Abstract
Conditions experienced during prenatal development can have long-lasting organizational effects on offspring. Maternal carotenoids deposited in the eggs of birds and other oviparous species play an important role during fast embryonic growth and chick development through their antioxidant properties. However, the long-term consequences of variation in maternal carotenoid transfer for the offspring have seldom been considered. Since plasma carotenoid levels at adulthood are known to influence testis size and yolk carotenoid levels influence the ability to extract carotenoids later in life, we hypothesized that maternally transmitted carotenoids might influence gonad size at adulthood. Here, we showed that male Japanese quail (Coturnix japonica) originating from a carotenoid-enriched egg had smaller testes than control individuals at adulthood. This result shows that yolk carotenoids have long-term organizational effects. In addition, given that carotenoid intake at sexual maturity increases sperm quality and that a decreased testis size is associated with a lower sperm production, we propose that carotenoid exposure during embryo development might influence a trade-off between ejaculate size and sperm quality.
Keywords: carotenoids, maternal effects, long-term effects, testis size
1. Introduction
Conditions experienced during embryo development can have major organizational effects that can last until adulthood [1]. However, although numerous studies have documented short-term effects of prenatal conditions on offspring phenotype [2], the long-term consequences for fitness-related traits are still poorly understood.
Maternally transmitted antioxidants might influence embryonic developmental trajectory owing to their capacity to scavenge the reactive oxygen species produced during development and the challenging period of rapid growth [3]. Several studies have examined the importance of these compounds during development using dietary carotenoid supplementation of laying females to indirectly manipulate yolk antioxidants levels (i.e. carotenoids) and have shown effects of this treatment on nestlings' condition or carotenoid levels just a few weeks after hatching [4–6]. However, these results must be considered cautiously since these effects may have been mediated by other effects of these dietary manipulations on maternal physiology and/or the differential allocation of other maternally transmitted compounds.
So far, only two studies have directly manipulated yolk carotenoid concentrations through in ovo injections, showing that these maternally transmitted compounds have the potential to influence chick growth, immunocompetence and antioxidation capacity [7–9]. The potential long-term effects of prenatal antioxidant exposure have so far only been examined in one study in which male barn swallows (Hirundo rustica) that hatched from eggs injected with vitamin E arrived earlier at their breeding grounds than controls [10]. We thus need more studies where the long-term consequences of yolk antioxidant manipulations (through in ovo injections) are examined to determine the potential organizational effect of these maternally transmitted compounds.
Nutritional conditions during development and at adulthood have been shown to influence gonadal development in a variety of taxa (cockroach, [11], humans [12], mallard [13]), but information on the importance of specific antioxidants (such as carotenoids) on gonadal growth is scarce. Carotenoids are present in both testes and seminal fluid and it has been proposed that these molecules might limit oxidative stress and allow optimal cell growth in testes [14], a tissue where the high rate of cell division might generate high levels of free radicals [15]. Given that variation in yolk carotenoid levels can influence the ability to extract or assimilate these compounds later in life [4] and that dietary carotenoid availability at adulthood influenced testes size [16], we hypothesized that maternally transmitted carotenoid may influence gonad size at adulthood. To test this hypothesis, we experimentally manipulated yolk lutein levels in Japanese quail eggs and measured the consequences of this treatment for the sons' testis size at adulthood.
2. Material and methods
Unincubated Japanese quail (Coturnix japonica) eggs were collected from 55 females of our captive breeding population and injected with either 15 µg of carotenoids (FloraGLO Lutein 20%, Kemin Foods, Des Moines, IA, USA) dissolved in 15 µl of safflower oil or with only safflower oil as a control. Lutein was chosen because it is the most abundant carotenoid found in Japanese quail eggs and the dose injected represents approximately 1 s.d. of the yolk carotenoid content in this species [17]. The overall hatching success was 41.1% (control = 38.5% and carotenoid = 44.1%) and comparable to previous studies in Japanese quail [18,19]. See the electronic supplementary material for details on the incubating and rearing conditions. One year post-hatch, 48 males were randomly selected and euthanized (29 control and 19 birds from carotenoid-injected eggs), from 40 females and 430 eggs injected (226 control-injected and 204 carotenoid-injected). Both testes were collected and weighed to the nearest 1 mg. Tarsus length was measured to the nearest 0.1 mm.
(a). Statistics
Testis mass was significantly repeatable between the left and right sides (F1,45 = 23.45, p < 0.001), we thus used average values per bird for our analyses. In order to avoid pseudo-replication, we used the mean average testis mass of all male offspring per mother in our analyses because six mothers had more than one son. We ran general linear models to test whether the yolk carotenoid manipulation and bird size (tarsus length), as well as their interaction, affected testis size. All statistical analyses were run in R 3.01 (R Core Team, 2013).
