Abstract
Purpose
Entinostat is an oral isoform selective histone deacetylase inhibitor that targets resistance to hormonal therapies in estrogen receptor–positive (ER+) breast cancer. This randomized, placebo-controlled, phase II study evaluated entinostat combined with the aromatase inhibitor exemestane versus exemestane alone.
Patients and Methods
Postmenopausal women with ER+ advanced breast cancer progressing on a nonsteroidal aromatase inhibitor were randomly assigned to exemestane 25 mg daily plus entinostat 5 mg once per week (EE) or exemestane plus placebo (EP). The primary end point was progression-free survival (PFS). Blood was collected in a subset of patients for evaluation of protein lysine acetylation as a biomarker of entinostat activity.
Results
One hundred thirty patients were randomly assigned (EE group, n = 64; EP group, n = 66). Based on intent-to-treat analysis, treatment with EE improved median PFS to 4.3 months versus 2.3 months with EP (hazard ratio [HR], 0.73; 95% CI, 0.50 to 1.07; one-sided P = .055; two-sided P = .11 [predefined significance level of .10, one-sided]). Median overall survival was an exploratory end point and improved to 28.1 months with EE versus 19.8 months with EP (HR, 0.59; 95% CI, 0.36 to 0.97; P = .036). Fatigue and neutropenia were the most frequent grade 3/4 toxicities. Treatment discontinuation because of adverse events was higher in the EE group versus the EP group (11% v 2%). Protein lysine hyperacetylation in the EE biomarker subset was associated with prolonged PFS.
Conclusion
Entinostat added to exemestane is generally well tolerated and demonstrated activity in patients with ER+ advanced breast cancer in this signal-finding phase II study. Acetylation changes may provide an opportunity to maximize clinical benefit with entinostat. Plans for a confirmatory study are underway.
INTRODUCTION
Hormonal therapies are the foundation of estrogen receptor (ER) –positive breast cancer (BC) treatment. Because of the clinical activity and favorable adverse effect profile of hormonal agents, the standard of care typically involves sequencing hormonal agents until either resistance develops and/or visceral crises necessitate switching to chemotherapy. In postmenopausal women, aromatase inhibitors (AI) are a preferred class of antiestrogen therapy that functions by blocking endogenous estrogen synthesis. Exemestane is a steroidal AI that irreversibly binds and inactivates the aromatase enzyme with demonstrated efficacy in the metastatic setting after progression on a nonsteroidal AI (NSAI; ie, letrozole or anastrozole).1
Developing resistance to hormone therapies in advanced BC is a significant challenge. Putative mechanisms of resistance include estrogen-independent growth, hypersensitivity to low estrogen concentrations, cyclin D1 overexpression, constitutive nuclear factor kappa B activation, upregulation of growth factor–signaling pathways, and downregulation of estrogen receptor alpha (ERα) expression. These pathways and mechanisms provide potential targets for therapeutic interventions. Entinostat is a novel, oral inhibitor of histone deacetylases (HDAC), with high specificity toward class 1 HDACs and a unique pharmacologic profile allowing for weekly dosing. HDAC inhibition leads to elevated protein lysine acetylation in tumor and peripheral-blood cells, which serves as a surrogate potential pharmacodynamic marker of activity. Entinostat's class 1 specificity distinguishes it from the US Food and Drug Administration–approved HDAC inhibitors (HDACi) vorinstat (Zolinza; Merck, Whitehouse Station, NJ) and romidepsin (Istodax; Celgene, Summit, NJ). Preclinically, entinostat inhibits ERα-positive tumor growth and restores hormone sensitivity as a result of the downregulation of estrogen-independent growth factor signaling pathways, normalization of ERα levels, and increases in aromatase enzyme levels.2,3 We hypothesized that combining entinostat with exemestane in ER-positive breast cancer could overcome hormone therapy resistance, thereby sensitizing cells to antiestrogen therapy with exemestane.
PATIENTS AND METHODS
Study Design
This was a phase II, randomized, double-blind, placebo-controlled study of exemestane ± entinostat in patients with locally advanced or metastatic BC whose disease had progressed while taking an NSAI. One hundred thirty patients were enrolled onto the study between June 2008 and July 2010 at 38 sites in North America, Central Europe, and Russia. All patients provided written informed consent. Patients were randomly assigned using a blocked randomization scheme 1:1 to exemestane plus entinostat (EE; n = 64) or exemestane plus placebo (EP; n = 66). Randomization was stratified by prior NSAI treatment setting (adjuvant/metastatic), metastases in bone only (yes/no), and geographic region (North America/Central Europe and Russia). The randomization schedule was prepared and maintained by an independent statistical service provider. The protocol allowed approximately 20% of patients with nonmeasurable disease to enroll. Treatment with exemestane 25 mg by mouth once daily plus entinostat 5 mg or placebo by mouth once weekly continued until progressive disease (PD) or unacceptable toxicity.
