Abstract
Purpose
The addition of bevacizumab to fluorouracil-based chemotherapy is a standard of care for previously untreated metastatic colorectal cancer. Continuation of bevacizumab beyond progression is an accepted standard of care based on a 1.4-month increase in median overall survival observed in a randomized trial. No United States–based cost-effectiveness modeling analyses are currently available addressing the use of bevacizumab in metastatic colorectal cancer. Our objective was to determine the cost effectiveness of bevacizumab in the first-line setting and when continued beyond progression from the perspective of US payers.
Methods
We developed two Markov models to compare the cost and effectiveness of fluorouracil, leucovorin, and oxaliplatin with or without bevacizumab in the first-line treatment and subsequent fluorouracil, leucovorin, and irinotecan with or without bevacizumab in the second-line treatment of metastatic colorectal cancer. Model robustness was addressed by univariable and probabilistic sensitivity analyses. Health outcomes were measured in life-years and quality-adjusted life-years (QALYs).
Results
Using bevacizumab in first-line therapy provided an additional 0.10 QALYs (0.14 life-years) at a cost of $59,361. The incremental cost-effectiveness ratio was $571,240 per QALY. Continuing bevacizumab beyond progression provided an additional 0.11 QALYs (0.16 life-years) at a cost of $39,209. The incremental cost-effectiveness ratio was $364,083 per QALY. In univariable sensitivity analyses, the variables with the greatest influence on the incremental cost-effectiveness ratio were bevacizumab cost, overall survival, and utility.
Conclusion
Bevacizumab provides minimal incremental benefit at high incremental cost per QALY in both the first- and second-line settings of metastatic colorectal cancer treatment.
INTRODUCTION
Colorectal cancer is the third most common cancer and the third leading cause of cancer death in men and women in the United States.1 In 2010, $14 billion was spent in the United States on management of colorectal cancer.2
Fluorouracil (FU) combined with oxaliplatin (FOLFOX) is the most commonly used regimen in first-line therapy for metastatic colorectal cancer (mCRC).3 This regimen is essentially equivalent in efficacy to capecitabine plus oxaliplatin (XELOX).4 Bevacizumab, a monoclonal antibody against the vascular endothelial growth factor A, is commonly added to the regimen based on data from randomized clinical trials.5–7 FU and irinotecan (FOLFIRI) is commonly administered as a second-line regimen for patients with mCRC.8 Continuation of bevacizumab beyond first progression has an overall survival benefit,9 and administration of bevacizumab in addition to chemotherapy in both first- and second-line settings is now a standard practice. The modest survival benefit and high cost of bevacizumab have raised concerns regarding the cost effectiveness of this approach.
Although some international studies have evaluated the cost effectiveness of bevacizumab in mCRC,10–12 it has not been evaluated using a modeling approach in the United States. In this study, we estimate the cost effectiveness of bevacizumab from the perspective of the US payer.
METHODS
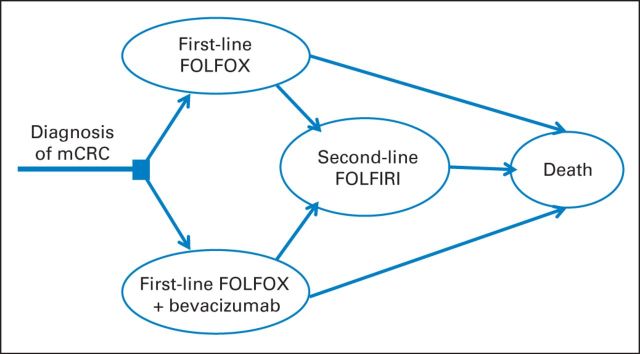
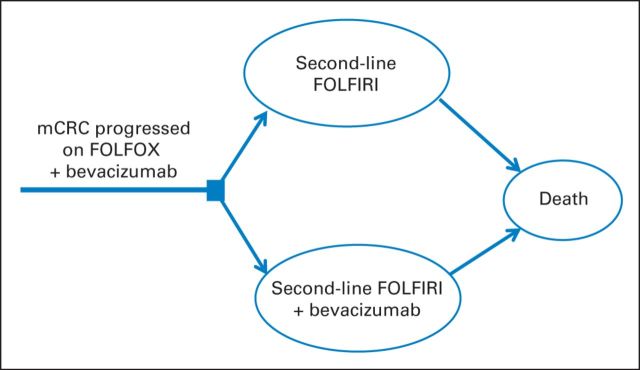
We developed two Markov models to evaluate the additional costs and effectiveness of bevacizumab as first- and second-line therapies. In the first-line model, we compared FOLFOX with or without bevacizumab in patients with newly diagnosed mCRC. On progression of disease, both groups received FOLFIRI without bevacizumab and subsequently experienced progression until death (Fig 1). In the second-line model, we compared FOLFIRI with or without bevacizumab, with subsequent progression to death, in patients who had experienced progression during first-line chemotherapy with bevacizumab (Fig 2).
Fig 1.
Markov model diagram for first-line model. FOLFIRI, fluorouracil plus irinotecan; FOLFOX, fluorouracil plus oxaliplatin; mCRC, metastatic colorectal cancer.
Fig 2.
Markov model diagram for second-line model. FOLFIRI, fluorouracil plus irinotecan; FOLFOX, fluorouracil plus oxaliplatin; mCRC, metastatic colorectal cancer.
