Abstract
Purpose
This study was conducted to characterize the effect of food on the relative bioavailability of lapatinib.
Patients and Methods
A single 1,500-mg, oral dose of lapatinib was administered to 27 patients with advanced solid tumors on each of three occasions that were 1 week apart, in random order: after an overnight fast, with a low-fat breakfast, and with a high-fat breakfast.
Results
The low-fat breakfast produced mean increases in lapatinib area under the concentration-time curve (AUC) of 167% (2.67-fold) and maximum concentration (Cmax) of 142% (2.42-fold). The high-fat breakfast produced mean increases in lapatinib AUC of 325% (4.25-fold) and Cmax of 203% (3.03-fold) compared with the fasted state. Increased bioavailability in the fed state did not significantly decrease relative variability. Therefore, absolute variability in systemic exposure was increased.
Conclusion
These large increases in lapatinib bioavailability and absolute variability support the recommendation for dosing in the fasted state to achieve consistent therapeutic exposure. Prescribers and patients should consider the potential consequences of toxicity or diminished efficacy that might result from dosing without regard to variations in diet.
INTRODUCTION
Metastatic breast cancer is the leading cause of cancer-related death among women worldwide.1 Breast, lung, and ovarian cancers that overexpress the human epidermal growth factor receptor 2 (ie, ErbB2, HER-2, HER-2/neu,) are associated with a higher risk of aggressive disease and shortened survival.2 Lapatinib (Tykerb/Tyverb, GlaxoSmithKline, Bentford, United Kingdom) is a 4-anilinoquinazoline competitive inhibitor of both ErbB1 and ErbB2 tyrosine kinases that is approved for use in combination with capecitabine to treat advanced metastatic breast cancer.
The pharmacokinetic parameters of lapatinib have been characterized previously in both healthy participants and patients under fasted conditions. After ingestion of an oral dose, measurable lapatinib concentrations appeared in plasma after a 15-minute lag time, and they reached a maximum value at 4 hours postdose. Lapatinib elimination half-life increased with dose from 6 hours to 14 hours after single doses that ranged from 10 to 250 mg, and it increased with time after repeated dosing to an effective accumulation half-life of 24 hours.3 Lapatinib is highly (> 99%) bound to albumin and alpha-1 acid glycoprotein, and it is extensively metabolized by CYP3A4 and CYP3A5; less than 2% of the dose is excreted in urine. It is also a substrate and inhibitor of the efflux transporters, breast cancer resistance protein (BCRP, ABCG2) and P-glycoprotein (Pgp, ABCB1).4 The bioavailability of lapatinib is susceptible to alteration when administered with food, as with other low-solubility, low-permeability drugs. Preclinical studies in rats and dogs indicated that dosing with food decreased lapatinib bioavailability. In contrast, preliminary clinical studies indicated that food increased lapatinib bioavailability. The effect of food on lapatinib pharmacokinetics in humans was first examined in 19 healthy participants who were given single 100-mg doses with a high-fat breakfast. Food caused a 60% increase in the relative bioavailability of lapatinib.5 On the basis of this finding, patients in phase II trials were restricted from taking lapatinib with food that was high in fat or caloric content, and they were instructed to take the dose either in a fasted state or with a light snack, if needed. The effect of a low-fat breakfast on the bioavailability of a single 1,250-mg dose was examined later in a small cohort of six cancer patients, which indicated a three-fold (200%) increase in the area under the concentration-time curve (AUC).5 On the basis of this observation, patients in phase III clinical trials were instructed to take lapatinib without food, at least 1 hour before or after a meal. This study was performed to help resolve discrepancies between these results and to characterize more fully the effects of the low-fat and high-fat meals on the relative bioavailability of a clinically relevant lapatinib dose in cancer patients.
PATIENTS AND METHODS
Patient Population
Patients with advanced solid tumors were recruited from the oncology clinics at the Dartmouth-Hitchcock Medical Center (Norris Cotton Cancer Center, Lebanon, NH) and the Fox Chase Cancer Center (Philadelphia, PA). The respective institutional review boards approved the study protocol, and all patients provided written informed consent before they participated in the study.