Eligibility
Postmenopausal women with ER-positive BC (as determined locally) who were experiencing disease relapse or progression while receiving an NSAI were eligible. Either patients had relapsed after adjuvant NSAI treatment administered for at least 12 months or their disease had progressed after NSAI treatment administered for at least 3 months in the metastatic/advanced setting. One prior line of chemotherapy in the metastatic setting was permitted if administered before the most recent NSAI. Within 4 weeks before starting study treatment, patients must have had at least one measurable lesion (≥ 20 mm by conventional techniques or ≥ 10 mm by spiral computed tomography scan); or in the case of bone-only metastases, patients needed a positive bone scan confirmed by magnetic resonance imaging or positron emission tomography–computed tomography. Additional requirements included an Eastern Cooperative Oncology Group performance status of 0 or 1; adequate hematologic parameters; and creatinine, AST, and ALT less than 2.5 times the upper limit of normal. Patients with prior exemestane, entinostat, or any other HDACi were excluded.
Procedures and Treatment
Treatment cycles were 28 days. Patients were evaluated on days 1, 8, and 15 during cycle 1 and on day 1 of all subsequent cycles. Peripheral blood samples were taken in a subset of patients before and after dosing on days 1, 8, or 15 of cycle 1. Patient/disease response assessments were performed on day 22 of cycle 2 and every other cycle thereafter. After completing study treatment, patients entered into post-treatment follow-up for evaluation of overall survival and subsequent therapies. Patients were to be observed until death, withdrawal of consent, or study closure by the sponsor.
Assessments
Safety assessment.
Safety was assessed via adverse events (AEs; using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0), ECGs, hematology and serum chemistries, Eastern Cooperative Oncology Group performance status, and vital signs.
Efficacy assessment.
Disease was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0. Contrast-enhanced CT scans were obtained at baseline, every other cycle for 12 months, and every third cycle thereafter. PD also was assessed by bone scan and clinical symptoms, as appropriate.
End points.
The primary end point was progression-free survival (PFS), defined as number of months from random assignment to documented PD or death as a result of any cause. Secondary end points included overall response (OR; complete response plus partial response) and clinical benefit rates (CBR; OR plus stable disease for ≥ 6 months). Overall Survival (OS) was an exploratory end point. Predefined subgroups included NSAI sensitive (patients who had a complete response, partial response, or stable disease for 6 months preceding NSAI therapy in the advanced setting or who relapsed at least 1 year after completing NSAI therapy in the adjuvant setting) and NSAI resistant (all other patients).
Exploratory Pharmacodynamics
Protein lysine acetylation was measured by multiparameter flow cytometry4 in peripheral blood mononuclear cells (PBMCs; CD19+ B cells, CD3+ T cells, and CD14+ monocytes) that were collected pre- and post-treatment on days 1, 8, and 15 of cycle 1 to explore the association with PFS.
Statistical Methods
Chia et al,1 reported a median PFS of 3.7 months with exemestane in the treatment of advanced BC demonstrating PD or recurrence following NSAI therapy. It was hypothesized that adding entinostat to exemestane would increase median PFS by 2.3 months (ie, from 3.7 to 6.0 months), corresponding to a target hazard ratio (HR) of 0.62. For primary analysis of PFS, a total of 77 progression events were required to detect such an improvement in the HR with ≥ 80% power, one-sided significance level of .10, and log-rank test. A total of 92 events were required for 85% power, and 112 events were required for 90% power. The initial type 1 and 2 error rates chosen and the size of targeted treatment effect are consistent with Rubenstein5 and Korn6 for phase II screening studies.
PFS was summarized descriptively using the Kaplan-Meier method7 and was reported based on 116 progression events as of March 2012 (Appendix [online-only]). The HR was estimated from a stratified Cox proportional hazards model, with placebo serving as the reference in the calculation. The primary inferential comparison between groups was made using the log-rank test, stratified by the three randomization factors. For patients who died before PD documentation, date of death was used as the PD date. Duration of PFS was right-censored at last disease assessment for patients who started nonprotocol-defined anticancer therapy, were lost to follow-up, or did not have documentation of PD. Multivariate Cox models were used to determine whether the reduced hazard rate for PFS and OS attributed to entinostat in the univariate model was still present after accounting for patient-, disease-, and prior treatment-related factors. The OR and CBR estimate determinations are described in the Appendix. Efficacy analyses were performed using the intention-to-treat population, defined as all randomly assigned patients. All reported P values are two-sided and levels ≤ .05 are regarded as statistically significant, except for the primary PFS end point, which was evaluated using a one-sided test and used a .10 threshold for significance. Safety analyses were performed using the safety population (all patients who received ≥ one dose of entinostat/placebo). Safety was assessed by an independent data safety monitoring board. All participating investigators and patients remain blinded to the assigned study treatment, as post-treatment follow-up for OS is continuing.