Each health state was assigned a health utility score based on published studies. Only direct medical costs were considered and are stated in 2013 US dollars. All costs and outcomes were discounted by 3% annually. Each model cycle represents 2 weeks, because patients receive chemotherapy biweekly in clinical practice. The primary outputs of the models included total cost, life-years (LYs), quality-adjusted LYs (QALYs), and incremental cost-effectiveness ratios (ICERs). The Markov models were implemented in C++ language, and statistical analyses were performed in R software (http://www.r-project.org).
Patients and Treatment Regimens
We based our assumption describing the survival benefits associated with first-line FOLFOX plus bevacizumab versus FOLFOX on the results from the N01966 trial, which demonstrated an improved median overall survival (OS) of 1.4 months when bevacizumab was added to FOLFOX and XELOX.6 Patient characteristics, regimen information, and treatment outcomes for trials used in the models are summarized in Appendix Table A1 (online only). We based our assumption about the benefit of second-line bevacizumab on the ML18147 trial, which demonstrated a median OS benefit of 1.4 months when bevacizumab was continued beyond progression in combination with second-line FU-based chemotherapy.9
Mortality Estimates
The overall mortality rate corresponded to the probability of transition from any state to the death state, which was estimated as the cause-specific mortality from mCRC and background mortality resulting from other causes. The cause-specific mortalities of each treatment strategy were derived from the OS curves in the studies associated with each treatment regimen. Engauge Digitizer software (http://digitizer.sourceforge.net) was used to extract the data points from each OS plot, and these data points were then used to fit parametric survival models. We found that Weibull and log-logistic models provided a good fit for all curves according to the Akaike information criterion and the Schwarz Bayesian criterion.13 Between the Weibull and log-logistic models, we selected the Weibull distribution because it can have an increasing hazard rate and is suitable for modeling the events occurring early during follow-up periods. On the basis of the fitted Weibull OS model, denoted as OS(t), we computed the cause-specific mortality m(t) at cycle t as follows:
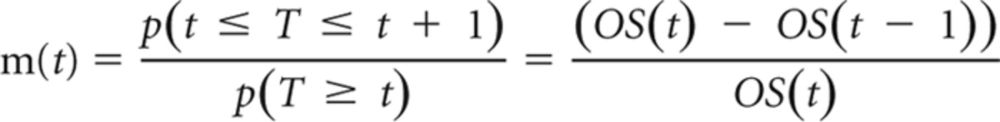 |
We estimated the background mortality for each age group based on US life-tables.14 The models sampled the initial age at diagnosis of mCRC in each simulation run using the age distribution for mCRC from SEER data.15
Progression Risk
In the first-line model, the transitions from first- to second-line therapy in each cycle were determined by the estimates of the progression risks based on N01966. Estimates of mortality and progression risk beyond the follow-up time in the clinical trials were extrapolated based on the fitted survival models.
Utility Estimates
To compute the total QALYs in the Markov models, survival time was adjusted by health-related quality of life. We used previously published mean utilities of 0.85 and 0.65 for patients in first- and second-line settings, respectively.16,17
Cost Estimates
Direct costs included drug, administration, and adverse event (AE) costs. In the first-line setting, the intravenous drug costs for each 2-week cycle of FOLFOX were based on the following doses: oxaliplatin 85 mg/m2, leucovorin 200 mg/m2, and FU 400 mg/m2 bolus and 600 mg/m2 continuously over 46 hours. In the second-line setting, the costs for each 2-week cycle of FOLFIRI were based on the following doses: irinotecan 180 mg/m2, leucovorin 400 mg/m2, and FU 400 mg/m2 bolus and 2,400 mg/m2 continuously over 46 hours. When bevacizumab was added, the cost was based on dosing at 5 mg/kg. We used a body-surface area of 1.86 m2 and body weight of 82 kg, based on mean US values.18 We did not round up drugs to the full vial size. To estimate the unit price of each drug, we used the 2013 average sales price from the Centers for Medicare and Medicaid Services.19
Administration and AE costs were calculated according to the Medicare physician fee schedule for 2013. Each chemotherapy infusion was assumed to last 4 hours. The fees for outpatient physician visits were based on Current Procedure Terminology codes.20 The methods used for these cost calculations were previously described by Tumeh et al.21 The fees for physician visits and chemotherapy administration are listed in Appendix Table A2 (online only).
The costs for grade 3 to 4 AEs were based on diagnosis-related group codes.21 The N01966 trial reported only toxicities related to bevacizumab that had a significant difference between arms.6 The model projected that patients with venous thromboembolic events would be treated with enoxaparin (1 mg/kg twice daily) for 6 months according to American Society of Clinical Oncology guidelines.22 Hypertension was assumed to be managed with amlodipine in the outpatient setting (Table 1).
Table 1.
