. 2003;101:2521–2528. doi: 10.1182/blood-2002-05-1357. [DOI] [PubMed] [Google Scholar]
- 32.Bernard J, Weil M, Boiron M, et al. Acute promyelocytic leukemia: Results of treatment by daunorubicin. Blood. 1973;41:489–496. [PubMed] [Google Scholar]
- 33.Sanz MA, Vellenga E, Rayon C, et al. All-trans retinoic acid and anthracycline monochemotherapy for the treatment of elderly patients with acute promyelocytic leukemia. Blood. 2004;104:3490–3493. doi: 10.1182/blood-2004-04-1642. [DOI] [PubMed] [Google Scholar]
- 34.Mandelli F, Latagliata R, Avvisati G, et al. Treatment of elderly patients (> or =60 years) with newly diagnosed acute promyelocytic leukemia: Results of the Italian multicenter group GIMEMA with ATRA and idarubicin (AIDA) protocols. Leukemia. 2003;17:1085–1090. doi: 10.1038/sj.leu.2402932. [DOI] [PubMed] [Google Scholar]
- 35.Thomas X, Le QH, Fiere D. Anthracycline-related toxicity requiring cardiac transplantation in long-term disease-free survivors with acute promyelocytic leukemia. Ann Hematol. 2002;81:504–507. doi: 10.1007/s00277-002-0534-8. [DOI] [PubMed] [Google Scholar]
- 36.Andersen MK, Pedersen-Bjergaard J. Therapy-related MDS and AML in acute promyelocytic leukemia. Blood. 2002;100:1928–1929. doi: 10.1182/blood-2002-03-0962. author reply 1929. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Manero G, Kantarjian HM, Kornblau S, et al. Therapy-related myelodysplastic syndrome or acute myelogenous leukemia in patients with acute promyelocytic leukemia (APL) Leukemia. 2002;16:1888. doi: 10.1038/sj.leu.2402616. [DOI] [PubMed] [Google Scholar]
- 38.Latagliata R, Petti MC, Fenu S, et al. Therapy-related myelodysplastic syndrome-acute myelogenous leukemia in patients treated for acute promyelocytic leukemia: An emerging problem. Blood. 2002;99:822–824. doi: 10.1182/blood.v99.3.822. [DOI] [PubMed] [Google Scholar]