Eligible patients were men or women aged 18 through 70 years who had histologically confirmed solid tumors that were refractory to standard therapy or for which there was no standard therapy. Additional inclusion criteria were a Karnofsky performance status ≥ 70; adequate physiologic organ function, as evidenced by hemoglobin ≥ 9 g/dL (5 mmol/L); absolute neutrophil count ≥ 1,500/mm3 (1.5 × 109/L); platelet count ≥ 100,000/mm3 (100 × 109/L); estimated creatinine clearance (≥ 30 mL/min); total bilirubin ≤ 1.5 mg/dL; and an ALT or AST less than three times the upper limit of the reference range. Patients were ineligible to participate if they had any condition that compromised esophageal or gastrointestinal function; had exposure to any investigational drug within 4 weeks before the study; had received chemotherapy or radiation therapy within 3 weeks before the study (or 6 weeks for nitrosoureas and mitomycin); had class III or IV heart failure, as defined by the New York Heart Association functional classification system; a left ventricular ejection fraction less than 40% on the basis of multigated angiogram or echocardiogram; evidence of uncontrolled brain metastases or leptomeningeal disease; other serious illness or conditions, including clinically significant cardiac disease (ie, angina or prior myocardial infarction), psychiatric disorder, active infection, or required concurrent therapy with potent CYP3A4 inhibitors or inducers during the study.
Study Design
This was an open-label, three-period, randomized, crossover study with a treatment continuation phase for patients who tolerated initial therapy. Each patient received a single 1,500-mg oral dose of lapatinib (six 250-mg tablets) on three separate occasions that were 1 week apart (on study days 1, 8, and 15) under one of three randomly assigned prandial conditions—an overnight fast maintained for 4 hours postdose; immediately after a low-fat breakfast; and immediately after a high-fat breakfast. The low-fat breakfast consisted of one cup of cereal (ie, Special K), 8 ounces of skimmed milk, one piece of toast with jam (no butter or marmalade), apple juice, and one cup of coffee or tea (2 g fat, 17 g protein, 93 g of carbohydrate, 520 calories). The high-fat breakfast consisted of two eggs fried in butter, two strips of bacon, two slices of toast with butter, 4 ounces of hash brown potatoes, 8 ounces of whole milk, and one cup of coffee or tea (54 g fat, 46 g protein, 72 g carbohydrate, 1,036 calories). It was estimated that complete data from 24 patients would provide 93% power to determine a 20% difference between fed and fasted conditions at the 5% significance level on the basis of data from the previously described, six-patient study cohort. After completion of the initial part of the study on day 16, patients began daily treatment with lapatinib (1,500 mg or lower at the discretion of the clinical investigator) administered at least 1 hour before or after breakfast, and they continued treatment as outpatients until they withdrew consent or experienced either disease progression or treatment-related toxicities.
Safety Assessment
Each patient's medical condition was assessed by physical examination, ECG, vital signs, and clinical laboratory tests at screening; before each dose; and 4, 24, and 48 hours after each dose on days 1, 8, and 15. These clinical and safety assessments were continued at regular intervals after the food-effect component of the study, while patients remained on lapatinib therapy, and again 30 days after lapatinib treatment was stopped. Adverse events were assessed by the investigator throughout the study and the subsequent treatment for relationship to treatment and severity, and events were graded according to the National Cancer Institute Common Toxicity Criteria, version 2 (Cancer Therapy Evaluation Program, 2003).
Blood Sampling and Lapatinib Assay
Blood samples (2 mL) were obtained from an indwelling peripheral venous catheter before lapatinib dosing and at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 16, 24, 36, and 48 hours postdose. Blood was centrifuged at 720 × g for 10 minutes at room temperature, and plasma aliquots were stored at or below −20°C until analysis for lapatinib concentration was performed, according to a previously described liquid chromatography–mass spectrometry method6 that had a linear range of 5 to 5,000 ng/mL and precision and accuracy within 15% of nominal.