Model Parameters: Baseline Values, Ranges, and Distributions for Sensitivity Analysis
| Variable | Value | Minimum | Maximum | Study | Distribution |
|---|---|---|---|---|---|
| AE incidence | |||||
| First-line FOLFOX | |||||
| Hypertension | 0.010 | 0.001 | 0.090 | N01966 | 0.49 to 48.51* |
| Venous thromboembolic event | 0.050 | 0.036 | 0.069 | N01966 | 35.050 to 665.950* |
| First-line FOLFOX plus bevacizumab | |||||
| Hypertension | 0.040 | 0.013 | 0.114 | N01966 | 2.84 to 68.16* |
| Venous thromboembolic event | 0.080 | 0.062 | 0.102 | N01966 | 55.92 to 643.08* |
| Second-line FOLFIRI | |||||
| Previously treated with bevacizumab | |||||
| Hypertension | 0.010 | 0.004 | 0.025 | ML18147 | 4.09 to 404.91* |
| Venous thromboembolic event | 0.030 | 0.017 | 0.051 | ML18147 | 12.270 to 396.730* |
| No previous treatment with bevacizumab | |||||
| Hypertension | 0.018 | 0.008 | 0.041 | E3200 | 5.130 to 279.870* |
| Venous thromboembolic event | 0.025 | 0.012 | 0.050 | E3200 | 7.125 to 277.875* |
| Second-line FOLFIRI plus bevacizumab† | |||||
| Hypertension | 0.020 | 0.01 | 0.039 | ML18147 | 8.02 to 392.98* |
| Venous thromboembolic event | 0.050 | 0.0323 | 0.076 | ML18147 | 20.05 to 380.95* |
| Costs per cycle, $ | |||||
| Administration‡ | 284.77 | 177.70 | 375.44 | 28.295 to 10.06§ | |
| FOLFOX | 435.05 | 348.04 | 522.06 | 100 to 4.351§ | |
| Bevacizumab | 2,649.42 | 2,119.54 | 3,179.30 | 64 to 6.25§ | |
| FOLFIRI | 392.60 | 314.08 | 471.12 | 100 to 3.926§ | |
| AE costs, $ | |||||
| Hypertension | 51.89 | 41.51 | 62.27 | 100 to 0.519§ | |
| Venous thromboembolic event | 5,567.00 | 4,453.60 | 6,680.40 | 100 to 55.670§ | |
| Utility | |||||
| Before progression | 0.85 | 0.68 | 1 | Ramsey et al16 | 14.15 to 2.497* |
| Beyond progression | 0.65 | 0.52 | 0.78 | Ramsey et al16,17 | 34.35 to 18.496* |
| Discount factor | 0.03 | 0 | 0.05 | 0 to 0.05‖ |
Abbreviations: AE, adverse event; FOLFIRI, fluorouracil plus irinotecan; FOLFOX, fluorouracil plus oxaliplatin.
Beta.
Assuming previous treatment with bevacizumab.
Clinical visit plus chemotherapy.
Gamma.
Uniform.
Model Validation
We performed external and internal model validations. In the external validation, we compared the survival curves used in this study with those for the same treatment in other published studies. N01966 OS curves were compared with those from studies by Schmoll et al23 and Tournigand et al.8 The internal validation showed that the progression-free survival and OS curves generated by the Markov model simulation closely approximated those presented in the clinical trials (Appendix Fig A1, online only).
Sensitivity Analysis
A series of sensitivity analyses were performed to evaluate the robustness of the model and address uncertainty in the estimation of variables. Utilities were varied over their 95% CIs. Drug costs were varied within ± 20% of their baseline values as in a similar study.24 Physician fees were varied across the range for all provider sites in the Centers for Medicare and Medicaid Services database as previously described.25 All ranges and distributions used in sensitivity analyses are summarized in Table 1.
We addressed the uncertainty of survival probabilities in the models. First, we identified the range for each survival curve based on the CI for the corresponding median survival. We adjusted the curve S(t) by a hazard ratio γ such that the adjusted survival [S(t)]γ had a median survival within the reported CI. We then re-estimated the risk of progression or death in each model cycle based on the adjusted survival curves [S(t)]γ with the lowest and highest values of hazard ratio γ.
In univariable sensitivity analyses, we varied the value of one parameter at a time over its defined range and examined the effect on the ICER. In probabilistic sensitivity analyses (PSAs), we ran the model 10,000 times, each time randomly varying all parameters simultaneously. We used gamma distribution for cost parameters, beta distribution for parameters bounded between 0 and 1, and uniform distribution for the discount factor. The baseline values, ranges, and distributions of model parameters are listed in Table 1.
We performed an additional analysis to evaluate the robustness of the model by altering the data sources for the first-line model (using sequential nonrandomized results of TREE1 [Three Regimens of Eloxatin Evaluation] and TREE2 [Three Regimens of Eloxatin Evaluation with bevacizumab]) studies.5 Furthermore, we compared the costs and effectiveness for patients who received bevacizumab in the first- and second-line settings versus those who did not receive bevacizumab in either setting. We used the control arm of the E3200 study to define the outcomes of second-line therapy without receipt of prior bevacizumab.26 Although the E3200 study used second-line FOLFOX, we felt that it was acceptable to use these data to model second-line outcomes, because FOLFOX and FOLFIRI have similar efficacy in this setting.27 For patients who had received first-line bevacizumab, we used the ML18147 trial to define the outcomes of second-line FOLFIRI plus bevacizumab.9 Although the reliability of these results is limited by the data being derived from different trials, these analyses were used only to assess whether the direction and magnitude of the differences in cost effectiveness of bevacizumab use in mCRC were similar to our primary analyses.