Association of PFS with degree of change in protein lysine acetylation from baseline in PBMCs was evaluated as an exploratory, posthoc analysis using the Cox proportional hazards model (Appendix).
RESULTS
Patient Characteristics
A total of 130 patients were randomly assigned, 64 to EE and 66 to EP (Fig 1). Treatment groups were generally well balanced (Table 1) with the exception of visceral disease (EE, 53% v EP, 67%), median duration since initial BC diagnosis (EE, 7.9 years v EP, 4.6 years), and median duration since diagnosis of advanced BC (EE, 19.5 v EP, 17.2 months).
Fig 1.
CONSORT diagram. (*)One patient received no study drug and was excluded from the safety set. ITT, intention-to-treat.
Table 1.
Demographics and Baseline Disease Characteristics in the Intention-to-Treat Population
| Parameter/Statistic | Treatment Group |
|||
|---|---|---|---|---|
| Exemestane Plus Entinostat (n = 64) |
Exemestane Plus Placebo (n = 66) |
|||
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 63.0 | 62.0 | ||
| Range | 37-85 | 37-88 | ||
| < 65 | 35 | 55 | 40 | 61 |
| ≥ 65 | 29 | 45 | 26 | 39 |
| ECOG performance status | ||||
| 0 | 40 | 63 | 50 | 76 |
| 1 | 24 | 38 | 16 | 24 |
| Region | ||||
| North America | 42 | 66 | 43 | 65 |
| Central Europe/Russia | 22 | 34 | 23 | 35 |
| Advanced/metastatic disease at study entry | 64 | 100 | 65 | 98 |
| Measurable disease | 52 | 81 | 54 | 82 |
| Metastatic disease* | ||||
| Bone | 49 | 77 | 47 | 71 |
| Bone only | 13 | 20 | 11 | 17 |
| Visceral disease, lung and/or liver | 34 | 53 | 44 | 67 |
| Other† | 42 | 66 | 42 | 64 |
| Hormone receptor status | ||||
| Estrogen receptor–positive | 63 | 98 | 65 | 98 |
| Progesterone receptor–positive | 52 | 81 | 50 | 76 |
| HER2-positive | 5 | 8 | 7 | 11 |
| Median time since breast cancer diagnosis | ||||
| Since initial diagnosis, years | 7.9 | 4.6 | ||
| Since advanced disease diagnosis, months | 19.5 | 17.2 | ||
| Prior breast cancer treatment | ||||
| Chemotherapy | 37 | 58 | 44 | 67 |
| Adjuvant/neoadjuvant setting | 22 | 34 | 28 | 42 |
| Advanced disease setting | 22 | 34 | 21 | 32 |
| Both | 6 | 9 | 5 | 8 |
| Hormone therapy | 64 | 100 | 66 | 100 |
| Adjuvant/neoadjuvant setting | 33 | 52 | 32 | 48 |
| Advanced disease setting | 54 | 84 | 57 | 86 |
| Both | 23 | 36 | 23 | 35 |
| No. of hormone therapies in advanced setting | ||||
| 1 | 42 | 66 | 44 | 67 |
| ≥ 2 | 12 | 19 | 13 | 20 |
| NSAI progression treatment setting | ||||
| Relapsed after > 12 months of adjuvant therapy | 10 | 16 | 9 | 14 |
| PD after > 3 months of therapy for advanced disease | 54 | 84 | 57 | 86 |
| Sensitive to most recent NSAI therapy‡; | 45 | 70 | 40 | 61 |
| Resistant to most recent NSAI therapy‡ | 19 | 30 | 26 | 39 |
Abbreviations: CR complete response ECOG Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; NSAI, nonsteroidal aromatase inhibitor; PD, progressive disease; PR, partial response
Patients may be counted in more than one row.
Includes metastases in the CNS, pleura, lymph nodes, spleen, colon, rectum, breast, abdomen, bladder, adrenal/kidney, skin, ovary, or uterus.
NSAI-sensitive disease was defined by patients who had a CR, PR, or stable disease for 6 months in the metastatic setting or patients who relapsed at least 1 year after completion of an NSAI in the adjuvant setting. All other patients were defined as NSAI-resistant.
Of the 130 patients randomly assigned, 85 (EE, n = 45; EP, n = 40) met the study-specified definitions of NSAI sensitivity (see End Points section). Of the NSAI-sensitive group, one patient's disease had progressed after adjuvant NSAI and 84 patients' disease had progressed after metastatic NSAI. Forty-five patients (EE, n = 19; EP, n = 26) were NSAI-resistant; 18 patients had PD after adjuvant NSAI and 27 had PD after metastatic NSAI.
