Abstract
Purpose
There are no global screening recommendations for esophageal squamous cell carcinoma (ESCC). Endoscopic screening has been investigated in areas of high incidence in China since the 1970s. This study aimed to evaluate whether an endoscopic screening and intervention program could reduce mortality caused by ESCC.
Methods
Residents age 40 to 69 years were recruited from communities with high rates of ESCC. Fourteen villages were selected as the intervention communities. Ten villages not geographically adjacent to intervention villages were selected for comparison. Participants in the intervention group were screened once by endoscopy with Lugol's iodine staining, and those with dysplasia or occult cancer were treated. All intervention participants and a sample consisting of one tenth of the control group completed questionnaires. We compared cumulative ESCC incidence and mortality between the two groups.
Results
Three thousand three hundred nineteen volunteers (48.62%) from an eligible population of 6,827 were screened in the intervention group. Seven hundred ninety-seven volunteers from an eligible population of 6,200 in the control group were interviewed. Six hundred fifty-two incident and 542 fatal ESCCs were identified during the 10-year follow-up. A reduction in cumulative mortality in the intervention group versus the control group was apparent (3.35% v 5.05%, respectively; P < .001). Furthermore, the intervention group had a significantly lower cumulative incidence of ESCC versus the control group (4.17% v 5.92%, respectively; P < .001).
Conclusion
We showed that endoscopic screening and intervention significantly reduced mortality caused by esophageal cancer. Detection and treatment of preneoplastic lesions also led to a reduction in the incidence of this highly fatal cancer.
INTRODUCTION
Esophageal cancer is the eighth most common cancer worldwide, with an estimated 456,000 new cases in 2012, and the sixth most common cause of death, with an estimated 400,000 deaths in 2012.1 The two primary histologic subtypes, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma, account for 94% of all histologically specified esophageal cancers.2 In many economically developed countries, the incidence rate of esophageal adenocarcinoma has increased dramatically over the last 35 years, especially in white men3; in sharp contrast, the incidence of ESCC has steadily decreased among virtually all racial/ethnic groups.4,5 The majority of the worldwide burden of esophageal cancer remains ESCC, and the greatest burden of ESCC occurs in the “Asian Esophageal Cancer Belt,” which extends from northern Iran, east to China, and north to Russia.6
During the last three decades, as a result of socioeconomic development and lifestyle changes, mortality caused by ESCC has decreased by 41.6% in China. However, China still accounts for approximately 50% of the world's esophageal cancers, and ESCC remains the fourth most common cancer in both urban and rural China, with the majority of cancers arising in the resource-limited populations and poverty-stricken rural areas.7
Esophageal cancer is usually fatal, with poor 5-year survival (18.3% in Germany and 17.4% in the United States).8 This poor survival is mainly a result of the late stage at diagnosis when the tumor is no longer amenable to surgical resection. In China, the survival rate is less than 10% when diagnosed at an advanced stage but is as high as 85% if detected at an earlier stage.9 Therefore, the development of appropriate early screening methods for curable lesions offers a great opportunity to reduce ESCC mortality.
Since the 1970s, several screening methods have been developed and tested in the high-risk areas of China, including balloon cytology with smears,10–13 liquid-based balloon cytology,14 occult blood detection,15,16 and endoscopic examination with Lugol's iodine staining and biopsy.17,18 These studies showed that endoscopic examinations with iodine staining have the best sensitivity and specificity for detecting esophageal squamous dysplasia, the precursor lesion for ESCC, and ESCC.19,20
Although previous studies have evaluated various aspects of these screening methods, including endoscopic screening,21 none of them tested for or reported whether the method can achieve a significant reduction in esophageal cancer incidence or mortality. Our group from the Cancer Institute at the Chinese Academy of Medical Sciences (Beijing, China) completed a 10-year follow-up study to assess the efficacy of endoscopic screening for the reduction of esophageal cancer incidence or mortality in a high-risk population in China.