RESULTS
Base Case Results
As shown in Appendix Table A3 (online only), drug costs for FOLFOX, FOLFIRI, and bevacizumab were estimated at $435, $393, and $2,649, respectively, per 2-week cycle. In the primary analysis, the effectiveness and costs were compared in the first-line model for the FOLFOX and FOLFOX-plus-bevacizumab groups. FOLFOX provided 1.31 QALYs (1.78 LYs) at a cost of $32,561. FOLFOX plus bevacizumab provided 1.41 QALYs (1.92 LYs) at a cost of $92,112 (Table 2). The ICER for FOLFOX plus bevacizumab was $571,240 per QALY. In the second-line model, FOLFIRI provided 0.64 QALYs (0.99 LYs) at a cost of $7,443. FOLFIRI plus bevacizumab provided 0.75 QALYs (1.15 LYs) at a cost of $46,764. The ICER for FOLFIRI plus bevacizumab was $364,083 per QALY.
Table 2.
Base Case Results
| Result | First-Line Model |
Second-Line Model |
||||
|---|---|---|---|---|---|---|
| FOLFOX | FOLFOX Plus Bevacizumab | Incremental | FOLFIRI | FOLFIRI Plus Bevacizumab | Incremental | |
| LYs | 1.78 | 1.92 | 0.14 | 0.99 | 1.15 | 0.16 |
| QALYs | 1.31 | 1.41 | 0.10 | 0.64 | 0.75 | 0.11 |
| Total cost of regimen, $* | 32,561 | 93,112 | 60,551 | 7,443 | 46,764 | 39,321 |
| ICER, $ | ||||||
| Per LY | 438,779 | 235,455 | ||||
| Per QALY | 571,240 | 364,083 | ||||
Abbreviations: FOLFIRI, fluorouracil plus irinotecan; FOLFOX, fluorouracil plus oxaliplatin; ICER, incremental cost-effectiveness ratio; LY, life-year; QALY, quality-adjusted life-year.
Total cost of regimen in first-line model = first-line chemotherapy + second-line chemotherapy + adverse events. Total cost of regimen in second-line model = second-line chemotherapy + adverse events.
Sensitivity Analyses
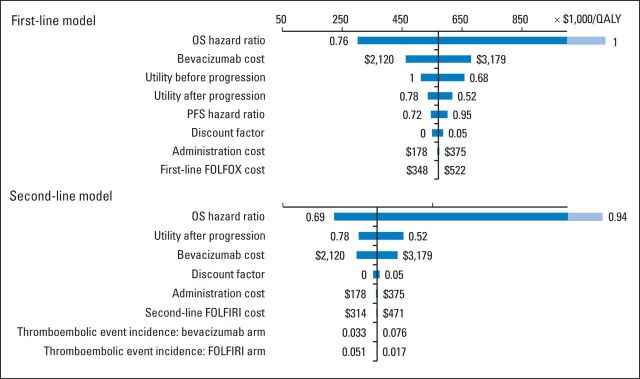
The results of univariable sensitivity analyses are shown in the tornado diagrams (Fig 3). The parameters with the greatest influence on the ICERs were similar in both models: median OS for each regimen, drug cost of bevacizumab, and utility values for living with mCRC. Across broad variation in the ranges for each parameter, the ICER remained > $200,000 per QALY. The median PFS and the discount factor had a minor influence on the ICER in the first-line model. The effects of other parameters were negligible.
Fig 3.
Univariable sensitivity analyses of variables with greatest influence on model. Bar shaded lighter blue indicates that actual value is beyond range of axis. FOLFIRI, fluorouracil plus irinotecan; FOLFOX, fluorouracil plus oxaliplatin; OS, overall survival; PFS, progression-free survival; QALY, quality-adjusted life-year.
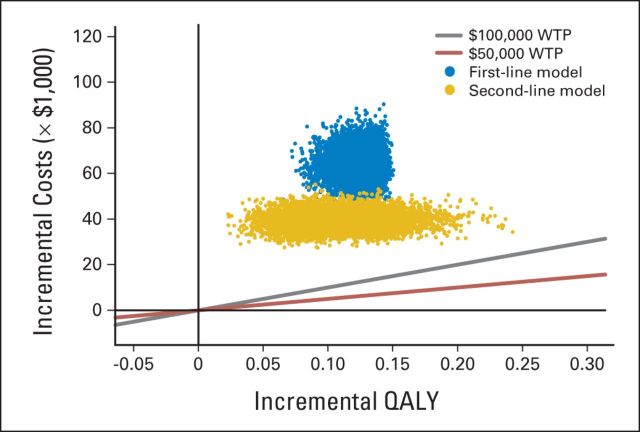
The ICERs for 10,000 samples in the PSA are shown in the scatterplot (Fig 4), with quadrants indicating the differences in costs and effectiveness and a line indicating the level of $100,000 per QALY. For each model, all the points were above the $100,000-per-QALY line. The cost-effectiveness acceptability curve is shown in Appendix Figure A2 (online only) for varying values of willingness to pay per QALY. In the structural sensitivity analysis using data from other studies to compare ICERs with the base case analyses, our alternative first-line model demonstrated an ICER of $240,810 per QALY. In the analysis comparing bevacizumab in the first- and second-line settings versus bevacizumab in neither line of therapy, the ICER for the addition of bevacizumab was $1.2 million per QALY.
Fig 4.
Scatter plot of probabilistic sensitivity analysis. Red and gray lines indicate threshold of willingness to pay (WTP) of $50,000 and $100,000 per quality-adjusted life-year (QALY), respectively. Each point in scatterplot corresponds to one sample of parameter values. Points above reference line indicate higher incremental cost-effectiveness ratio than threshold value.