Efficacy
In the intention-to-treat population, median PFS was 4.3 months for the EE group versus 2.3 months for the EP group (Fig 2A; Table 2), with an HR of 0.73 (95% CI, 0.50 to 1.07; one-sided P = .055; two-sided P = .11; significant according to prespecified design criteria). PFS benefit in favor of EE was consistent across all subgroups of prognostic importance (Fig 3), including patients with acquired resistance (NSAI-sensitive, n = 85; HR, 0.85) and primary resistance (NSAI-resistant, n = 45; HR, 0.47). The OR and CBR were similar for the EE and EP groups (OR, 6.3% and 4.6%, respectively; CBR, 28.1% and 25.8%, respectively; Table 2). Median OS was 28.1 months for the EE group and 19.8 months for the EP group (HR, 0.59; 95% CI, 0.36 to 0.97; P = .036; Fig 2B; Table 2) with the incidence of death at 42% for the EE group and 65% for the EP group. Multivariate analyses indicated the favorable PFS and OS outcomes for EE versus EP were preserved when adjusted for baseline factors, including visceral disease and duration of diagnosis of advanced BC.
Fig 2.
Kaplan-Meier estimates of (A) progression-free survival (PFS) and (B) overall survival (OS). (A) Vertical tick marks represent the PFS time of patients without progressive disease. (B) Vertical tick marks represent the survival time of patients alive or lost to follow-up as of the last contact.
Table 2.
Summary of Clinical Outcomes in the Intention-to-Treat Population
| Endpoint/Statistic | EE (n = 64) | EP (n = 66) | P |
|---|---|---|---|
| PFS, months* | |||
| Median | 4.3 | 2.3 | |
| 95% CI | 3.3 to 5.4 | 1.8 to 3.7 | |
| Hazard ratio†‡ | 0.73 | ||
| 95% CI | 0.50 to 1.07 | ||
| Log-rank testठ| |||
| One-sided | .055 | ||
| Two-sided | .11 | ||
| OS, months* | |||
| Median | 28.1 | 19.8 | |
| 95% CI | 21.2 to NR | 17.0 to 26.7 | |
| Hazard ratio | 0.59 | ||
| 95% CI | 0.36 to 0.97 | ||
| Log-rank testठ| .036 | ||
| Objective response rate, % | 6.3 | 4.6 | .58ठ|
| Clinical benefit rate, % | 28.1 | 25.8 | .78ठ|
Abbreviations: EE, exemestane plus entinostat; EP, exemestane plus placebo; NR, not reached; OS, overall survival; PFS, progression-free survival
Measured from the date of random assignment. Median was estimated using the Kaplan-Meier method. Median duration of follow-up period for OS was 24.0 months for the EE group and 26.4 months for the EP group.
Hazard ratio estimated from a Cox proportional hazards model. Placebo group served as the reference group for interpretation of the hazard ratio.
Stratified by the randomization stratification factors.
All P values are two-sided except for the primary PFS end point, which was evaluated using a one-sided test and a .10 threshold for significance.
Fig 3.
Forest plot of progression-free survival for key subgroups. EE, exemestane plus entinostat; EP, exemestane plus placebo; NSAI, nonsteroidal aromatase inhibitor.
Safety
A total of 129 patients (EE, n = 63; EP, n = 66) were in the safety population. One EE patient withdrew from study before receiving treatment. Compared with the EP group, the EE group had a higher rate of AEs (95% v 85%), grade 3 AEs (44% v 23%), grade 4 AEs (6% v 3%), AEs leading to dose modification (35% v 6%), and AEs leading to study discontinuation (11% v 2%), irrespective of study drug relationship. AEs leading to the majority of EE dose modifications included neutropenia (14%), thrombocytopenia (14%), and fatigue (6%). AEs leading to EE study discontinuation included two patients owing to nausea and vomiting and one patient each owing to neutropenia, worsening weakening in extremities, hypoxia and radiation pneumonitis, fatigue, and mucositis. One EP patient discontinued study treatment owing to fatigue, anemia, thrombocytopenia, and leukopenia. The entinostat AE profile was consistent with previous clinical experiences.8,9 The most frequent AEs occurring in the EE group (> 15% of patients; Table 3 ) were fatigue, nausea, neutropenia, peripheral edema, vomiting, anemia, dyspnea, thrombocytopenia, decreased weight, diarrhea, and pain. Neutropenia was most commonly attributed to entinostat (13 of 19 patients; 68%). The incidence of serious AEs was similar (EE, 16%;EP, 12%). Four EE patients (6%) each experienced a grade 4 AE, including fatigue, leukopenia, neutropenia, and hypercalcemia. One fatal AE occurred in each treatment arm; the EE arm event was considered related to PD.