Pharmacokinetic and Statistical Analyses
Lapatinib plasma concentration and time data were analyzed by using WinNonlin Version 4.0 software (Pharsight, Mountain View, CA). Noncompartmental analysis was performed to determine AUC extrapolated to infinity (AUC∞), peak concentration (Cmax), time at Cmax (tmax), absorption lag time (tlag), and elimination half-life (t1/2). Statistical analysis of pharmacokinetic parameters was carried out with SAS software (Version 8.2; SAS Institute, Cary, NC) after log-transformation to compare each fed state to the fasted state by using analysis of variance, with the exception of observed tmax and tlag, which were compared without transformation by using the Wilcoxon matched-pairs test.
RESULTS
Patient Demographics
Twenty-seven patients were enrolled to allow for the possibility that some patients might not complete all treatments. The demographic characteristics of the 27 patients who completed at least two of the three prandial state treatments are listed in Table 1.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | No. of Patients (N = 27) |
|---|---|
| Age, years | |
| Median | 63 |
| Range | 34–71 |
| Sex | |
| Male | 10 |
| Female | 17 |
| Ethnicity | |
| White | 27 |
| BMI, kg/m2 | |
| Median | 25 |
| Range | 18–47 |
| Karnofsky score | |
| Median | 80 |
| Range | 70–100 |
| Number of prior treatments | |
| 0-3 | 8 |
| 4-6 | 9 |
| 7-9 | 6 |
| > 9 | 4 |
| Tumor type | |
| Lung | 8 |
| Breast | 4 |
| Pancreas | 3 |
| Melanoma | 2 |
| Head and neck | 2 |
| Liver | 1 |
| Renal | 1 |
| Prostate | 1 |
| Colon | 1 |
| Parotid | 1 |
| Chondroblastic sarcoma | 1 |
| Follicular lymphoma | 1 |
| Ovarian adenocarcinoma | 1 |
Abbreviation: BMI, body mass index.
Lapatinib Pharmacokinetics
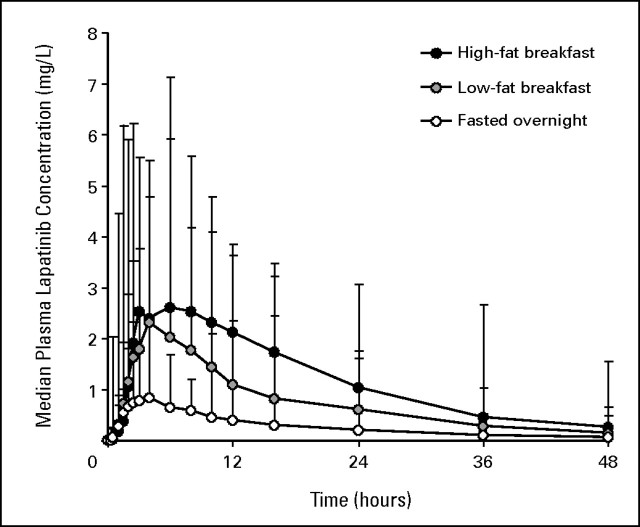
The median and upper ranges of plasma lapatinib concentrations versus time for each prandial state are shown in Figure 1. Lapatinib pharmacokinetic parameters in each prandial state are listed and compared in Table 2. Relative to the fasted state, the low- and high-fat breakfasts were associated with 2.67-fold (167%) and 4.25-fold (325%) geometric mean increases in apparent AUC∞, respectively. The absolute magnitude and variability of the effects of low- and high-fat breakfasts are illustrated in Figure 2, which shows individual changes in AUC∞ that ranged from a 24% decrease to a 473% (5.73-fold) increase (coefficient of variation [CV] 52%) after the low-fat breakfast and from 70% to 2,255% (1.7- to 23.55-fold) increases (CV 52%) after the high-fat breakfast relative to fasting (CV 68%). The corresponding geometric mean increases in Cmax were 2.42-fold (142%) and 3.03-fold (203%), respectively, and individual increases ranged up to 786% (8.86-fold) and 1,087% (11.87-fold), respectively. The tlag was 50% longer after each breakfast, but apparent t1/2 (approximately 12 hours) was unaffected.
Fig 1.
Median and upper ranges of plasma lapatinib concentrations versus time following a 1,500-mg dose administered after fasting overnight, after a low-fat breakfast, and after a high-fat breakfast.
Table 2.