METHODS
Study Design and Population
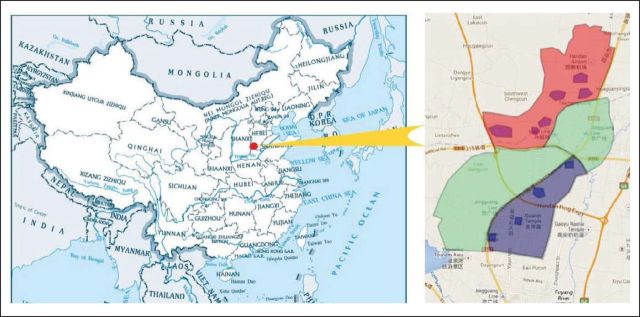
The study was conducted in a region with high rates of ESCC, specifically in the Taihang Mountain area at the grand junction between Henan, Hebei, and Shanxi provinces in northern China. This was a community assignment, controlled endoscopic screening trial for esophageal cancer in Cixian County, Hebei Province. Forty villages in this rural region were divided into three groups according to their geographic character. Fourteen villages located in the north were selected as the intervention communities. Ten villages in the south were used as the control communities. The control group seemed to be equivalent to the intervention group in population structure, morbidity and mortality of ESCC, and socioeconomic status. There were 16 villages between the intervention and control communities that served as a buffer region to help avoid contamination. Men and women age 40 to 69 years with no contraindications for endoscopic examinations (eg, history of reaction to iodine or lidocaine) and who were mentally and physically competent to provide written informed consent were eligible for enrollment (Appendix Fig A1, online only).
The primary end points were the incidence of ESCC and mortality caused by ESCC alone. The secondary outcomes were incidence and mortality as a result of all cancers.
Cohort Establishment and Surveillance
The total population covered in the selected villages was determined using the household registration system at local police stations, which can provide demographic information including name, sex, age, village, and information that would facilitate follow-up. All information about patients with cancer outside the screening exams was collected by the cancer registration systems. International Classification of Diseases (10th revision) codes were used throughout. Follow-up data, including date of onset, disease coding, diagnostic hospital, diagnostic materials, date of death, death code, hospital of death diagnosis, and other information, were double-entered and logic-checked to ensure accurate coding.
Screening Procedure
All endoscopic examinations and therapies were conducted by local doctors after training by and under the supervision of experienced doctors from the Cancer Institute. Participants were recruited and surveyed between November 1999 and May 2000. Endoscopy screening was accomplished from June 2000 to November 2001. After completion of the informed consent process, screened participants were provided a local anesthetic (5 mL of 1% lidocaine by mouth for 5 minutes). They were placed in the left lateral position, and the entire esophagus and stomach were visually examined including careful examination of the lesser curvature of the cardia because it is a frequent site of gastric adenocarcinoma in this region. Next, Lugol's iodine (1.2%) solution was used to stain the full length of the esophagus, which leaves suspicious lesions unstained.19,20 Unstained foci were targeted for biopsy, and the number of biopsies taken depended on the size of the lesion but ranged from one to three biopsies. Participants were monitored during recovery and then sent home.
Biopsy specimens were fixed in 10% buffered formalin, embedded in paraffin, cut in 5-μm sections, and stained with hematoxylin and eosin. The biopsy slides were read by two pathologists without knowledge of the visual endoscopic findings. The histologic criteria were as previously described.18,19
When early lesions were histologically diagnosed, participants were recalled to the clinic, and intervention methods appropriate to the lesions' severity were used. For severe dysplasia/carcinoma in situ or intramucosal carcinomas, endoscopic mucosal resection and/or argon plasma coagulation treatments were used as local therapies. Endoscopic follow-up was done 6 and 12 months after the first therapy as part of the clinical follow-up of the original intervention. For submucosal cancers and advanced esophageal cancers, therapies included esophagectomy, radiotherapy, and other conventional treatments.
Data Collection and Data Management
A uniform questionnaire was administered by trained interviewers for all participants who underwent the intervention and for a 10% sample of the target population in the control group who provided informed consent before completion of the questionnaire concurrently with the screening group. Each completed questionnaire was checked before participants left the interview room. Ten percent of participants were selected to perform a second survey, and concurrence rates were greater than 90% for each question.
Statistical Analyses
SAS version 8.0 software (SAS Institute, Cary, NC) was used for statistical analysis. χ2 tests and t tests were used to compare categorical and continuous variables between the two groups, respectively. Cumulative mortality and incidence between the two groups were compared using the PROC Lifetest and PROC Phreg in SAS. The proportional hazards assumption for the Cox proportional hazards regression model was assessed using the Kaplan-Meier survival curve, the curve of log[–logS(t)] to t, and the time-dependent covariate test. The variable of screened/not screened did not meet the proportional hazards assumption (P < .05) in specific analysis. Therefore, the Cox regression analysis with time-varying coefficients was chosen to estimate the adjusted screening effects when the coefficient for intervention group varied over time.22 Finally, we report two hazard ratios for two periods for three of the four points studied. We used an α = .05 and two-sided tests throughout. The time to incidence or death from ESCC was defined from cohort accrual (January 1, 2000) to the date of end point onset. Participants who had no ESCC incidence or death at the closing date (December 31, 2009) were censored. The study was approved by the institutional review board of the China Cancer Foundation, and the study used the Protocol Registration System in ClinicalTrials.Gov (identifier: NCT02094105).