DISCUSSION
We performed a cost-effectiveness analysis of bevacizumab in addition to chemotherapy in mCRC. In both the first- and second-line settings, bevacizumab provided modest incremental benefit at high incremental cost per QALY. In both the first- and second-line models, adding bevacizumab prolonged the median OS by 6 weeks and increased costs by $60,000 and $40,000, respectively. Bevacizumab is more cost effective in the second line than in the first line because of a decreased period of treatment. In the best-case scenario for the most sensitive variables in the univariable sensitivity analysis, the ICER remained > $200,000 per QALY. The PSA revealed that the probability of bevacizumab being cost effective was 0% in the first- and second-line settings for a willingness-to-pay threshold of $100,000 per QALY. This uncertainty analysis suggests a high likelihood that bevacizumab exceeds the usually accepted values for cost-effective incremental costs of care.
Other studies have analyzed the cost effectiveness of bevacizumab in mCRC in different settings.10–12 A British study found that adding bevacizumab to FOLFIRI in first-line management cost £62,857 (US$102,000) per QALY.12 A Canadian study found that the addition of bevacizumab had an ICER of Canadian $131,600 (US$120,000) per QALY,10 whereas in Japan, it was ¥13.5 million ($113,000) per QALY.11 A United States–based retrospective analysis found that adding bevacizumab to chemotherapy cost $75,303 per LY.28 Were quality of life to be incorporated, this ICER would be expected to increase. Because of the differences in the modeling approaches, costs, and health care systems among countries, conclusions drawn from one country cannot be applied to another.29 In addition, these analyses provided information in the first-line setting only. To our knowledge, our study is the first modeling study from a US payer perspective and the first worldwide to examine bevacizumab when continued beyond progression.
In advanced non–small-cell lung cancer, bevacizumab is approved in addition to chemotherapy based on the ECOG 4599 trial.30 A United States–based analysis demonstrated an ICER of $560,000 per QALY.24 Although informative, these results in lung cancer cannot be extrapolated to colon cancer.
When irinotecan was approved for mCRC, it was found to have an ICER of $49,000 per LY when added to FU.31 When FOLFOX demonstrated a survival benefit over irinotecan and bolus FU, the ICER was $112,000 per QALY.32 Anti–epidermal growth factor receptor therapies such as cetuximab can also be added to chemotherapy, with an ICER of $2.9 million per LY.33 When using KRAS testing as a biomarker to select patients most likely to benefit, the ICER was reduced to $2.8 million per LY.33 The magnitude of ICERs when new drugs gained approval more than a decade ago is significantly less than the ICERs for more modern monoclonal antibodies, such as bevacizumab and cetuximab. Although inflation in the costs of health care services accounts for some of this increase in cost, it should not substantially influence the incremental costs of these interventions over contemporary alternatives.
The threshold of $50,000 to $100,000 per QALY is frequently justified as a definition for a cost-effective health care intervention based on the cost effectiveness of dialysis.34 A more recent analysis revealed that the ICER for dialysis is $130,000 per QALY.35 Ultimately, the willingness of payers, patients, and other stakeholders to cover the costs of expensive cancer therapies will establish a new benchmark for cost-effective care.
As with any model, our analysis has limitations, which are governed by data availability and our assumptions.36 We used reimbursement rates provided by Medicare, the largest public payer in the United States, which are generally lower than reimbursement rates for private insurers.37 We are unaware of research comparing physician-administered drug reimbursement rates between Medicare and private payers. Our model was static and assumed no differences in drug acquisition costs over time. This may have resulted in overestimation of the cost of bevacizumab when it goes off patent. Our model provides a framework that can be used to assess the cost effectiveness of bevacizumab for any value of the drug price; the sensitivity analyses included wide variability in the cost of bevacizumab.
As with most modeling studies, these results are limited by the fact that the models were based on data from previously published trials and not collected prospectively. However, a benefit of this approach is that we were able to use national averages for costs, thus accounting for regional variations.
Patients recruited to the ML18147 trial received standard first-line FU-based chemotherapy with either oxaliplatin or irinotecan.9 On progression, patients were switched between oxaliplatin and irinotecan. Given the similar efficacy and AE profile of these regimens,8 we felt it was justified to simplify the model to FOLFOX followed by FOLFIRI. Our model was based on the use of FOLFOX4, as in the N01966 study; however, it has become more standard to use the modified FOLFOX6 regimen, which contains a higher dose of bolus FU.5 There is only a $9 difference in cost per cycle for these regimens, and with similar outcomes, these differences would be well accounted for in the sensitivity analyses. We included in the model only those AEs that commonly affect cost or health utility. For example, peripheral neuropathy, proteinuria, and intestinal perforation were excluded from the model. Moreover, although our model provides a good framework for the management of mCRC, recently approved agents have slightly changed the treatment paradigm. Notably, regorafenib is now commonly used as monotherapy in the third-line setting.38 Cetuximab and panitumumab are also approved in patients without KRAS-mutated disease and represent alternative options to bevacizumab.39,40
The ICER of bevacizumab potentially could be improved with the use of an effective biomarker to select patients most likely to benefit. This has been demonstrated previously with other therapies. For example, in mCRC, testing the tumor for KRAS gene mutation status indicates which patients are more likely to benefit from therapy such as cetuximab or panitumumab, and KRAS testing improves the cost effectiveness of these interventions.33 Several attempts have been made to find a biomarker that predicts response to bevacizumab; however, no predictive biomarker has been validated.41
Our study demonstrates the high incremental cost with low incremental benefit of bevacizumab in mCRC over wide variations in the assumptions incorporated into the models. In the era of personalized medicine, the needs of patients with mCRC or other malignancies warrant the development of new therapeutic technologies, but with the dramatic rises in drug prices over the last decades, the use of new treatments should be tailored to the patients who are most likely to benefit. In the current environment, costly drugs face few barriers to coverage and adoption by physicians. However, the process for new drug approval and the incorporation of drugs into treatment formularies and guidelines may eventually force physicians and policymakers to confront tradeoffs between cost and benefit more explicitly. Data from this study and others like it provide a starting point for such discussions.