Table 3.
Most Common Adverse Events in the Safety Population
| MedDRA Preferred Term | Adverse Events by Grade and Treatment Group |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exemestane Plus Entinostat (n = 63) |
Exemestane Plus Placebo (n = 66) |
|||||||||||
| Any |
Grade 3 |
Grade 4 |
Any |
Grade 3 |
Grade 4 |
|||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Fatigue | 30 | 48 | 7 | 11 | 1 | 2 | 17 | 26 | 2 | 3 | 0 | |
| Nausea | 25 | 40 | 3 | 5 | 0 | 10 | 15 | 1 | 2 | 0 | ||
| Neutropenia | 19 | 30 | 8 | 13 | 1 | 2 | 0 | 0 | 0 | |||
| Peripheral edema | 13 | 21 | 0 | 0 | 3 | 5 | 0 | 0 | ||||
| Vomiting | 13 | 21 | 3 | 5 | 0 | 3 | 5 | 0 | 0 | |||
| Anemia | 12 | 19 | 1 | 2 | 0 | 8 | 12 | 1 | 2 | 1 | 2 | |
| Dyspnea | 12 | 19 | 2 | 3 | 0 | 7 | 11 | 0 | 0 | |||
| Thrombocytopenia | 12 | 19 | 1 | 2 | 0 | 4 | 6 | 0 | 1 | 2 | ||
| Weight decreased | 12 | 19 | 0 | 0 | 12 | 18 | 0 | 0 | ||||
| Diarrhea | 11 | 17 | 0 | 0 | 8 | 12 | 1 | 2 | 0 | |||
| Back pain | 10 | 16 | 0 | 0 | 11 | 17 | 1 | 2 | 0 | |||
| Pain | 10 | 16 | 1 | 2 | 0 | 4 | 6 | 1 | 2 | 0 | ||
| Pain in extremity | 10 | 16 | 0 | 0 | 4 | 6 | 0 | 0 | ||||
| Arthralgia | 7 | 11 | 1 | 2 | 0 | 11 | 17 | 0 | 0 | |||
| Constipation | 6 | 10 | 0 | 0 | 10 | 15 | 1 | 2 | 0 | |||
Abbreviation: MedDRA, Medical Dictionary for Regulatory Activities.
Biomarker Analysis
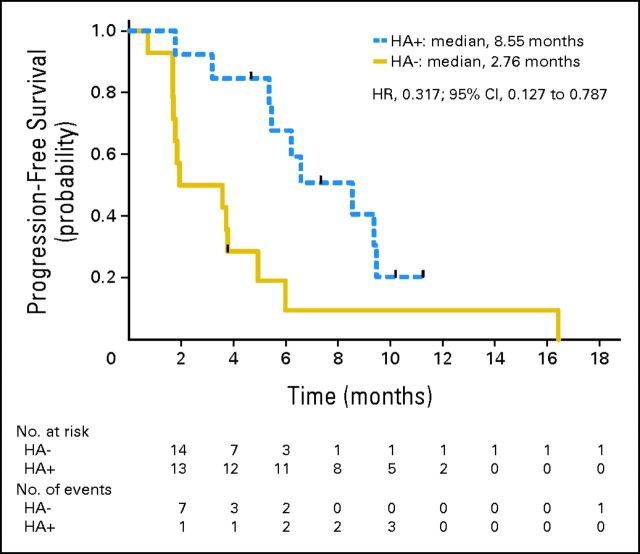
Cycle 1 pre- and post-treatment samples were obtained in a subset of 49 patients (EE, n = 27; EP, n = 22). Baseline characteristics were consistent with the overall study population. Hyperacetylation in EE patients was associated with a prolonged median PFS consistent across all cell types tested: 8.5 months for high acetylators versus 2.7 months for low acetylators (B cells; HR, 0.32; 95% CI, 0.13 to 0.79; Fig 4), 6.6 months for high acetylators versus 3.6 months for low acetylators (T cells; HR, 0.44; 95% CI, 0.18 to 1.08), and 6.2 months for high acetylators versus 3.6 months for low acetylators (monocytes; HR, 0.50; 95% CI, 0.21 to 1.20). Plasma entinostat concentrations at time points used for acetylation evaluation were generally at or below the assay detection limit (< 0.5 ng/mL), preventing correlation between entinostat concentration and acetylation status.
Fig 4.
Kaplan-Meier estimates of progression-free survival (PFS) in exemestane plus entinostat (EE) –treated acetylation subset. HA+, percent change in protein lysine acetylation at cycle 1 day 15 greater than the study derived median (hyperacetylation +). HA−, patients with percent change in protein lysine acetylation at cycle 1 day 15 less than the study derived median (hyperacetylation −). Vertical tick marks represent the PFS time of patients without progressive disease.
