Abstract
The identification of species at risk of extinction is a central goal of conservation. As the use of data compiled for IUCN Red List assessments expands, a number of misconceptions regarding the purpose, application and use of the IUCN Red List categories and criteria have arisen. We outline five such classes of misconception; the most consequential drive proposals for adapted versions of the criteria, rendering assessments among species incomparable. A key challenge for the future will be to recognize the point where understanding has developed so markedly that it is time for the next generation of the Red List criteria. We do not believe we are there yet but, recognizing the need for scrutiny and continued development of Red Listing, conclude by suggesting areas where additional research could be valuable in improving the understanding of extinction risk among species.
Keywords: climate change, geographical range, population decline, rarity, spatial autocorrelation, uncertainty
1. Introduction
Quantitative criteria for the IUCN Red List of Threatened Species (hereafter Red List) were developed recognizing the need for rigour and objectivity in the assessment of extinction risk of species [1]. With the Red List, IUCN fulfils its goal to ‘provide information and analyses on the status, trends and threats to species in order to inform and catalyse action for biodiversity conservation’. Over 79 000 species have been assessed (figure 1), with growing coverage of less well-known groups of invertebrates, plants and fungi, to complement comparatively better-known groups of vertebrates. This resource for biodiversity conservation is being widely used to inform global and regional biodiversity targets, aid conservation planning, evaluate conservation actions and inform legislative frameworks to protect species [2].
Figure 1.
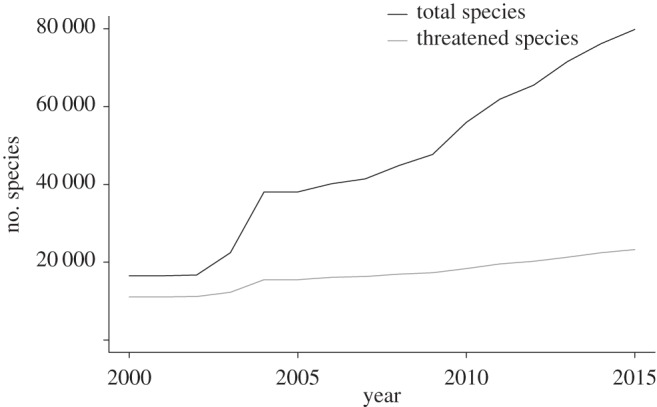
Temporal trend in assessments on IUCN Red List.
We outline five classes of misconceptions that have arisen regarding the purpose, application, and use of the Red List categories and criteria. The most consequential misconceptions drive proposals for revised versions of the criteria, which would render assessments among different species incomparable.
(a). Goals of criteria
The Red List criteria were established to measure the relative risk of extinction among a broad array of eukaryotic taxa. Species are allocated to broad categories of extinction risk by applying simple quantitative rules (table 1), relating to population size, range area and rate of decline of both. Misconceptions surrounding the goals of the criteria include the notion that the Red List represents a prioritization mechanism for species conservation; it explicitly does not. Conservation prioritization strategies seek to balance a variety of competing factors. Extinction risk may contribute to such decisions, alongside cost, chance of success and other metrics (e.g. abundance, rarity, endemism). The Red List categories were designed to reflect likelihood of extinction under prevailing circumstances [1].
Table 1.
The IUCN Red List categories and criteria for Critically endangered, Endangered, Vulnerable. n.a., not applicable.