RESULTS
Baseline Cohort Information
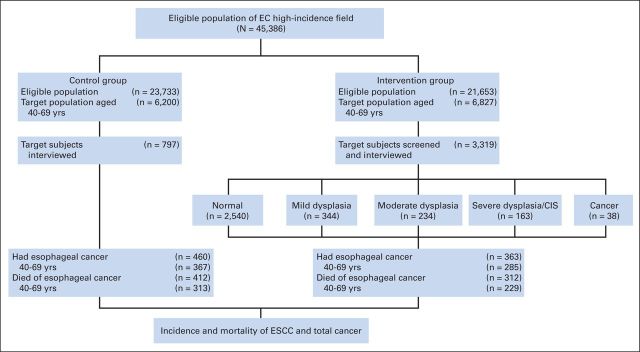
Fourteen villages were selected for the intervention group, which covered a total population of 21,653 people. All residents age 40 to 69 years were all selected as the target population, which included 6,827 people. In the target population of the intervention group, 3,319 participants were enrolled, and screening compliance was 48.62% (3,319 of 6,827 people). We selected 10 villages as a control group, which covered a total population of 23,733 people, of whom 6,200 were in the target age range. The control villages were similar to the intervention villages in population structure, socioeconomic status, and mortality rates of ESCC.21 All participants were observed for 10 years, from January 1, 2000, to December 31, 2009. No participants were lost to follow-up during this period (Fig 1).
Fig 1.
Enrollment and outcomes according to study procedure. CIS, carcinoma in situ; EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma.
Risk factors listed in Table 1 were compared between the intervention group and control group. Three thousand three hundred nineteen participants in the intervention group and 797 comparison participants in the control group were interviewed. No significant differences were apparent for sex, body mass index, number of household members, drinking water source, smoking tobacco, alcohol drinking, and history of diseases of the digestive system. We found that three factors had statistically significant differences between the intervention group and control group. Compared with the control group, participants interviewed in the intervention group had more education and more frequently reported a family history of cancer. In contrast, compared with the intervention group, participants interviewed in the control group had more household income.
Table 1.
Comparison of Baseline Characteristics Between the Intervention Population and a 10% Sample of the Control Population
| Characteristic | Intervention Group |
Control Group |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sex | .1852 | ||||
| Male | 1,629 | 49.08 | 412 | 51.69 | |
| Female | 1,690 | 50.92 | 385 | 48.31 | |
| Body mass index, kg/m2 | .0543 | ||||
| < 24 | 1,786 | 53.81 | 459 | 57.59 | |
| ≥ 24 | 1,533 | 46.19 | 338 | 42.41 | |
| Household members, No. | .2758 | ||||
| < 6 | 2,660 | 80.14 | 625 | 78.42 | |
| ≥ 6 | 659 | 19.86 | 172 | 21.58 | |
| Household income | < .001 | ||||
| ≤ ¥5,000 | 2,701 | 81.38 | 552 | 69.26 | |
| > ¥5,000 | 618 | 18.62 | 245 | 30.74 | |
| Education | .0013 | ||||
| No primary education | 1,059 | 31.91 | 302 | 37.89 | |
| Primary education or more | 2,260 | 68.09 | 495 | 62.01 | |
| Drinking water | .1413 | ||||
| Well or lake water | 3,282 | 98.89 | 783 | 98.24 | |
| Piped water | 37 | 1.11 | 14 | 1.76 | |
| Smoke | .4121 | ||||
| No | 2,282 | 68.76 | 536 | 67.25 | |
| Yes | 1,037 | 31.24 | 261 | 32.75 | |
| Drink alcohol | .1301 | ||||
| No | 2,879 | 86.74 | 675 | 84.69 | |
| Yes | 440 | 13.26 | 122 | 15.31 | |
| Diseases of digestive system | .6964 | ||||
| No | 2,966 | 89.36 | 716 | 89.84 | |
| Yes | 353 | 10.64 | 81 | 10.16 | |
| Family history of cancer | < .001 | ||||
| No | 2,028 | 61.10 | 621 | 77.92 | |
| Yes | 1,291 | 38.90 | 176 | 22.08 | |
NOTE. All participants in the targeted intervention cohort and a 10% sample of the control cohort were individually interviewed. The intervention villages and control villages were separated by a defined geographic distance (see Methods), which likely led to some differences in the two groups.