DISCUSSION
In vitro studies have demonstrated that HDAC inhibitors enhance the activity of and restore sensitivity to hormonal therapies in ER-positive cell lines.10,11 In our randomized phase II study, which was designed as a screening study to identify signals of clinical activity for combining entinostat with exemestane, adding entinostat to exemestane improved clinical outcomes in postmenopausal patients with ER-positive advanced BC who demonstrated PD on previous NSAI. The risk of disease progression was reduced by 27%, translating into a 2-month prolongation of median PFS. Importantly, the PFS benefit in the EE arm was consistent across all subsets of prognostic importance, including the subset with primary NSAI-resistant disease in which a differential treatment effect (HR, 0.47) was observed relative to the NSAI-sensitive subgroup (HR, 0.85). Both human epidermal growth factor 2 (HER2) and lack of ER expression have been implicated as causes of primary resistance to hormonal therapies. Through downregulation of HER2 and induction of ER expression, entinostat has effectively targeted both of these resistance mechanisms in preclinical studies. Restoration of ER expression and estrogen dependency confers sensitivity to not only ER-targeted therapies but also to AIs. Clinically, agents targeting HER2 signaling have resulted in promising outcomes when combined with AIs, particularly in tumors expressing both HER2 and ER.12 More recently, targeting activated growth factor signaling by inhibiting mammalian target of rapamycin has also effectively extended the benefit of exemestane in ER-positive postmenopausal BC progressing on a prior NSAI.13
The outcome of 2.3 months in the exemestane-only arm was in the low range of expected values and lower than the 3.7 months observed for exemestane in the Evaluation of Faslodex Versus Exemestane Clinical Trial (EFECT) study and 3.2 months in the BOLERO-2 (Breast Cancer Trials of Oral Everolimus-2) study. Unlike the EFECT trial however, prior fulvestrant was permitted in our trial. In addition, though we have not identified a specific patient characteristic that drives the differences, our specific criteria of having patients enter our study directly when their disease demonstrated progression on prior nonsteroidal AI may have skewed the patient population to patients with generally more AI-resistant tumors. The randomized, placebo controlled phase II design was essential in our ability to observe a treatment effect of entinostat combined with exemestane and reflects the value of this type of screening design.
Additional secondary end points including response rate and clinical benefit rate did not demonstrate a significant difference between EE versus EP. Interestingly, overall survival, an exploratory end point, was prolonged in the EE arm versus the EP arm (28.1 v 19.8 months; HR, 0.59; 95% CI, 0.36 to 0.97; P = .036). Although an effect of minor imbalances in patient and/or disease characteristics cannot be excluded, multivariate analyses indicated the favorable PFS and OS outcomes for patients randomly assigned to receive EE versus EP were preserved when adjusted for baseline factors, including visceral disease and duration of diagnosis of advanced BC. In addition, a detailed examination of the poststudy treatment therapies prescribed in the follow-up period indicates no apparent differences between treatment arms. It is plausible that the prolonged OS benefit relative to PFS may be attributable to longer-term effects of entinostat on tumor phenotype, cancer stem cell, or progenitor cell pool, and sensitization to subsequent poststudy treatments.
HDAC inhibitors have been studied in a number of tumor indications and combinations, and investigators commonly measured histone acetylation as a surrogate marker of HDACi activity. To date, this measurement has not been successfully linked to clinical outcome. In our study, analysis of protein lysine acetylation in pre- and post-treatment samples collected in a subset of 49 patients demonstrated that hyperacetylation of protein lysines in PBMCs was associated with improved clinical outcome, as shown by the prolonged PFS in hyperacetylators versus low acetylators. Elevated levels of protein lysine acetylation maintained in certain patients despite entinostat levels at or below the level of detection at the time of sampling seem to reflect the durability and potency of the pharmacodynamic effects that low sustained concentrations of entinostat can elicit.
The AE profile of entinostat in our study was consistent with previous clinical experience and noted HDACi class effects.14–21 These consisted of fatigue, gastrointestinal disturbances, and hematologic toxicities and were easily addressed by entinostat dose reductions or interruptions, with only one patient using growth factor support for neutropenia. Though cardiovascular and electrocardiographic effects including QT (interval corrected for heart rate) prolongation have been previously reported with HDACi,22–26 in our study, 31% of the population had previously received an anthracycline and the incidence of reported cardiac disorders was similar on the EE and EP arms (17% v 15%, respectively). Among EE-treated patients, the most common cardiac disorders were tachycardia and myocardial ischemia (each 5%) and sinus tachycardia (3%); all events were grade 1 or 2 and most were unrelated to entinostat.