| critically endangered | endangered | vulnerable | |
|---|---|---|---|
| (A) population reduction | |||
| declines measured over the longer of 10 years or 3 generations | |||
| A1 (%) | ≥90 | ≥70 | ≥50 |
| A2, A3 and A4 (%) | ≥80 | ≥50 | ≥30 |
| (B) geographical range either EOO or AOO | |||
| (B1) extent of occurrence (EOO; km2) | <100 | <5000 | <20 000 |
| (B2) area of occupancy (A00; km2) | <10 | <500 | <2000 |
| and 2 of the following: | |||
| (a) severely fragmented or no. of locations | = 1 | ≤5 | ≤10 |
| (b) continuing decline in (i) EOO; (ii) AOO; (iii) area, extent and/or quality of habitat; (iv) no. of locations or subpopulations; and (v) no. of mature individuals | |||
| (c) extreme fluctuations in (i) EOO; (ii) AOO; (iii) no. of locations or subpopulations; and (iv) no. of mature individuals | |||
| (C) small population size and decline | |||
| no. of mature individuals | <250 | <2500 | <10 000 |
| and either C1 or C2: | |||
| (C1) estimated continuing decline: up to a maximum of 100 years | 25% in 3 years or 1 generation | 20% in 5 years or 2 generations | 10% in 10 years or 3 generations |
| (C2) continuing decline and (a) and/or (b): | |||
| (a) (i) no. of mature individuals in all subpopulations: | ≤50 | ≤250 | ≤1000 |
| (ii) % individuals in one subpopulation | >90–100% | 95–100% | 100% |
| (b) extreme fluctuations in the number of mature individuals | |||
| (D) very small or restricted population | |||
| (1) no. mature individuals | <50 | <250 | <1000 |
| or | |||
| (2) restricted AOO | n.a. | n.a. | AOO <20 km2 or no. of locations ≤ 5 |
| (E) quantitative analysis | |||
| indicating probability of extinction in the wild: | ≥50% in 10 years or 3 generations (100 years max.) | ≥20% in 20 years or 5 generations (100 years max.) | ≥10% in 100 years |
The Red List classifies extinction risk rather than rarity. Rarity is an important metric for biodiversity that is not directly reflected in the Red List classification. Species can be rare in markedly different ways, and rarity does not consistently lead to high extinction risk [3]. Extremely rare species (very small population size) are captured under criterion D, irrespective of population trend. Although criteria B and C incorporate different metrics pertaining to rarity (e.g. restricted range, few locations, severe fragmentation, small population size), the subcriteria recognize instances where rare species decline rapidly to extinction, and others where they maintain populations for long periods. Conversely, criterion A (population reduction) deals with species that are at risk because of a steep rate of decline, irrespective of whether they are currently abundant or rare. The criteria employ symptoms of high risk that may covary with rarity, in order to classify species consistently.
(b). Structure of criteria
One of the most frequent misconceptions regarding structure is the perception that the criteria cannot work consistently for species in different taxonomic groups [4]. The five criteria were, however, developed based on the principles of population dynamics and derived from a wide review of risk-promoting factors across a broad range of species with diverse life histories. The criteria were structured to recognize the major differences between species, and the symptoms indicative of risk [1].
While the major drivers of extinction are known, risk changes nonlinearly with these pressures. Differences in ecology and geography have substantial influence and vary among taxonomic groups [5]. These interactions were impossible to simplify for a broadly applicable scheme [1]. Where high-quality data are available, criterion E enables quantification of interactions among different threats, although this criterion has seldom been used (figure 2a). It is crucial to evaluate all criteria for which data are available to exploit the ensemble properties of the criteria to identify species on different pathways to extinction.
Figure 2.
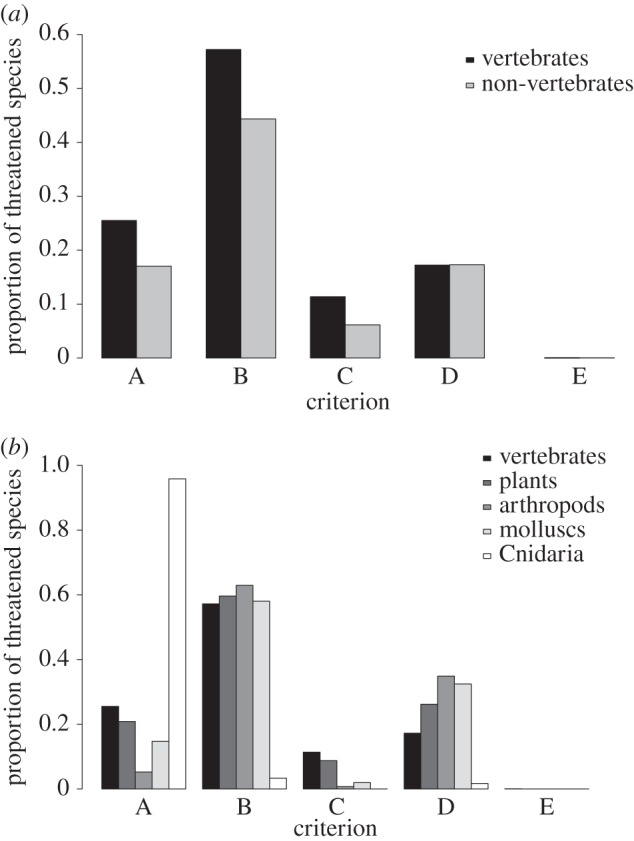
Proportion of threatened species meeting each criterion: (a) vertebrates and non-vertebrates; and (b) non-vertebrates subdivided.