3. Results
Larger birds had larger testes (F1,37 = 7.77, p = 0.008). Furthermore, testis size was significantly affected by the carotenoid treatment (F1,37 = 4.52, p = 0.04), with males originating from carotenoid-injected eggs having smaller testes than males originating from control eggs (figure 1). The interaction effect between carotenoid treatment and tarsus length was non-significant (F1,36 = 0.030, p = 0.86). Yolk carotenoid treatment did not influence body size at adulthood (F1, 38 = 1.41, p = 0.24).
Figure 1.
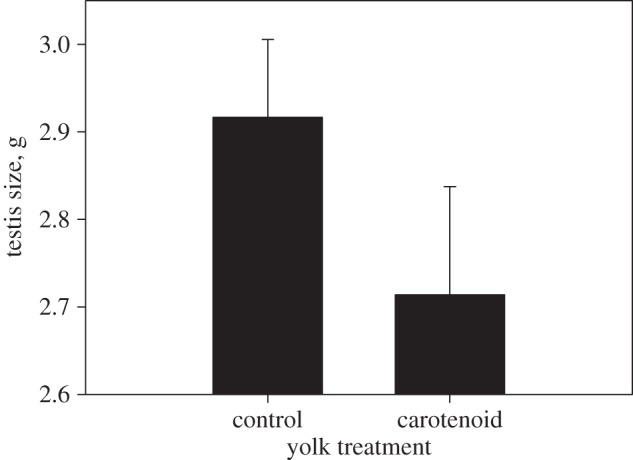
Effect of yolk carotenoid injection on testis size 1 year post-hatching. Means +1 s.e. are shown.
4. Discussion
Here, we show that prenatal exposure to carotenoids has long-term effects on a primary sexual trait. Male Japanese quail originating from a carotenoid-injected egg had smaller testes than controls 1 year post-hatching. This result is in line with the idea that yolk carotenoids have organizational effects that last until adulthood, potentially owing to their antioxidant properties ([20], but see [21]) and/or their effect on gene expression, cell proliferation and cell–cell communication [22,23].
Previous studies have indirectly manipulated yolk carotenoid levels through supplementation of laying females and showed that chick absorption and utilization of carotenoids, immunocompetence and plumage coloration were influenced by this dietary manipulation several weeks after hatching [4,5,24]. Our result confirms these long-lasting effects of yolk carotenoid levels using a direct in ovo manipulation of these maternally transmitted compounds and by measuring a primary sexual trait several months after the end of the developmental phase. Given that only one carotenoid has been manipulated in our study, further work is needed to investigate whether other yolk carotenoids also have long-term effects on fitness-related traits.
We propose three non-mutually exclusive hypotheses that might explain the reduction of testis size in males originating from carotenoid-enriched eggs. First, carotenoids may influence a trade-off between ejaculate size and sperm quality. Recent studies have shown that an increased dietary intake of carotenoid leads to a reduction of testes size in mallards (Anas platyrhynchos) [13] and that testis size and testis carotenoid concentrations are negatively correlated in house finches (Haemorhous mexicanus) [16]. Given that testis size is positively associated with sperm production in birds [25], this strongly suggests that high carotenoid intake decreases ejaculate size in birds. However, studies in various taxa have also shown that an increased carotenoid intake at sexual maturity increases sperm quality [26–28], potentially through their antioxidant properties or the recycling of vitamin E molecules, a major actor in spermatozoa protection from oxidative damage [28]. Thus, carotenoids seem to stimulate the production of high-quality sperm, but in smaller amounts. Future studies should test this hypothesis by simultaneously measuring ejaculate size and sperm quality in individuals supplemented or not with carotenoid at different life stages (before or after hatching). Second, prenatal exposure to high levels of carotenoid may influence the trade-off between self-maintenance and survival towards a reduced reproductive investment during the first breeding event. However, this hypothesis seems unlikely since carotenoid supplementation has been shown to increase several components of reproductive investment in various species [29]. Finally, while we acknowledge the possibility that prenatal carotenoid exposure may have detrimental consequences for testes maturation and thus sperm production in our study, we believe that it is inconsistent with the accumulating evidence that carotenoid supplementation improves sperm quality ([26,28], but see [30]). In addition, the injected carotenoid dose was well within the natural range [17] and yolk carotenoid levels after injection were not unnaturally high since females were fed with a low-carotenoid diet during the whole experiment.
To conclude, we show for the first time that yolk carotenoid levels have long-term effects on a primary sexual trait, strongly suggesting that maternally transmitted antioxidants influence offspring fitness.