Glossary Terms
- overall survival:
the duration between random assignment and death.
- quality-adjusted life-year (QALY):
a common measure of health improvement used in economic evaluation that measures life expectancy adjusted for quality of life.
- utility:
a measure of the preference for, or desirability of, a specific level of health status or specific health outcome.
Appendix
Table A1.
Patient Demographic and Clinical Characteristics by Treatment Regimen
| Characteristic | First-Line Treatment |
Second-Line Treatment |
|||||
|---|---|---|---|---|---|---|---|
| FOLFOX/XELOX (n = 701) | FOLFOX/XELOX Plus Bevacizumab (n = 699) | FOLFOX (n = 111) | FOLFOX Plus Bevacizumab (n = 713) | FOLFIRI (n = 411) | FOLFIRI (n = 291) | FOLFIRI Plus Bevacizumab (n = 409) | |
| Study | N01966 (2008) | N01966 (2008) | Tournigand et al8 (2004) | Schmoll et al23(2012) | ML18147 (2013) | E3200 (2007) | ML18147 (2013) |
| Age, years | |||||||
| Median | 60 | 60 | 65 | 60 | 63 | 61 | 63 |
| Range | 18-83 | 18-86 | 40-75 | 22-88 | 21-84 | 25-84 | 27-84 |
| Male sex, % | 56 | 60 | 72 | 58 | 63 | 61 | 65 |
| Performance status 0 to 1, % | 100 | 100 | 94 | 100 | 95 | 94 | 95 |
| OS, months | |||||||
| Median | 19.9 | 21.3 | 20.6 | 21.3 | 9.8 | 10.8 | 11.2 |
| Range | 17.7-24.6 | 8.9-10.7 | 10.4-12.2 | ||||
| PFS, months | |||||||
| Median | 8.0 | 9.4 | 8.0 | 10.3 | 4.1 | 4.7 | 5.7 |
| Range | 6.2-9.4 | 3.7-4.4 | 5.2-6.2 | ||||
Abbreviations: FOLFIRI, fluorouracil plus irinotecan; FOLFOX, fluorouracil plus oxaliplatin; OS, overall survival; PFS, progression-free survival; XELOX, capecitabine plus oxaliplatin.
Table A2.
Costs of Drug Administration and Routine Management
| Description | CPT Code | Cost ($) | Range ($) |
|---|---|---|---|
| Outpatient follow-up visit | 99213 | 49.67 | 43.28-66.32 |
| Inpatient first visit | 99222 | 134.73 | 116.73-179.17 |
| Inpatient follow-up visit | 99232 | 70.09 | 61.58-94.16 |
| Inpatient discharge visit | 99238 | 70.77 | 60.98-93.4 |
| Chemotherapy administration | |||
| IV, first hour | 96413 | 143.24 | 99.45-190.62 |
| IV, additional hours | 96415 | 30.62 | 22.7-39.5 |
NOTE. Assumptions were as follows: national payment amount (ie, GPCI), 1; conversion factor (for 2013), 34.023; data from year 2013.
Abbreviations: CPT, Common Procedure Terminology; GPCI, geographic practice cost index; IV, intravenous.
Table A3.
Drug Costs for 2-Week Cycle
| Drug and Dose | Cost ($) | Total Cost ($) |
|---|---|---|
| First-line FOLFOX | ||
| Oxaliplatin 85 mg/m2 | 0.535 per 0.5 mg | 169.16 |
| Leucovorin 200 mg/m2 | 4.501 per 50 mg | 36.01 |
| FU 400 mg/m2 bolus | 1.995 per 500 mg | 2.97 |
| FU 600 mg/m2 continuous infusion | 1.995 per 500 mg | 4.45 |
| Palonosetron 250 mcg IV | 19.384 per 25 mcg | 193.84 |
| Dexamethasone 12 mg orally | 0.64 per 4 mg | 1.92 |
| Prochlorperazine 30 × 10 mg tablets | 0.89 per 10 mg | 26.70 |
| First-line bevacizumab | ||
| Bevacizumab 5 mg/kg | 64.62 per 10 mg | 2649.42 |
| Second-line FOLFIRI | ||
| Irinotecan 180 mg/m2 | 4.46 per 20 mg | 74.66 |
| Leucovorin 400 mg/m2 | 4.501 per 50 mg | 66.97 |
| FU 400 mg/m2 bolus | 1.995 per 500 mg | 2.97 |
| FU 2,400 mg/m2 continuous infusion | 1.995 per 500 mg | 17.81 |
| Palonosetron 250 mcg IV | 19.384 per 25 mcg | 193.84 |
| Dexamethasone 12 mg orally | 0.64 per 4 mg | 1.92 |
| Prochlorperazine 30 × 10 mg tablets | 0.89 per 10 mg | 26.70 |
| Atropine 0.4 mg IV | 1.73 | 1.73 |
| Loperamide 2 to 4 mg once every 4 hours (30 tablets) | 0.20 × 30 | 6.00 |
| Total | 392.60 |
Abbreviations: FOLFIRI, fluorouracil plus irinotecan; FOLFOX, fluorouracil plus oxaliplatin; FU, fluorouracil; IV, intravenous.