The ca 79 000 species assessments on the Red List suggest broad applicability. Threatened vertebrates are assessed in broadly similar proportions under each of the five criteria as threatened non-vertebrates, a pattern consistent for plants, arthropods and molluscs (figure 2b). The one exception is cnidarians, where criterion A was applied more frequently because of the anticipated impact of a single threat. Variations within major taxa likely reflect that certain variables are more readily estimated for some taxa, e.g. area of occupancy for large sessile rather than small mobile organisms; rates of decline for taxa with slow rather than rapid population turnover.
(c). Use of standard metrics
The argument that one type of risk assessment cannot work for all taxa tends to hinge on two biological measures that differ markedly across species: life history and geographical range. The argument is made that the criteria could be improved by adopting different parameter thresholds for different taxa. However, this would reduce generality. For example, broadcast spawning fish are viewed as more fecund than most other species; however, high levels of fecundity do not consistently lead to low extinction risk in marine fish [6], so idiosyncratic thresholds may not improve assessments. Accounting for variability is important, and is accomplished by using bespoke definitions to account for variation in biological characteristics. Failure to consider correctly these definitions causes the majority of misconceptions regarding standardized metrics. Species responses to threatening processes are scaled to generation length to accommodate variation in population turnover [7] (although, for practicality, A3, A4, C1 and E limit the time horizon for future declines to 100 years, regardless of generation length). Arbitrarily changing the time horizon would produce inconsistent outcomes—extinction risk could not be compared among taxa [8]. An alternative would be taxon-specific modified sets of parameters. These would render cross-species comparisons invalid and make the large task of assessing a representative set of species far more onerous [9].
A bespoke definition is used to calculate extent of occurrence (EOO)—area contained within the shortest continuous boundary encompassing all the known, inferred or projected sites of occurrence of a species. EOO reflects the spatial spread of risk from threats across the species range. It is therefore an index of insurance against spatially explicit threats, and not intended as an accurate depiction of the range of a species [10].
Comparable application of the criteria requires that EOO be estimated consistently across different species. It remains unclear whether research that develops the measurement of range size results in improved indices of risk-spreading, but applying different measures to Red List thresholds compromises cross-taxon comparability. Improved consistency in the measurement of EOO is leading to hundreds of bird, mammal and amphibian species being down-listed [11].
(d). Application of criteria
Most assessments are based on a range of quantitative estimates derived from a variety of sources. A common misconception is that categories are assigned based on unstructured expert opinion—listings are not assigned directly through expert opinion. The Red List criteria are frequently applied by groups of assessors in workshops, in which available data for a species are compared against the quantitative criteria thresholds. Taking into account uncertainty, specialist expertise on the species or the threats it faces are used to estimate parameter values based on incomplete data, or to interpret certain qualifiers to these criteria (e.g. infer whether habitat degradation observed in a species' range impacts that species and leads to a decline in habitat quality—a qualifier in the B criterion). Quantitative thresholds ensure that these are transparent and falsifiable.
Uncertainty (natural variability or measurement error) in estimation of parameters, and the impacts that those uncertainties have on classification, can be incorporated in a number of ways. Analytically, parameter estimates can be made using bounds and best estimates together with fuzzy logic to assign a range of plausible categories [12]. Probably the largest source of variation in Red List assessments is due to variation in risk tolerance of assessors. Attitudes to risk span a continuum from precautionary (evidence needed to classify a species as non-threatened) to evidentiary (evidence needed to classify as threatened). Inconsistency in risk tolerance is most evident when assessing valuable exploited species [6].