Limitations of this study include the lack of tissue collection before study entry. Clearly centralized ER, progesterone receptor, HER2, and tumor proliferative assessments would have potentially aided in linking the observed clinical benefit to molecular subsets of BC, allowing potential further refinement of treatment strategies and patient selection opportunities. Confirmatory studies will include tissue analyses. An additional limitation was the inability to correlate changes in PBMC protein lysine acetylation with entinostat plasma levels or intratumoral effects. Ongoing clinical pharmacology studies will provide insight into the relationship between entinostat concentrations and pharmacodynamic effects. Finally, though the data warrant confirmatory phase III studies, the limitations inherent in the screening nature and size of the randomized, phase II design employed in our study do not allow for conclusive evidence of clinical benefit. In conclusion, adding entinostat to exemestane prolonged PFS and OS in postmenopausal women with ER-positive advanced BC that had progressed after treatment with an NSAI. These results demonstrate for the first time that the addition of an epigenetic therapy (ie, entinostat) to antiestrogen therapy may be an effective approach to targeting resistance pathways in BC, particularly in hormone-positive disease. Although entinostat added toxicity to the hormone therapy, it was felt to have an acceptable safety profile for this patient population. More importantly and for the first time, an association of HDAC inhibition with entinostat-induced protein lysine acetylation and improved clinical outcomes was demonstrated in a subset of patients. These data support the continuing development of entinostat in BC as well as in other solid tumors, with plans for confirmatory studies underway.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Denise A. Yardley, Syndax Pharmaceuticals (U); Pamela M. Klein, Syndax Pharmaceuticals (C); Scott Cruickshank, Syndax Pharmaceuticals (C); Kathy D. Miller, AstraZeneca (C), Bristol-Myers Squibb (C), Clovis Oncology (C), Nektar (C) Stock Ownership: Scott Cruickshank, Syndax Pharmaceuticals Honoraria: None Research Funding: Jane B. Trepel, Syndax Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Pamela N. Munster, Pamela M. Klein, Scott Cruickshank
Provision of study materials or patients: Denise A. Yardley, Roohi R. Ismail-Khan, Bohuslav Melichar, Mikhail Lichinitser, Kathy D. Miller
Collection and assembly of data: Denise A. Yardley, Roohi R. Ismail-Khan, Bohuslav Melichar, Mikhail Lichinitser, Scott Cruickshank, Kathy D. Miller, Min J. Lee, Jane B. Trepel
Data analysis and interpretation: Denise A. Yardley, Pamela M. Klein, Scott Cruickshank, Min J. Lee, Jane B. Trepel
Manuscript writing: All authors
Final approval of manuscript: All authors
Supplementary Material
Appendix
At the time of the primary progression-free survival analysis (January 2011), a total of 104 progression events had occurred among the 130 patients randomly assigned. Seventeen patients were continuing on blinded-study treatment without evidence of progressive disease at that time. A follow-up analysis including a total of 116 progression events was performed in March 2012, including all progression events, safety data, and deaths reported after database lock for the primary progression-free survival analysis. No inconsistencies were noted between the results of the primary and follow-up analyses.(27) To avoid duplication of results and streamline reporting study results, only the results of the follow-up analysis are reported in this article.
The overall response rate was estimated based on the proportion of patients whose best response during treatment was a complete response or a partial response, as defined by RECIST version 1.0. The clinical benefit rate was estimated based on the proportion of patients whose best response was a complete response, a partial response, or stable disease lasting at least 6 months. For both response end points, comparisons between treatment arms were made using the Cochran-Mantel-Haenszel test stratified for the randomization stratification factors.
The percent change in acetylation from baseline was determined based on the last sample obtained. The degree of change in acetylation was then dichotomized into high (ie, above the median or hyperacetylators) and low (ie, below the median) subgroups using a nonmodel-based approach; patients with a change from baseline that was greater than or equal to the 50th percentile (median) of the overall distribution were assigned to the high group, and patients with a change less than the 50th percentile were assigned to the low group. The cut point for the analysis (50th percentile) was determined a priori but was not based on findings from earlier studies. Analyses in all three cell types (B cells, T cells, monocytes) was performed for consistency of results and to aid in selection of optimal cell type for analysis in future studies.
Footnotes
Supported by Syndax Pharmaceuticals.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00676663.