Pharmacokinetic Parameters in Each Prandial State and in Comparisons of Each Fed State to the Fasted State
| Parameter | Prandial State |
Comparison |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FO |
LFB |
HFB |
LFB v FO |
HFB v FO |
||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | LS Mean Ratio | 90% CI | LS Mean Ratio | 90% CI | |
| AUC∞, h × mg/L | 14.5 | 11.8 to 17.8 | 38.6 | 32.6 to 45.8 | 60.9 | 50.2 to 74.0 | 2.67 | 2.26 to 3.16 | 4.25 | 3.60 to 5.02 |
| Cmax, mg/L | 0.94 | 0.77 to 1.14 | 2.38 | 1.96 to 2.89 | 2.90 | 2.44 to 3.45 | 2.42 | 2.02 to 2.90 | 3.03 | 2.53 to 3.63 |
| tmax, hours | 4.0 | 3.1 to 4.3 | 4.0 | 3.9 to 5.7 | 6.0 | 4.9 to 7.2 | 1.09 | 0.50 to 2.00 | 2.53 | 1.50 to 4.00 |
| tlag, minutes | 15 | 0 to 30 | 15 | 0 to 30 | 15 | 0 to 121 | 8 | 0 to 8 | 8 | 0 to 15 |
| t1/2, hours | 13.4 | 11.7 to 15.3 | 12.0 | 10.9 to 13.3 | 11.9 | 10.4 to 13.6 | 0.91 | 0.82 to 1.02 | 0.92 | 0.83 to 1.02 |
Note. For AUC, Cmax, and t1/2, comparision is the geometric LS mean ratio, and for tmax and tlag, comparision is the median difference.
Abbreviations: FO, fasted overnight; LFB, low-fat breakfast; HFB, high-fat breakfast; LS, least square; AUC∞, area under the concentration-time curve extrapolated to infinity; Cmax, peak concentration; tmax, time to peak concentration; tlag, absorption lag time; t1/2, elimination half life.
Fig 2.
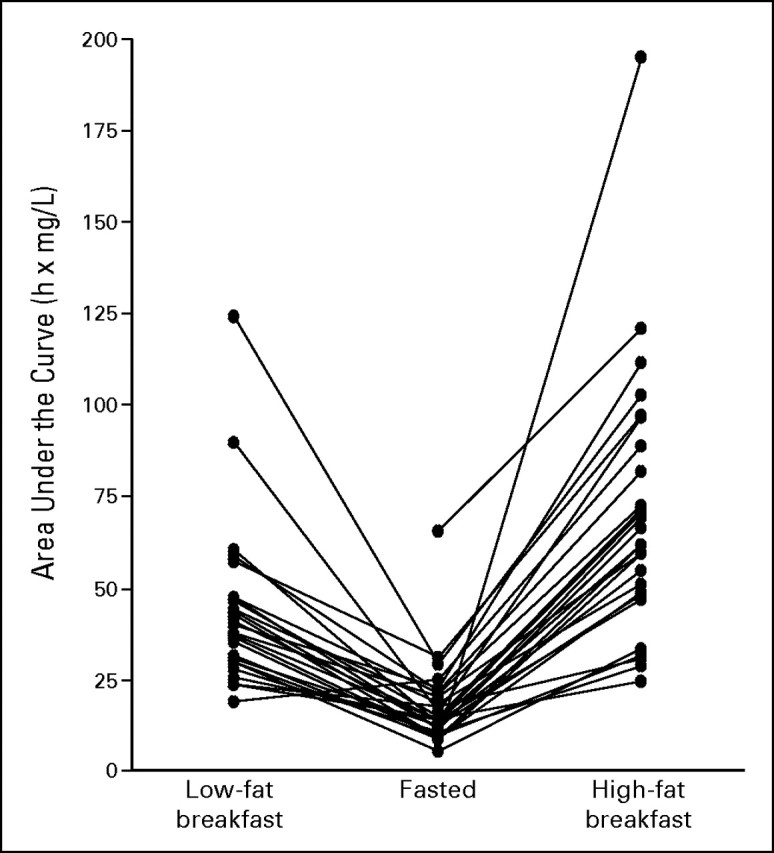
Magnitude and variability of the changes in observed systemic exposure (AUC) of lapatinib after administration with a low-fat breakfast (n = 26) and a high-fat breakfast (n = 27).