Supplementary Material
Acknowledgements
We thank Alison Pick and Pascale Hutter for help with data collection.
Ethics
All procedures conform to the relevant regulatory standards and were conducted under licences provided by the Veterinary Office of the Canton of Zurich, Switzerland (195/2010; 14/2014; 156).
Data accessibility
Data are available from the Dryad repository: http://dx.doi.org/10.5061/dryad.523t4.
Authors' contributions
M.G. and A-K.Z. collected the data; M.G. and B.T. designed the study; B.T. analysed the data. M.G. and B.T. wrote the manuscript. All authors agree to be held accountable for the content therein and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
The study was supported by the Swiss National Science Foundation (PP00P3_128386 and PP00P3_157455) and the Fonds zur Förderung des akademischen Nachwuchses.
References
- 1.Mousseau TA, Fox CW. 1998. Maternal effects as adaptations. New York, NY: Oxford University Press. [Google Scholar]
- 2.Groothuis TGG, Wendt M, von Engelhardt N, Carere C, Eising C. 2005. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329–352. ( 10.1016/j.neubiorev.2004.12.002) [DOI] [PubMed] [Google Scholar]
- 3.Surai PF, Speake BK, Sparks NHC. 2001. Carotenoids in avian nutrition and embryonic development. 2. Antioxidant properties and discrimination in embryonic tissues. J. Poultry Sci. 38, 117–145. ( 10.2141/jpsa.38.117) [DOI] [Google Scholar]
- 4.Koutsos EA, Clifford AJ, Calvert CC, Klasing KC. 2003. Maternal carotenoid status modifies the incorporation of dietary carotenoids into immune tissues of growing chickens (Gallus gallus domesticus). J. Nutr. 133, 1132–1138. [DOI] [PubMed] [Google Scholar]
- 5.McGraw KJ, Adkins-Regan E, Parker RS. 2005. Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colorful songbird. Naturwissenschaften 92, 375–380. ( 10.1007/s00114-005-0003-z) [DOI] [PubMed] [Google Scholar]
- 6.Grether GF, Kolluru GR, Lin K, Quiroz MA, Robertson G, Snyder AJ. 2008. Maternal effects of carotenoid consumption in guppies (Poecilia reticulata). Funct. Ecol. 22, 294–302. ( 10.1111/j.1365-2435.2007.01365.x) [DOI] [Google Scholar]
- 7.Saino N, Ferrari RP, Romano M, Martinelli R, Møller AP. 2003. Experimental manipulation of egg carotenoids affects immunity of barn swallow nestlings. Proc. R. Soc. Lond. B 270, 2485–2489. ( 10.1098/rspb.2003.2534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saino N, Romano M, Caprioli M, Rubolini D, Ambrosini R. 2011. Yolk carotenoids have sex-dependent effects on redox status and influence the resolution of growth trade-offs in yellow-legged gull chicks. Behav. Ecol. 22, 411–421. ( 10.1093/beheco/arq220) [DOI] [Google Scholar]
- 9.Romano M, Caprioli M, Ambrosini R, Rubolini D, Fasola M, Saino N. 2008. Maternal allocation strategies and differential effects of yolk carotenoids on the phenotype and viability of yellow-legged gull chicks in relation to sex and laying order. J. Evol. Biol. 21, 1826–1840. ( 10.1111/j.1420-9101.2008.01599.x) [DOI] [PubMed] [Google Scholar]
- 10.Møller AP, Biard C, Karadas F, Rubolini D, Saino N, Surai PF. 2011. Maternal effects and changing phenology of bird migration. Clim. Res. 49, 201–210. ( 10.3354/cr01030) [DOI] [Google Scholar]
- 11.Barrett ELB, Hunt J, Moore AJ, Moore PJ. 2009. Separate and combined effects of nutrition during juvenile and sexual development on female life-history trajectories: the thrifty phenotype in a cockroach. Proc. R. Soc. B 276, 3257–3264. ( 10.1098/rspb.2009.0725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lummaa V. 2003. Early developmental conditions and reproductive success in humans: downstream effects of prenatal famine, birthweight, and timing of birth. Am. J. Hum. Biol. 15, 370–379. ( 10.1002/ajhb.10155) [DOI] [PubMed] [Google Scholar]