P values come from χ2 tests or two-sample t tests, as appropriate, for categorical and continuous variables.
Table 2 shows that ESCC accounted for 58.69% (652 of 1,111 cancers) of the total cancer incidence in the target population. Fatal ESCC accounted for 57.48% of total cancer deaths (542 of 943 cancer deaths) in the target population. These figures demonstrate that ESCC is the most common cancer in the studied population.
Table 2.
Incident and Fatal Cancers for All Cancers and ESCC in Cohort Populations (January 1, 2000, to December 31, 2009)
| Group | Cohort Size |
All Cancer |
ESCC |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Cohort |
TP |
Total |
TP |
|||||||
| Total No. | No. in TP | No. of Incident Cancers (%) | No. of Fatal Cancers (%) | No. of Incident Cancers (%) | No. of Fatal Cancers (%) | No. of Incident Cancers (%) | No. of Fatal Cancers (%) | No. of Incident Cancers (%) | No. of Fatal Cancers (%) | |
| Intervention | 21,653 | 6,827 | 687 (3.17) | 610 (2.82) | 528 (7.73) | 444 (6.50) | 363 (1.68) | 312 (1.44) | 285 (4.17) | 229 (3.35) |
| Control | 23,733 | 6,200 | 780 (3.29) | 688 (2.90) | 583 (9.40) | 499 (8.05) | 460 (1.94) | 412 (1.74) | 367 (5.92) | 313 (5.05) |
| Total | 45,386 | 13,027 | 1,467 (3.23) | 1,298 (2.86) | 1,111 (8.53) | 943 (7.24) | 823 (1.81) | 724 (1.60) | 652 (5.00) | 542 (4.16) |
Abbreviations: ESCC, esophageal squamous cell carcinoma; TP, target population.
Cumulative incidence of ESCC was 4.17% (285 of 6,827 participants) and 5.92% (367 of 6,200 participants) in the intervention and control groups, respectively. Cumulative mortality as a result of ESCC was 3.35% (229 of 6,827 participants) and 5.05% (313 of 6,200 participants) in the intervention and control groups, respectively.
Adjusted Screening Effect Estimates
Because of the potential differences in the intervention and control groups, we also calculated adjusted hazard ratios. Statistically significant associations were observed for both ESCC incidence and mortality and total cancer incidence (Table 3). Compared with the control group, the hazard ratio for incident ESCC in the screened group in the first time period was 0.61 and even lower in the second time period at 0.15. There were similar patterns for ESCC mortality and total cancer incidence. Total cancer mortality did not show evidence for time-varying effects, and the hazard ratio for being in the screened group was 0.51.
Table 3.
Multivariable* HRs and 95% CIs From the Cox Regression Model With Time-Varying Coefficients for the Effect of the Intervention on Each Outcome
| Category | No. of Participants | Baseline Period |
Time-Varying Coefficient Period |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| ESCC incidence | .01 | .02 | |||||
| Control group† | 48 | 1.00 | 1.00 | ||||
| Intervention group‡ | 109 | 0.61§ | 0.42 to 0.89 | 0.15§ | 0.07 to 0.32 | ||
| ESCC mortality | < .001 | .03 | |||||
| Control group† | 39 | 1.00 | 1.00 | ||||
| Intervention group‡ | 66 | 0.45‖ | 0.29 to 0.71 | 0.13‖ | 0.03 to 0.62 | ||
| Total cancer incidence | .02 | < .001 | |||||
| Control group† | 81 | 1.00 | 1.00 | ||||
| Intervention group‡ | 199 | 0.72¶ | 0.54 to 0.95 | 0.14¶ | 0.041 to 0.44 | ||
| Total cancer mortality | < .001 | ||||||
| Control group† | 65 | 1.00 | |||||
| Intervention group‡ | 140 | 0.51# | 0.37 to 0.69 | ||||
Abbreviations: ESCC, esophageal squamous cell carcinoma; HR, hazard ratio.
Adjusted for sex, body mass index (< v ≥ 24 kg/m2), household members (one to five v ≥ six members), income (≤ v > ¥5,000), education (no primary education v primary education or more), drinking water (piped water v well or lake water), cigarette smoking (ever v never), alcohol drinking (any v none), disease of digestive system (yes v no), and family history of cancer (yes v no).