References
- 1.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor–positive, advanced breast cancer: Results from EFECT. J Clin Oncol. 2008;26:1664–1670. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 2.Sabnis GJ, Goloubeva O, Chumsri S, et al. Functional activation of the estrogen receptor-α and aromatase by the HDAC inhibitor entinostat sensitizes ER-negative tumors to letrozole. Cancer Res 71:1893- 1903;2011 doi: 10.1158/0008-5472.CAN-10-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabnis GJ, Kazi A, Goloubeva O, et al. HDAC inhibitor entinostat restores responsiveness of letrozole resistant MCF-7Ca xenografts to AIs through modulation of Her-2.; Presented at the 33rd Annual San Antonio Breast Cancer Symposium; San Antonio, TX. 2010. Dec 8-12, abstr PD05-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung EJ, Lee S, Sausville EA, et al. Histone deacetylase inhibitor pharmacodynamic analysis by multiparameter flow cytometry. Ann Clin Lab Sci. 2005;35:397–406. [PubMed] [Google Scholar]
- 5.Rubinstein LV, Korn EL, Freidlin B, et al. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23:7199–7206. doi: 10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
- 6.Korn EL, Arbuck SG, Pluda JM, et al. Clinical trial designs for cytostatic agents: Are new approaches needed? J Clin Oncol. 2001;19:265–272. doi: 10.1200/JCO.2001.19.1.265. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–481. [Google Scholar]
- 8.Knipstein J, Gore L. Entinostat for treatment of solid tumors and hematologic malignancies. Expert Opin Investig Drugs. 2011;20:1455–1467. doi: 10.1517/13543784.2011.613822. [DOI] [PubMed] [Google Scholar]
- 9.Wardley A, Stein R, McCaffrey J, et al. Results of a phase 2 study of entinostat, an oral, class 1 selective histone deacetylase inhibitor, added to maintained aromatase inhibitor therapy in patients whose breast cancer is progressing on hormone therapy. J Clin Oncol. 2010;28:126s. (suppl; abstr 1052) [Google Scholar]
- 10.Hodges-Gallagher L, Valentine CD, Bader SE, et al. Inhibition of histone deacetylase enhances the anti-proliferative action of antiestrogens on breast cancer cells and blocks tamoxifen-induced proliferation of uterine cells. Breast Cancer Res Treat. 2007;105:297–309. doi: 10.1007/s10549-006-9459-6. [DOI] [PubMed] [Google Scholar]
- 11.Thomas S, Thurn KT, Biçaku E, et al. Addition of a histone deacetylase inhibitor redirects tamoxifen-treated breast cancer cells into apoptosis, which is opposed by the induction of autophagy. Breast Cancer Res Treat. 2011;130:437–447. doi: 10.1007/s10549-011-1364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor–positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 13.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kummar S, Gutierrez M, Gardner ER, et al. Phase I trial of MS-275, a histone deacetylase inhibitor, administered weekly in refractory solid tumors and lymphoid malignancies. Clin Cancer Res. 2007;13:5411–5417. doi: 10.1158/1078-0432.CCR-07-0791. [DOI] [PubMed] [Google Scholar]
- 15.Ryan QC, Headlee D, Acharya M, et al. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J Clin Oncol. 2005;23:3912–3922. doi: 10.1200/JCO.2005.02.188. [DOI] [PubMed] [Google Scholar]
- 16.Zain J, O'Connor OA. Targeting histone deacetylases in the treatment of B- and T-cell malignancies. Invest New Drugs. 2010;28:s58–s78. doi: 10.1007/s10637-010-9591-3. (abstr S58) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan J, Cang S, Ma Y, et al. Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. J Hematol Oncol. 2010;3:5. doi: 10.1186/1756-8722-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acharya MR, Sparreboom A, Venitz J, et al. Rational development of histone deacetylase inhibitors as anticancer agents: A review. Mol Pharmacol. 2005;68:917–932. doi: 10.1124/mol.105.014167. [DOI] [PubMed] [Google Scholar]
- 19.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 20.Piekarz RL, Frye R, Prince HM, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117:5827–5834. doi: 10.1182/blood-2010-10-312603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4485–4491. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 22.Piekarz RL, Frye AR, Wright JJ, et al. Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res. 2006;12:3762–3773. doi: 10.1158/1078-0432.CCR-05-2095. [DOI] [PubMed] [Google Scholar]
- 23.Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molife R, Fong P, Scurr M, et al. HDAC inhibitors and cardiac safety. Clin Cancer Res. 2007;13:1068. doi: 10.1158/1078-0432.CCR-06-1715. [DOI] [PubMed] [Google Scholar]
- 25.Sandor V, Bakke S, Robey RW, et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res. 2002;8:718–728. [PubMed] [Google Scholar]
- 26.Giles F, Fischer T, Cortes J, et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12:4628–4635. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- 27.Yardley DA, Ismail-Khan RR, Klein PM. Entinostat, a novel histone deacetylase inhibitor, added to exemestane improves PFS in advanced breast cancer in a randomized, phase II, double-blind study. Cancer Res. 2011;71:118s. (suppl; abstract PD01-04) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.