- 13.Butler MW, Karanfilian B, Homsher M, McGraw KJ. 2013. Carotenoid supplementation during adulthood, but not development, decreases testis size in mallards. Comp. Biochem. Physiol. A 166, 465–469. ( 10.1016/j.cbpa.2013.07.024) [DOI] [PubMed] [Google Scholar]
- 14.Blount JD, Møller AP, Houston DC. 2001. Antioxidants, showy males and sperm quality. Ecol. Lett. 4, 393–396. ( 10.1046/j.1461-0248.2001.00255.x) [DOI] [Google Scholar]
- 15.Taylor CT. 2001. Antioxidants and reactive oxygen species in human fertility. Environ. Tox. Pharm. 10, 189–198. ( 10.1016/S1382-6689(01)00099-0) [DOI] [PubMed] [Google Scholar]
- 16.Rowe M, Tourville EA, McGraw KJ. 2012. Carotenoids in bird testes: links to body carotenoid supplies, plumage coloration, bodymass and testes mass in house finches. Comp. Biochem. Physiol. B 163, 285–291. ( 10.1016/j.cbpb.2012.06.005) [DOI] [PubMed] [Google Scholar]
- 17.Peluc SI, Reed WL, McGraw KJ, Gibbs P. 2012. Carotenoid supplementation and GnRH challenges influence female endocrine physiology, immune function, and egg-yolk characteristics in Japanese quail (Coturnix japonica). J. Comp. Physiol. B. 182, 687–702. ( 10.1007/s00360-011-0638-3) [DOI] [PubMed] [Google Scholar]
- 18.Daisley JN, Bromundt V, Mostl E, Kotrschal K. 2005. Enhanced yolk testosterone influences behavioral phenotype independent of sex in Japanese quail chicks Coturnix japonica. Horm. Behav. 47, 185–194. ( 10.1016/j.yhbeh.2004.09.006) [DOI] [PubMed] [Google Scholar]
- 19.Hegyi G, Schwabl H. 2010. Do different yolk androgens exert similar effects on the morphology or behaviour of Japanese quail hatchlings Coturnix japonica? J. Avian Biol 41, 258–265. ( 10.1111/j.1600-048X.2009.04787.x) [DOI] [Google Scholar]
- 20.Halliwell B, Gutteridge J. 2007. Free radicals in biology and medicine. Oxford, UK: Oxford University Press. [Google Scholar]
- 21.Costantini D, Møller AP. 2008. Carotenoids are minor antioxidants for birds. Funct. Ecol. 22, 367–370. ( 10.1111/j.1365-2435.2007.01366.x) [DOI] [Google Scholar]
- 22.Edge R, McGarvey DJ, Truscott TG. 1997. The carotenoids as antioxidants: a review. J. Photochem. Photobiol. B 41, 189–200. ( 10.1016/S1011-1344(97)00092-4) [DOI] [PubMed] [Google Scholar]
- 23.Chew BP, Park JS. 2004. Carotenoid action on the immune response. J. Nutr. 134, 257S–261S. [DOI] [PubMed] [Google Scholar]
- 24.Biard C, Surai PF, Møller AP. 2007. An analysis of pre- and post-hatching maternal effects mediated by carotenoids in the blue tit. J. Evol. Biol. 20, 326–339. ( 10.1111/j.1420-9101.2006.01194.x) [DOI] [PubMed] [Google Scholar]
- 25.Møller AP. 1988. Testes size, ejaculate quality and sperm competition in birds. Biol. J. Linn. Soc. 33, 273–283. ( 10.1111/j.1095-8312.1988.tb00812.x) [DOI] [Google Scholar]
- 26.Helfenstein F, Losdat S, Møller AP, Blount JD, Richner H. 2010. Sperm of colourful males are better protected against oxidative stress. Ecol. Lett. 13, 213–222. ( 10.1111/j.1461-0248.2009.01419.x) [DOI] [PubMed] [Google Scholar]
- 27.Taş M, Güney Saruhan B, Kurt D, Yokuş B, Denl M. 2010. Protective role of lycopene on aflatoxin B1 induced changes spermcharacteristics and testicular damages in rats. Kafkas Univ. Vet. Fak. Derg. 16, 597–604. ( 10.9775/kvfd.2009.1236) [DOI] [Google Scholar]
- 28.Almbro M, Dowling DK, Simmons LW. 2011. Effects of vitamin E and beta-carotene on sperm competitiveness. Ecol. Lett. 14, 891–895. ( 10.1111/j.1461-0248.2011.01653.x) [DOI] [PubMed] [Google Scholar]
- 29.Blount JD, Houston DC, Surai PF, Møller AP. 2004. Egg-laying capacity is limited by carotenoid pigment availability in wild gulls Larus fuscus. Proc. R. Soc. Lond. B 271, S79–S81. ( 10.1098/rsbl.2003.0104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan M, Brown AC, Clotfelter ED. 2014. Dietary carotenoids do not improve motility or antioxidant capacity in cichlid fish sperm. Fish Physiol. Biochem. 40, 1399–1405. ( 10.1007/s10695-014-9934-7) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Dryad repository: http://dx.doi.org/10.5061/dryad.523t4.