Seven hundred ninety-seven comparison participants were interviewed in the control group.
Three thousand three hundred nineteen participants were interviewed in the intervention group.
At baseline, the HR for the intervention group compared with control group was 0.61, whereas it was 0.15 between 5.5 and 6.5 years.
The HR at baseline for the intervention group compared with control group was 0.45, whereas it was 0.13 between 7 and 8 years.
The HR at baseline for the intervention group compared with control group was 0.72, whereas it was 0.14 between 5 and 6 years.
Multivariable model of total cancer mortality met the proportional hazards assumption (P < .05). The Cox regression analysis was chosen.
Cumulative Incidence and Mortality Among the Target Population
Figure 2A shows that screening caused an initial excess incidence of ESCC in the intervention group compared with the control group, likely because more ESCCs were detected in the screened group. However, the curves began to cross and then separate in year 5, and cumulative incidence in the intervention group started to increase more slowly. Over the full follow-up period, the difference between the cumulative incidence of the two groups was statistically significant (log-rank test P < .001).
Fig 2.
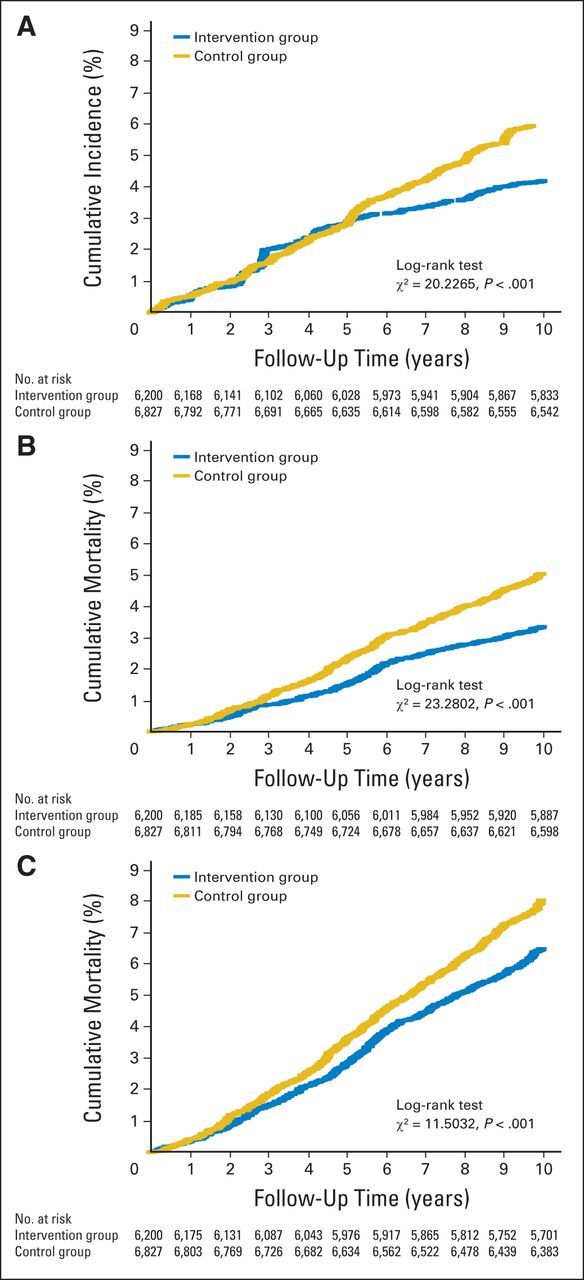
Cumulative incidence and mortality from esophageal squamous cell carcinoma (ESCC) and all cancers. (A) Cumulative incidence of ESCC among target population. (B) Cumulative mortality from ESCC among target population. (C) Cumulative mortality from all cancers among target population.
Figure 2B shows the cumulative mortality for ESCC. A reduction in mortality in the intervention group is apparent after the first year, and the differences were statistically significant (log-rank test, P < .001). Figure 2C shows that the difference in cumulative mortality of total cancer among the target population in the two groups was statistically significant (log-rank test, P < .001).
DISCUSSION
Esophageal cancer is a common cancer worldwide and has poor prognosis because of a lack of symptoms at early stages; thus, most patients are diagnosed too late for curative treatment. There is a clear need for practical techniques that can screen asymptomatic individuals from populations with high rates. However, there are no proven screening methods for ESCC at this time. Several screening methods have been investigated in the high-incidence areas of China since the 1970s, but up until now, there were no proven strategies that would lead to a reduction in disease morbidity and mortality.