Fig A1.
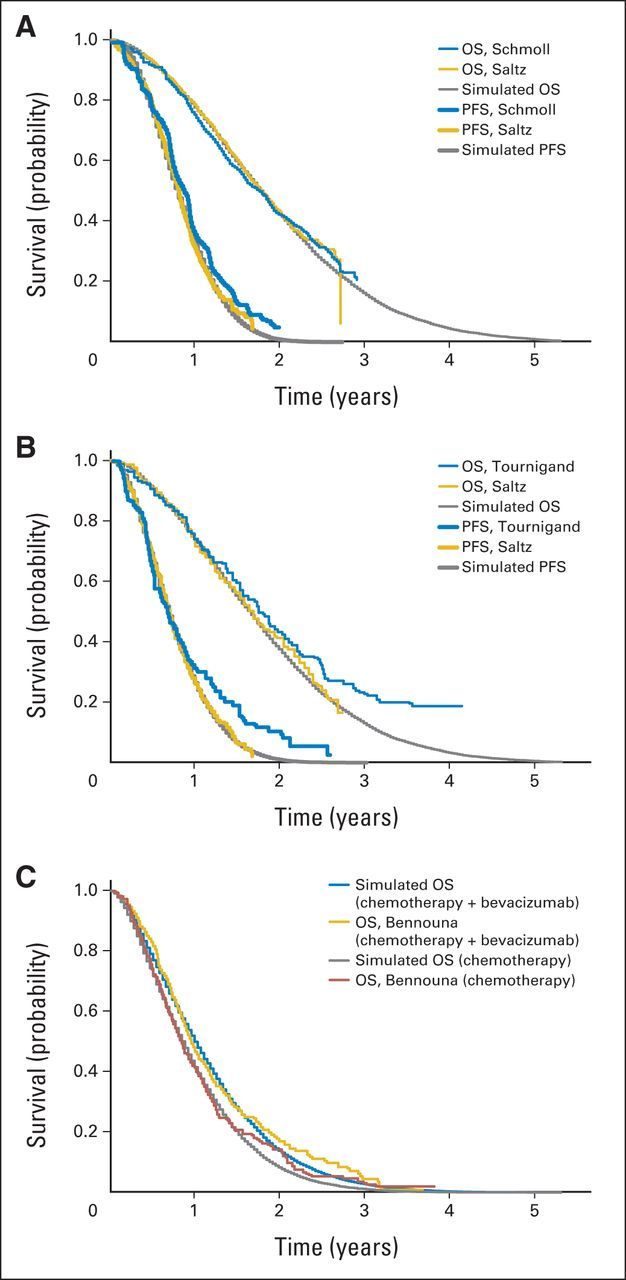
(A) Progression-free survival (PFS) inference and external validation for arm treated with fluorouracil plus oxaliplatin (FOLFOX) or capecitabine plus oxaliplatin (XELOX), with addition of bevacizumab (data adapted6,23), and (B) internal validation with simulation results for arm treated with FOLFOX or XELOX in first-line model (data adapted6,8). (C) Internal validation of overall survival (OS) in second-line model (data adapted9).
Fig A2.
Cost-effectiveness acceptability curve.
Footnotes
See accompanying editorial on page 1093
Presented orally at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel A. Goldstein, Qiushi Chen, Turgay Ayer, David H. Howard, Bassel F. El-Rayes, Christopher R. Flowers
Financial support: Turgay Ayer, Christopher R. Flowers
Administrative support: Christopher R. Flowers
Provision of study materials or patients: Christopher R. Flowers
Collection and assembly of data: Daniel A. Goldstein, Qiushi Chen, Christopher R. Flowers
Data analysis and interpretation: Daniel A. Goldstein, Qiushi Chen, Turgay Ayer, David H. Howard, Joseph Lipscomb, Christopher R. Flowers
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
First- and Second-Line Bevacizumab in Addition to Chemotherapy for Metastatic Colorectal Cancer: A United States–Based Cost-Effectiveness Analysis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Daniel A. Goldstein
No relationship to disclose
Qiushi Chen
No relationship to disclose
Turgay Ayer
No relationship to disclose
David H. Howard
Honoraria: Onyx Pharmaceuticals
Travel, Accommodations, Expenses: Onyx Pharmaceuticals
Joseph Lipscomb
No relationship to disclose
Bassel F. El-Rayes
Research Funding: Novartis (Inst), Roche (Inst), Synta (Inst), Bristol-Myers Squibb (Inst), Kirin (Inst), Pfizer (Inst), AVEO Pharmaceuticals (Inst)
Christopher R. Flowers
Consulting or Advisory Role: OptumRx, Algeta, Seattle Genetics, Celgene, Genentech/Roche, Gilead Sciences
Research Funding: Acerta (Inst), Infinity (Inst), Onyx Pharmaceuticals (Inst), Janssen Oncology (Inst), Gilead Sciences (Inst), Spectrum Pharmaceuticals (Inst), Celgene (Inst), TG Therapeutics (Inst), Pharmacyclics (Inst)
REFERENCES
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 4.Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 5.Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: Results of the TREE study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 6.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 8.Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 9.Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013;14:29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence D, Maschio M, Leahy KJ, et al. Economic analysis of bevacizumab, cetuximab, and panitumumab with fluoropyrimidine-based chemotherapy in the first-line treatment of KRAS wild-type metastatic colorectal cancer (mCRC) J Med Econ. 2013;16:1387–1398. doi: 10.3111/13696998.2013.852097. [DOI] [PubMed] [Google Scholar]
- 11.Shiroiwa T, Fukuda T, Tsutani K. Cost-effectiveness analysis of bevacizumab combined with chemotherapy for the treatment of metastatic colorectal cancer in Japan. Clin Ther. 2007;29:2256–2267. doi: 10.1016/j.clinthera.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Tappenden P, Jones R, Paisley S, et al. The cost-effectiveness of bevacizumab in the first-line treatment of metastatic colorectal cancer in England and Wales. Eur J Cancer. 2007;43:2487–2494. doi: 10.1016/j.ejca.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Auget J-L, Balakrishnan N, Mesbah M, et al. Advances in Statistical Methods for the Health Sciences: Applications to Cancer and AIDS Studies, Genome Sequence Analysis, and Survival Analysis. Boston, MA: Birkhäuser; 2007. [Google Scholar]
- 14.Arias E. National vital statistics reports: United States life tables, 2007. http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_09.pdf. [PubMed]
- 15.National Cancer Institute. SEER research data 2000-2010. http://seer.cancer.gov/data.
- 16.Ramsey SD, Andersen MR, Etzioni R, et al. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88:1294–1303. [PubMed] [Google Scholar]