Safety and Tolerability
Lapatinib was generally well tolerated during both the pharmacokinetic and the chronic-treatment phases of this study. Of the 27 patients enrolled, 26 completed the pharmacokinetic phase, and one withdrew after the second period (high-fat meal) because of grade 3 rash that resolved after 31 days, which was subsequently treated at a reduced lapatinib dose in the chronic-treatment phase. One patient experienced grade 3 orthostatic hypotension in the first period (fasted) that resolved without sequelae. This patient completed the pharmacokinetic phase and entered the chronic-treatment phase. Only one patient elected not to enter the chronic-treatment phase. The most commonly reported treatment-related adverse events are listed in Table 3. Corrected QT (QTc) interval data are listed in Table 4.
Table 3.
Drug-Related Adverse Events Observed in More Than One Patient
| Adverse Event* | Prandial State |
||
|---|---|---|---|
| Fasted Overnight (n = 27) | Low-Fat Breakfast (n = 26) | High-Fat Breakfast (n = 27) | |
| Diarrhea with all doses | |||
| Grades 1 and 2 | |||
| No. | 6 and 1 | 5 and 1 | 9 and 0 |
| % | 22 and 4 | 19 and 4 | 33 and 0 |
| Onset, days | |||
| Median | 0 | 0 | 0.5 |
| Range | 0–2 | 0–1 | 0–2 |
| Duration, days | |||
| Median | 2 | 3.5 | 2 |
| Range | 1–44 | 1–29 | 1–65 |
| Diarrhea with first dose only | |||
| Grades 1 and 2 | |||
| No. | 3 and 0 | 2 and 0 | 3 and 0 |
| % | 11 and 0 | 8 and 0 | 11 and 0 |
| Onset, days | |||
| Median | 0 | 0.5 | 1 |
| Range | 0–1 | 0–1 | 0–1 |
| Duration, days | |||
| Median | 2 | 2.5 | 2 |
| Range | 2–44 | 1–4 | 1–65 |
| Nausea | |||
| Grade 1 | |||
| No. | 5 | 1 | 2 |
| % | 19 | 4 | 7 |
| Fatigue | |||
| Grade 1 | |||
| No. | 3 | 1 | 1 |
| % | 11 | 4 | 4 |
| Rash | |||
| Grades 1 and 3 | |||
| No. | 1 and 0 | 1 and 0 | 0 and 1 |
| % | 4 and 0 | 4 and 0 | 0 and 4 |
Adverse events are categorized by severity, onset, and duration. Onset measured relative to preceding dose; duration measured relative to onset. Adverse events severity measured by National Cancer Institute Common Toxicity Criteria.
Table 4.
QTcF Duration Measurements
| QTcF (ms) | Prandial State |
Difference |
|||
|---|---|---|---|---|---|
| FO | LFB | HFB | LFB − FO | HFB − FO | |
| Predose baseline | |||||
| Median | 414 | 407 | 411 | −5 | −1 |
| Range | 381–497 | 376–451 | 380–468 | −102 to 33 | −80 to 34 |
| Postdose maximum | |||||
| Median | 424 | 417 | 421 | −3 | −5 |
| Range | 404–454 | 394–451 | 401–449 | −15 to 18 | −17 to 22 |
| Maximum change | |||||
| Median | 5 | 10 | 5 | 3 | −3 |
| Range | 1–24 | −9 to 36 | 3–55 | −11 to 21 | −5 to 46 |
Abbreviations: QTcF, Fridericia corrected QT interval; FO, fasted overnight; LFB, low-fat breakfast; HFB, high-fat breakfast.