One of the most ambitious attempts to screen for esophageal cancer in China was the mass screening using esophageal balloon cytology. From 1959 to 1979, approximately 500,000 people in China received balloon cytologic examinations of the esophagus.10 The sensitivity and specificity of cytologic detection with different kinds of balloons varied, ranging from 14% to 36% and 90% to 99% for biopsy-proven esophageal cancer, respectively.11–13 The latest study using liquid-based cytology methods conducted in Linxian showed that when more than atypical squamous cells of undetermined significance, favor neoplastic, was considered a positive result, the sensitivities and specificities of the mechanical versus inflatable balloons were 39% and 85% versus 46% and 84%, respectively, for detecting any squamous dysplasia or cancer. These esophageal cell samplers performed equivalently, but the accuracy was still too low for a primary screening test14 and was not promising enough to move forward into cancer control trials.
During the last few decades, many studies of screening using endoscopy examinations were conducted in Linxian, China. Results from these studies found that squamous dysplasia is the valid histologic precursor that can be used as an intermediate end point for early detection and screening.17,18 Endoscopy with mucosal iodine staining is a sensitive technique for identifying clinically relevant esophageal squamous lesions. The sensitivity and specificity of endoscopy examinations were 96% and 63%, respectively, for identifying high-grade (moderate and severe) squamous dysplasia and invasive squamous carcinoma,19 and the specificity is dramatically increased after biopsy and histologic diagnosis. Prospectively defined follow-up studies showed that increasing grades of dysplasia were strongly associated with increasing risk of future cancer, indicating that the histologic grading was clinically meaningful. Squamous dysplasia was the only histologic lesion associated with a significantly increased risk of developing ESCC within 13.5 years after endoscopy. Thus, documenting the identity of these precursor lesions of ESCC has assisted us in the development of effective screening strategies for this disease.23,24
Therefore, we conducted a population-based prevention study in an area with high risk of ESCC in China (reported here). Our study showed that the overall cumulative incidence of ESCC in the endoscopically screened group (4.17%) is lower than in the control group (5.92%). A reduction in cumulative mortality in the intervention group versus the control group was apparent (3.35% v 5.05%, respectively). Furthermore, because ESCC constitutes more than 50% of all cancers in the population, our screening program reduced total cancer incidence and mortality in the target population.
It is noteworthy that significant differences existed between the two groups at baseline for some risk factors. We think these differences may be apparent because of how the participants were selected for interview, but this may not reflect differences in the screened populations. For most of the compared factors, it seemed that the intervention group had more individuals at higher risk for developing ESCC. For example, the intervention group had less income but more frequently had a family history of cancer. The other major reason is that this study was funded by the government, and endoscopy and any necessary treatment were offered at no charge. This may have induced a different group to volunteer for the study and may have led to under-reporting of income during the interview. Higher income and lower family history of cancer were more frequent in the control group. These data suggest that, overall, the interviewed control participants had better socioeconomic status and would likely be at lower risk for developing ESCC. Therefore, if the populations did differ as shown, rather than being a result of a bias in those recruited to complete the questionnaire, we would have expected that our screening program would have to overcome an inherently higher risk in the population screened. To address this possibility, we generated risk estimates using crude data and with adjustment for the factors in the questionnaire (Table 3). These results showed that the baseline risk factor differences between the two groups (even if real) would be unlikely to affect our results.
In addition to potential differences between the two groups, our study has some other limitations. Social factors required us to use community assignment rather than individual-level random assignment, which would have produced a stronger test of the method. Furthermore, the methods used are complex and require highly skilled therapeutic endoscopists, which are not currently widely available in rural areas most in need of effective esophageal cancer screening. Therefore, translation from an experimental study to the general population has and will continue to require careful training and monitoring of implementation.
On the basis of the preliminary results of the studies conducted that developed the methods implemented here, the Ministry of Health of China launched a population-based endoscopy screening program in the year 2005. The primary program was first targeted to 11 high-incidence areas for esophageal cancer and was expanded to 110 sites in 29 provinces in the year 2013. One hundred eighty-two thousand people were screened, and 3,040 cancers were detected, including 2,201 cancers (72.4%) at an early stage (ie, carcinoma in situ and intramucosal or submucosal [T1N0M0] carcinoma); 2,573 patients who had precancerous lesions or carcinoma were treated with appropriate therapies.25
In summary, to our knowledge, this is the first population-based, community assignment study to evaluate the utility of an endoscopic screening on the incidence and mortality of ESCC. We found that one-time screening by endoscopic examination with iodine staining and biopsy followed by removal of preneoplastic lesions can decrease mortality from esophageal cancer. Also, because our program can detect noninvasive lesions and includes early intervention for these lesions, we also reduced the incidence of this highly fatal cancer. This study has led to a large-scale screening program in China and provides a practical and implementable program for screening in other rural high-incidence ESCC areas.