Red Listing has proved controversial in the debate surrounding the risk faced by small or range-restricted, stable populations (e.g. those on small oceanic islands) that nominally meet the criterion B area thresholds. There are many examples of naturally rare highly restricted species that have life-history strategies to enable long-term persistence [13], thus putting them at low risk of extinction, whereas others with large ranges may be high risk. Hence, species cannot be listed solely on the basis of size, and require other symptoms of risk to qualify for threatened status under criterion B.
Finally, applying the five criteria and listing under the highest-risk outcome has been criticized for not using best available information. Alternatives include averaging extinction risk across criteria, or ignoring some criteria based on differences in data quality. However, the different criteria were derived from a wide review through wide consultation with species experts aimed at detecting risk factors across the broad range of organisms and the diverse life histories they exhibit [1], thus producing an ensemble of criteria to identify the symptoms of risk. Broad consistency among them was sought [10]. Adopting the highest category returned by any criterion (i.e. relying on the worst symptoms with reliable data) ensures a more precautionary approach to making urgent decisions based on limited information. This approach is akin to emergency room doctors focusing their assessments of patients on the most severe symptoms, instead of an average, where the best symptoms cancel out the worst ones. Assessors are encouraged to document criteria under which a species meets lower categories of risk, as such information is critical to recovery planning.
(e). Interpretation of classifications
Subjectivity was a criticism of early unstructured versions of the Red List, and was the principal motivation for development of quantitative criteria [1]. Clear guidelines are given on how quantitative data are used to assign species to categories of risk [10]. There is subjectivity in the establishment of boundaries among the categories of risk, though there is no theoretical reason why they should not be subjective. These boundaries divide extinction risk, a continuous metric, into categorical blocks. The continuum could have been divided differently. However, the proportion of species in the three threatened categories show that the current boundaries are reasonable: for randomly or fully assessed groups, the proportion in each category is neither negligible nor overwhelming, meeting the Red List's goal to provide an informative index of extinction risk.
Criteria A–D are based on population size, geographical range size and rates of decline. Criterion E is based on quantitative models of extinction risk, e.g. population viability analyses. Some researchers have assumed that species assessed using criteria A–D (proxies of extinction risk) can be assigned the probability of extinction thresholds in criterion E. Because E is the only criterion that can potentially incorporate all factors and symptoms of extinction risk, and the only criterion that includes quantitative thresholds of extinction probability, the thresholds of criterion E should not be used to infer the probability of extinction for species under any of the criteria A–D [8]. Comparisons of thresholds across categories and criteria are complex because of uncertainties in the relationship between extinction probability (E) and extinction risk proxies (A–D) used to assess taxa.
2. Future focus for the development of extinction risk measures
The development of Red List criteria has promoted valuable thinking and empirical research on extinction risk. The scrutiny that the scientific community continues to bring to Red Listing is welcome, and much has been done to refine and develop the existing framework in response to such scrutiny. However, we are not yet at the point where understanding has developed so markedly that it is time for the next generation of the Red List criteria. We conclude by identifying several key areas requiring further research.
(1) Further standardization of parameter estimation methods, particularly methods that can use sparse, uncertain and qualitative information to estimate robustly variables such as population reduction.
(2) Exploiting new data: remote sensing, genetic sampling, citizen science and social media. Effectively using these will require both fundamental research and new practical methods for estimating the variables used in the criteria.
(3) Assessment of risk under changing and interacting threats. Climate change is expected to have profound effects on biodiversity. Novel combinations of threats are also likely to occur. Although a recent study [14] suggested that the Red List criteria can identify species that might go extinct owing to climate change, species may require more frequent and complete assessment. Methods are required to facilitate use of future climate and land-use change scenarios, e.g. through species distribution and population modelling.
(4) Better understanding of the relationship between spatial structure and population dynamics (common and rare species), in relation to the spatial patterns of human impacts. Such research would lead to more specific guidelines on determining the number of locations and degree of fragmentation.