DISCUSSION
Food can alter oral drug bioavailability by affecting drug solubility and gastrointestinal physiology.7–9 Lapatinib bioavailability is complicated in both of these aspects. It is a weak base with low, pH-dependent solubility that declines precipitously above pH 4. Food rapidly buffers gastric acid and, therefore, would be expected to decrease lapatinib bioavailability. Thus, the observed increase in bioavailability with food in this study suggests that pH is not an important factor for lapatinib. Food also delays gastric emptying, which can allow more time for tablet dissolution before the tablet enters the small intestine, where absorption occurs, and which would enhance bioavailability. The tlag in detectable lapatinib plasma concentration is similar to gastric emptying, which has a half-life of 15 minutes in the fasted state. On the basis of caloric content,10,11 the corresponding predicted half-lives of gastric emptying after the low- and high-fat breakfasts used in this study would be 22 and 36 minutes, respectively. This is consistent with the 90th percentile of tlag at 23 minutes. However, lapatinib tablet dissolution is sufficiently rapid (ie, 6-minute half-life in vitro) that this delay would have little impact on bioavailability. Small intestinal transit time is generally 3 to 4 hours, unless it is prolonged by a heavy meal.9 Decreased motility may contribute to the effects of the high-fat breakfast, which reflects the delay in tmax. Ingestion of a meal also stimulates increased blood flow to the intestinal mucosa, which would facilitate the uptake of drug into the circulation. However, neither of these phenomena could account for the magnitude of change from either the low- or high-fat breakfast.
The solubility of hydrophobic substances in the gut is enhanced by incorporation into micelles formed by bile salts. In response to a high-fat meal, bile salts increase from approximately 4 mmol/L (fasted) to 20 mmol/L.12 The release of bile salts into the duodenum could increase both the solubility and the permeability of lapatinib into enterocytes, which would lead to higher bioavailability. Bile acid turnover of 12 to 18 g/d (1 to 1.5 mmol/h), is sufficient to solubilize a 1,500 mg (2.58 mmol) dose of lapatinib. 13 Bile salt solubilization also may account for the opposite effect of food on lapatinib bioavailability in rats and dogs. These species have higher bile flow7,14 and greater capacity for solubilization that favors fecal excretion over absorption, which results in higher fecal recovery of unmetabolized lapatinib. Bile salts in excess of 15 mmol/L can decrease diffusion of micelles through the unstirred water layer to the enterocyte membrane, which limits the effect of bile acid solubilization on bioavailability.15 It is likely, therefore, that other mechanisms contribute to the large increases in lapatinib bioavailability with food.
The absence of a change in lapatinib apparent half-life suggests that these mechanisms are largely presystemic. Lapatinib bioavailability also is likely to be limited by ABCB1-mediated efflux and by CYP3A-mediated metabolism in enterocytes. Food-enhanced permeability could result, therefore, in lapatinib concentrations that saturate these mechanisms or that augment the autoinhibition of CYP3A4 observed in vitro with lapatinib. These additional mechanisms could account for the magnitude and dose-dependence of the food effect, and their combined effects may underlie the high variability observed in the lapatinib pharmacokinetic parameters.
The effect of food on lapatinib is large compared with that of many other drugs. Current literature indicates that, among oral tyrosine kinase inhibitors, the next largest effect of food is a 1.6-fold increase reported for erlotinib.16 In contrast, gefitinib and imatinib bioavailability are unaffected by food,17 and dasatinib bioavailability increased only 14% with a high-fat meal.18 Other drugs that exhibit three- to five-fold food effects include albendazole,19 praziquantel,20 atovaquone,21 and fenretinide (an experimental breast cancer treatment). 22 A three- to five-fold increase in bioavailability with food indicates poor bioavailability that could be improved by dosing with meals to achieve therapeutic concentrations. One advantage of this strategy in most instances would be decreased variability in systemic exposure as a consequence of approaching the limit of complete absorption. However, interpatient variability in lapatinib AUC was neither significantly nor meaningfully diminished with food (CV 68% fasted v 52% fed; F test P = .895). However, relative variability does not convey the impact of food on absolute variability, which is magnified by the size of the difference in bioavailability. Individual increases in bioavailability ranged from a 20% decrease to a 24-fold increase. This large inherent variability might be magnified additionally by differences in eating habits between patients and within a patient from one day to the next. Under these circumstances, we suggest that the potential risks of toxicity or loss of efficacy (secondary to loss of appetite) currently preclude the routine use of food to reliably and consistently improve lapatinib bioavailability for chronic therapeutic use. Additional work is planned to examine the effect of food during chronic dosing of lapatinib.