Glossary Terms
- Cox proportional hazards regression model:
a statistical model for regression analysis of censored survival data, examining the relationship of censored survival distribution to one or more covariates. This model produces a baseline survival curve, covariate coefficient estimates with their standard errors, risk ratios, 95% CIs, and significance levels.
- cumulative incidence:
a statistical measure of an event of interest (eg, relapse, death, second malignant neoplasm, a specific disease) occurring in a specified period of time in the population at risk. It is calculated using the formula: (number of new cases of the event of interest)/(total population at risk).
- prospectively defined:
refers to a study design (eg, study objectives, outcome measures, analytical methods, analysis plan) specified and documented prior to study conduct. Prospective definition of the study design and analysis plan is critical to produce level-1 evidence for clinical use of a biomarker as defined by Simon et al (Simon RM, et al: J Natl Cancer Inst 101:1446-1452, 2009).
- surveillance:
monitoring patients for cancer recurrence after treatment with physical exam, laboratory and imaging studies, and minor procedures (ie, cystoscopy, colonoscopy, prostate biopsy). The specific interval and type of testing used for monitoring are specific to the type of cancer. Recommendations are typically provided by national cancer organizations/associations.
Appendix
Fig A1.
Location of intervention community (red), control community (blue), and buffer area (green). This map does not show the complete territory of China.
Footnotes
Supported by Grant No. 2001BA703B10 from the Ministry of Science and Technology of the People's Republic of China.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Wen-Qiang Wei, Jun Hou, Guo-Qing Wang, Zhi-Wei Dong, You-Lin Qiao
Provision of study materials or patients: Dong-Mei Lin
Collection and assembly of data: Wen-Qiang Wei, Zhi-Feng Chen, Yu-Tong He, Hao Feng, Dong-Mei Lin, Xin-Qing Li, Cui-Lan Guo, Shao-Sen Li, Guo-Qing Wang
Data analysis and interpretation: Wen-Qiang Wei, Christian C. Abnet
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Follow-Up of a Community Assignment, One-Time Endoscopic Screening Study of Esophageal Cancer in China
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Wen-Qiang Wei
No relationship to disclose
Zhi-Feng Chen
No relationship to disclose
Yu-Tong He
No relationship to disclose
Hao Feng
No relationship to disclose
Jun Hou
No relationship to disclose
Dong-Mei Lin
No relationship to disclose
Xin-Qing Li
No relationship to disclose
Cui-Lan Guo
No relationship to disclose
Shao-Sen Li
No relationship to disclose
Guo-Qing Wang
No relationship to disclose
Zhi-Wei Dong
No relationship to disclose
Christian C. Abnet
No relationship to disclose
You-Lin Qiao
Consulting or Advisory Role: MSD
Travel, Accommodations, Expenses: MSD
REFERENCES
- 1.International Agency for Research on Cancer. Oesophageal Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*StatDatabase: Incidence—SEER 18 Regs Research National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2012, based on the November 2011 submission. http://www.seer.cancer.gov.