- 17.Ramsey SD, Berry K, Moinpour C, et al. Quality of life in long term survivors of colorectal cancer. Am J Gastroenterol. 2002;97:1228–1234. doi: 10.1111/j.1572-0241.2002.05694.x. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Faststats. http://www.cdc.gov/nchs/fastats.
- 19.Centers for Disease Control and Prevention. 2013 ASP drug pricing files. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2013ASPFiles.html.
- 20.Centers for Disease Control and Prevention. 2013 Medicare physician fee schedule. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html?redirect=/physicianfeesched.
- 21.Tumeh JW, Moore SG, Shapiro R, et al. Practical approach for using Medicare data to estimate costs for cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res. 2005;5:153–162. doi: 10.1586/14737167.5.2.153. [DOI] [PubMed] [Google Scholar]
- 22.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189–2204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 23.Schmoll HJ, Cunningham D, Sobrero A, et al. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: A double-blind, randomized phase III study (HORIZON III) J Clin Oncol. 2012;30:3588–3595. doi: 10.1200/JCO.2012.42.5355. [DOI] [PubMed] [Google Scholar]
- 24.Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value Health. 2011;14:836–845. doi: 10.1016/j.jval.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Younossi ZM, Boparai N, McCormick M, et al. Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am J Gastroenterol. 2001;96:579–583. doi: 10.1111/j.1572-0241.2001.03537.x. [DOI] [PubMed] [Google Scholar]
- 26.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 27.Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: A multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23:4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 28.Shankaran V, Mummy D, Koepl L, et al. Survival and lifetime costs associated with first-line bevacizumab use in older patients with metastatic colorectal cancer. Oncologist. 2014;19:892–899. doi: 10.1634/theoncologist.2013-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drummond M, Barbieri M, Cook J, et al. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health. 2009;12:409–418. doi: 10.1111/j.1524-4733.2008.00489.x. [DOI] [PubMed] [Google Scholar]
- 30.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 31.Vaiani M, Trippoli S, Messori A. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2001;344:305–306. doi: 10.1056/NEJM200101253440413. author reply 306-307. [DOI] [PubMed] [Google Scholar]
- 32.Hillner BE, Schrag D, Sargent DJ, et al. Cost-effectiveness projections of oxaliplatin and infusional fluorouracil versus irinotecan and bolus fluorouracil in first-line therapy for metastatic colorectal carcinoma. Cancer. 2005;104:1871–1884. doi: 10.1002/cncr.21411. [DOI] [PubMed] [Google Scholar]
- 33.Behl AS, Goddard KA, Flottemesch TJ, et al. Cost-effectiveness analysis of screening for KRAS and BRAF mutations in metastatic colorectal cancer. J Natl Cancer Inst. 2012;104:1785–1795. doi: 10.1093/jnci/djs433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkelmayer WC, Weinstein MC, Mittleman MA, et al. Health economic evaluations: The special case of end-stage renal disease treatment. Med Decis Making. 2002;22:417–430. doi: 10.1177/027298902236927. [DOI] [PubMed] [Google Scholar]
- 35.Lee CP, Chertow GM, Zenios SA. An empiric estimate of the value of life: Updating the renal dialysis cost-effectiveness standard. Value Health. 2009;12:80–87. doi: 10.1111/j.1524-4733.2008.00401.x. [DOI] [PubMed] [Google Scholar]
- 36.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS): Explanation and elaboration—A report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. http://www.medpac.gov/documents/reports/mar14_entirereport.pdf?sfvrsn=0.
- 38.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 40.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 41.Lambrechts D, Lenz HJ, de Haas S, et al. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31:1219–1230. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]