Lapatinib is indicated for treatment of advanced breast cancer in combination with capecitabine. Capecitabine dosing with food is recommended despite the resultant small decreases in the concentrations of its active metabolites.23 It appears advantageous, therefore, to administer both drugs in this combination in the fasted state. However, the effect of food on capecitabine is small and is likely overcome by the combined efficacy of these agents.
Diarrhea is the most common toxicity of lapatinib, and it appears to be related to dose and not to systemic exposure (ie, AUC),3 which suggests that it is provoked by drug in the gut. Incidence of diarrhea might be expected, therefore, to decrease with increased absorption. Although this study was neither large enough nor of sufficient duration to fully assess this toxicity, the incidence, onset, and duration of diarrhea experienced by 16 patients (of which 10 were after the first dose) were not diminished by the high-fat breakfast (Table 3).
Cardiotoxicity has been associated with agents that target HER-2. The QTc interval data obtained in this study showed no evidence of prolongation despite the high systemic exposures achieved. This is consistent with data from other studies (data on file, GlaxoSmithKline, Research Triangle Park, NC) that show no clinically significant effect of lapatinib on QTc interval duration at steady-state with daily doses up to 1,800 mg and with in vitro data that show lapatinib-activated AMP-kinase protection of cardiac cells from metabolic stress–induced apoptosis.24
The convenience of oral delivery of cancer therapies demands attention to the sources of variability in drug exposure that determine the balance between efficacy and toxicity. Patients should be alerted to these consequences by their prescribing physicians. The results of this study indicate that administration of 1,500 mg of lapatinib with food dramatically increased its relative bioavailability and the associated absolute variability in AUC. The current approved labeling for the administration of lapatinib in the fasted state is supported, therefore, by this data as the most reliable way to achieve consistent systemic exposure during chronic oral treatment with this targeted drug therapy.
Footnotes
Supported by GlaxoSmithKline.
Presented in part at the American Society of Clinical Pharmacology and Therapeutics (ASCPT) Annual Meeting, March 21-24, 2007, Anaheim, CA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Kevin M. Koch, GlaxoSmithKline (C) Consultant or Advisory Role: Lionel D. Lewis, GlaxoSmithKline (C) Stock Ownership: Kevin M. Koch, GlaxoSmithKline; Bonnie Whitehead, GlaxoSmithKline; Andrew Stead, GlaxoSmithKline Honoraria: None Research Funding: Roger B. Cohen, GlaxoSmithKline; Lionel D. Lewis, GlaxoSmithKline Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Kevin M. Koch, Andrew Stead
Administrative support: Bonnie Whitehead
Provision of study materials or patients: Nandi J. Reddy, Roger B. Cohen, Nancy L. Lewis, Kathleen Mackay, Andrew P. Beelen, Lionel D. Lewis
Collection and assembly of data: Kevin M. Koch, Roger B. Cohen, Nancy L. Lewis, Bonnie Whitehead, Kathleen Mackay, Andrew Stead, Andrew P. Beelen, Lionel D. Lewis
Data analysis and interpretation: Kevin M. Koch, Nandi J. Reddy, Roger B. Cohen, Andrew Stead, Lionel D. Lewis
Manuscript writing: Kevin M. Koch, Nandi J. Reddy, Roger B. Cohen, Lionel D. Lewis
Final approval of manuscript: Kevin M. Koch, Roger B. Cohen, Nancy L. Lewis, Andrew P. Beelen, Lionel D. Lewis
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, Hurwitz HI, Dees EC, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 4.Polli JW, Humphreys JE, Harmon KA, et al. The role of efflux and uptake transporters in [N-(3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine) (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:695–701. doi: 10.1124/dmd.107.018374. [DOI] [PubMed] [Google Scholar]
- 5.Smith DA, Koch KM, Lee D, et al. The effect of food on the pharmacokinetics of GW572016. Eur J Cancer. 2003;1(suppl):S169. abstr 558. [Google Scholar]