- 3.Drahos J, Wu M, Anderson WF, et al. Regional variations in esophageal cancer rates by census region in the United States, 1999-2008. PLoS One. 2013;8:e67913. doi: 10.1371/journal.pone.0067913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.González L, Magno P, Ortiz AP, et al. Esophageal cancer incidence rates by histological type and overall: Puerto Rico versus the United States Surveillance, Epidemiology, and End Results population, 1992-2005. Cancer Epidemiol. 2013;37:5–10. doi: 10.1016/j.canep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977-2005. Br J Cancer. 2009;101:855–859. doi: 10.1038/sj.bjc.6605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glenn TF. Esophageal cancer: Facts, figures, and screening. Gastroenterol Nurs. 2001;24:271–273. [PubMed] [Google Scholar]
- 7.Wei WQ, Yang J, Zhang SW, et al. Analysis of the esophageal cancer mortality in 2004-2005 and its trends during last 30 years in China. Zhonghua Yu Fang Yi Xue Za Zhi. 2010;44:398–402. [PubMed] [Google Scholar]
- 8.Hiripi E, Jansen L, Gondos A, et al. Survival of stomach and esophagus cancer patients in Germany in the early 21st century. Acta Oncol. 2012;51:906–914. doi: 10.3109/0284186X.2012.673732. [DOI] [PubMed] [Google Scholar]
- 9.Wang GQ, Jiao GG, Chang FB, et al. Long-term result of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann Thorac Surg. 2004;77:1740–1744. doi: 10.1016/j.athoracsur.2003.10.098. [DOI] [PubMed] [Google Scholar]
- 10.Shu YJ. Cytopathology of the esophagus: An overview of esophageal cytopathology in China. Acta Cytol. 1983;27:7–16. [PubMed] [Google Scholar]
- 11.Shen O, Liu SF, Dawsey SM, et al. Cytologic screening for esophageal cancer: Results from 12,877 subjects from a high-risk population in China. Int J Cancer. 1993;54:185–188. doi: 10.1002/ijc.2910540204. [DOI] [PubMed] [Google Scholar]
- 12.Dawsey SM, Shen Q, Nieberg RK, et al. Studies of esophageal balloon cytology in Linxian, China. Cancer Epidemiol Biomarkers Prev. 1997;6:121–130. [PubMed] [Google Scholar]
- 13.Roth MJ, Liu SF, Dawsey SM, et al. Cytologic detection of esophageal squamous cell carcinoma and precursor lesions using balloon and sponge samplers in asymptomatic adults in Linxian, China. Cancer. 1997;80:2047–2059. doi: 10.1002/(sici)1097-0142(19971201)80:11<2047::aid-cncr3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Pan QJ, Roth MJ, Guo HQ, et al. Cytologic detection of esophageal squamous cell carcinoma and its precursor lesions using balloon samplers and liquid-based cytology in asymptomatic adults in Linxian, China. Acta Cytol. 2008;52:14–23. doi: 10.1159/000325430. [DOI] [PubMed] [Google Scholar]
- 15.Qin DX, Wang GQ, Zuo JH, et al. Screening of esophageal and gastric cancer by occult blood bead detector. Cancer. 1993;71:216–218. doi: 10.1002/1097-0142(19930101)71:1<216::aid-cncr2820710133>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Qin DX, Wang GQ, Yuan FL. Screening for upper digestive cancer with an occult blood bead detector: Investigation of a normal north China population. Cancer. 1988;62:1030–1034. doi: 10.1002/1097-0142(19880901)62:5<1030::aid-cncr2820620533>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Dawsey SM, Wang GQ, Weinstein WM, et al. Squamous dysplasia and early esophageal cancer in the Linxian region of China: Distinctive endoscopic lesions. Gastroenterology. 1993;105:1333–1340. doi: 10.1016/0016-5085(93)90137-2. [DOI] [PubMed] [Google Scholar]
- 18.Dawsey SM, Lewin KJ, Liu FS, et al. Esophageal morphology from Linxian, China: Squamous histologic findings in 754 patients. Cancer. 1994;73:2027–2037. doi: 10.1002/1097-0142(19940415)73:8<2027::aid-cncr2820730803>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220–231. [PubMed] [Google Scholar]
- 20.Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: A multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566–1572. doi: 10.1200/JCO.2009.25.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou J, He YT, Li SS, et al. The dynamic analysis of esophageal cancer mortality from 1969 to 2000 in Cixian. J Pract Oncol. 2002;16:243–247. [Google Scholar]
- 22.Thomas L, Reyes EM. Tutorial: survival estimation for Cox regression models with time-varying coefficients using SAS and R. http://www.jstatsoft.org/v61/c01.
- 23.Dawsey SM, Lewin KJ, Wang GQ, et al. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus: A prospective follow-up study from Linxian, China. Cancer. 1994;74:1686–1692. doi: 10.1002/1097-0142(19940915)74:6<1686::aid-cncr2820740608>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Wang GQ, Abnet CC, Shen Q, et al. Histological precursors of oesophageal squamous cell carcinoma: Results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187–192. doi: 10.1136/gut.2004.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bureau of Disease Prevention and Control, National Health and Family Planning Commission. Cancer Early Detection and Treatment Program Report 2012/2013. Beijing, China: Bureau of Disease Prevention and Control, National Health and Family Planning Commission; 2013. [Google Scholar]