Acknowledgements
We thank Georgina Mace, Tom Brooks, Carlo Rondinini, Caroline Pollock, Jeff Hutchings, Robin Waples and Fangliang He.
Data accessibility
The data are available at www.iucnredlist.org.
Authors' contributions
Conceived and drafted the manuscript: B.C., N.K.D. and H.R.A. All authors contributed example misconceptions, made substantial contributions to acquisition of data, revised drafts for intellectual content, agree to be held accountable for the content and approve the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
There are no funders to report.
References
- 1.Mace G, Collar N, Gaston K, Hilton-Taylor C, Akcakaya H, Leader-Williams N, Milner-Gulland EJ, Stuart S. 2008. Quantification of extinction risk: IUCN's system for classifying threatened species. Conserv. Biol. 22, 1424–1442. ( 10.1111/j.1523-1739.2008.01044.x) [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann M, et al. 2010. The impact of conservation on the status of the world's vertebrates. Science 330, 1503–1509. ( 10.1126/science.1194442) [DOI] [PubMed] [Google Scholar]
- 3.Harnik P, Simpson C, Payne J. 2012. Long-term differences in extinction risk among the seven forms of rarity. Proc. R. Soc. B 279, 4969–4976. ( 10.1098/rspb.2012.1902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abeli T, Gentili R, Rossi G, Bedini G, Foggi B.. 2009. Can the IUCN criteria be effectively applied to peripheral isolated plant populations? Biodivers. Conserv. 18, 3877–3890. ( 10.1007/s10531-009-9685-4) [DOI] [Google Scholar]
- 5.Fisher D, Owens I. 2004. The comparative method in conservation biology. Trends Ecol. Evol. 19, 391–398. ( 10.1016/j.tree.2004.05.004) [DOI] [PubMed] [Google Scholar]
- 6.Dulvy N, Jennings S, Goodwin N, Grant A, Reynolds J. 2005. Comparison of threat and exploitation status in north-east Atlantic marine populations. J. Appl. Ecol. 42, 883–891. ( 10.1111/j.1365-2664.2005.01063.x) [DOI] [Google Scholar]
- 7.Yoccoz N, Nichols J, Boulinier T.. 2001. Monitoring of biological diversity in space and time. Trends Ecol. Evol. 16, 446–453. ( 10.1016/S0169-5347(01)02205-4) [DOI] [Google Scholar]
- 8.Akçakaya H, Butchart S, Mace G, Stuart S, Hilton-Taylor C.. 2006. Use and misuse of the IUCN Red List Criteria in projecting climate change impacts on biodiversity. Glob. Change Biol. 12, 2037–2043. ( 10.1111/j.1365-2486.2006.01253.x) [DOI] [Google Scholar]
- 9.Collen B, Baillie J. 2010. The barometer of life: sampling. Science 329, 140 ( 10.1126/science.329.5988.140-a) [DOI] [PubMed] [Google Scholar]
- 10.IUCN Standards and Petitions Subcommittee 2013. Guidelines for Using the IUCN Red List Categories and Criteria Version 10.1. Prepared by the Standards and Petitions Subcommittee. 1. http://jr.iucnredlist.org/documents/RedListGuidelines.pdf.
- 11.Joppa L, et al. 2015. Impact of alternative metrics on estimates of extent of occurrence for extinction risk assessment. Conserv. Biol. 30, 362–370. ( 10.1111/cobi.12591) [DOI] [PubMed] [Google Scholar]
- 12.Akçakaya H, Ferson S, Burgman M, Keith D, Mace G, Todd C. 2000. Making consistent IUCN classifications under uncertainty. Conserv. Biol. 14, 1001–1013. ( 10.1046/j.1523-1739.2000.99125.x) [DOI] [Google Scholar]
- 13.Gaston K. 1994. Rarity. London, UK: Chapman & Hall. [Google Scholar]
- 14.Stanton J, Shoemaker K, Pearson R, Akçakaya H.. 2015. Warning times for species extinctions due to climate change. Glob. Change Biol. 21, 1066–1077. ( 10.1111/gcb.12721) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available at www.iucnredlist.org.