- 6.Hsieh S, Tobien T, Koch K, et al. Increasing throughput of parallel on-line extraction liquid chromatography/electrospray ionization tandem mass spectrometry system for GLP quantitative bioanalysis in drug development. Rapid Commun Mass Spectrom. 2004;18:285–292. doi: 10.1002/rcm.1327. [DOI] [PubMed] [Google Scholar]
- 7.Jones HM, Parrott N, Ohlenbusch G, et al. Predicting pharmacokinetic food effects using biorelevant solubility media and physiologically based modelling. Clin Pharmacokinet. 2006;45:1213–1226. doi: 10.2165/00003088-200645120-00006. [DOI] [PubMed] [Google Scholar]
- 8.Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer agents: Underlying mechanisms and implications for oral chemotherapy. Clin Pharmacokinet. 2004;43:1127–1156. doi: 10.2165/00003088-200443150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Dressman J. Comparison of canine and human gastrointestinal physiology. Pharm Res. 1986;3:123–131. doi: 10.1023/A:1016353705970. [DOI] [PubMed] [Google Scholar]
- 10.Hunt JN, Stubbs DF. The volume and energy content of meals as determinants of gastric emptying. J Physiol. 1975;245:209–225. doi: 10.1113/jphysiol.1975.sp010841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calbet JA, MacLean DA. Role of caloric content on gastric emptying in humans. J Physiol. 1997;498:553–559. doi: 10.1113/jphysiol.1997.sp021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charman WN, Porter CJH, Mithanai S, et al. Physicochemical and physiological mechanisms for the effects of food on drug absorption: The role of lipids and pH. J Pharm Sci. 1997;86:269–282. doi: 10.1021/js960085v. [DOI] [PubMed] [Google Scholar]
- 13.Sievanen E. Exploitation of bile acid transport systems in prodrug design. Molecules. 2007;12:1859–1889. doi: 10.3390/12081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeSesso JM, Jacobson CF. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem Toxicol. 2001;39:209–228. doi: 10.1016/s0278-6915(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 15.Bakatselou V, Oppenheim RC, Dressman JB. Solubilization and wetting effects of bile salts on the dissolution of steroids. Pharm Res. 1991;8:1461–1469. doi: 10.1023/a:1015877929381. [DOI] [PubMed] [Google Scholar]
- 16.Johnson JR, Cohen M, Rajeshwari S, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic small-cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res. 2005;11:6414–6421. doi: 10.1158/1078-0432.CCR-05-0790. [DOI] [PubMed] [Google Scholar]
- 17.Swaisland HC, Smith RP, Laight A. Single-dose clinical pharmacokinetic studies of gefitinib. Clin Pharmacokinet. 2005;44:1165–1177. doi: 10.2165/00003088-200544110-00004. [DOI] [PubMed] [Google Scholar]
- 18.Dasatinib Package Insert. http//www.sprycel.com, revised November 2008.
- 19.Lange H, Eggers R, Bircher J. Increased systemic availability of albendazole when taken with a fatty meal. Eur J Clin Pharmacol. 1988;34:315–317. doi: 10.1007/BF00540964. [DOI] [PubMed] [Google Scholar]
- 20.Castro N, Medina R, Sotelo J, et al. Bioavailability of praziquantel increases with concomitant administration of food. Antimicrob Agents Chemother. 2000;44:2903–2904. doi: 10.1128/aac.44.10.2903-2904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolan PE, Mercer AJ, Weatherley BC, et al. Investigation of the factors responsible for a food-induced increase in absorption of a novel antiprotozoal drug 566C80. Br J Clin Pharmacol. 1992;33:534P–535P. doi: 10.1111/j.1365-2125.1994.tb04232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doose DR, Minn FL, Stellar S, et al. Effects of meals and meal composition on the bioavailability of fenretinide. J Clin Pharmacol. 1992;32:1089–1095. [PubMed] [Google Scholar]
- 23.Reigner B, Verweij J, Dirix L, et al. Effect of food on the pharmacokinetics of capecitabine and its metabolites following oral administration in cancer patients. Clin Cancer Res. 1998;4:941–948. [PubMed] [Google Scholar]
- 24.Spector NL, Yarden Y, Smith B, et al. Activation of AMP-activated protein kinase by human EGF receptor 2/EGF receptor tyrosine kinase inhibitor protects cadiac cells. Proc Natl Acad Sci U S A. 2007;104:10607–10612. doi: 10.1073/pnas.0701286104. [DOI] [PMC free article] [PubMed] [Google Scholar]