Abstract
Pollinator shifts are considered to drive floral trait evolution, yet little is still known about the modifications of petal epidermal surface at a biogeographic region scale. Here we investigated how independent shifts from insects to passerine birds in the Macaronesian Islands consistently modified this floral trait (i.e. absence of papillate cells). Using current phylogenies and extensive evidence from field observations, we selected a total of 81 plant species and subspecies for petal microscopy and comparative analysis, including 19 of the 23 insular species pollinated by opportunistic passerine birds (Macaronesian bird-flowered element). Species relying on passerine birds as the most effective pollinators (bird-pollinated) independently evolved at least five times and in all instances associated with a loss of papillate cells, whereas species with a mixed pollination system (birds plus insects and/or other vertebrates) evolved at least five times in Macaronesia and papillate cells were lost in only 25% of these transitions. Our findings suggest that petal micromorphology is a labile trait during pollinator shifts and that papillate cells tend to be absent on those species where pollinators have limited mechanical interaction with flowers, including opportunistic passerine birds that forage by hovering or from the ground.
Keywords: Islands, mixed pollination, opportunistic passerine birds, conical cells, pollinator shift
1. Introduction
The evolutionary relevance of the act of pollination in flowering plant species relies on the concurrence in time and space of most of the isolation barriers to gene flow [1]. In plant–pollinator interactions, petal micromorphology has a critical role, either enhancing flower visitation or contributing to the exclusion of less efficient pollinators [2,3]. Current evidence indicates that papillate cell types (conical) have a multifunctional role [4], enhancing flower visibility, as a tactile cue, improving grip and petal handling, influencing intrafloral microclimate and also affecting the degree of petal reflexing [2,4–7].
Despite this evidence, little is still known about the evolutionary transitions of petal epidermal types in relation to specific pollinators within a given biogeographic region. Thus, petal micromorphology is probably one of the less studied floral traits during pollinator shifts. Loss of papillate cells have been reported in several species in association with a shift to different pollinator types in Solanales [4], Antirrhineae [4,8] and Lotus species [3]. These previous studies were examined within a relatively narrow taxonomic context, and thus it is still unclear whether a similar association exists across independent plant lineages in a biogeographic region, where a set of plant species are under the selective pressure of a similar pollinator group (opportunistic passerine birds).
In this study, we examined the modifications of petal epidermal surface in a group of species that collectively seem to have converged to attract opportunistic passerine birds [9–11]. The so-called Macaronesian bird-flowered element shares similar floral traits, such as flower colour, longevity, and nectar sugar volume and composition that indicate an adaptation to attract birds [10,12–15], yet the role of birds varies among these plant species. Here, we tested whether shifts of pollination systems in the Macaronesian bird-flowered element are associated with changes in petal epidermal surface considering the adaxial (usually exposed to pollinators) and the abaxial sides. In particular, we tested whether changes from insect to bird or mixed pollination systems are associated with modifications from papillate to tabular cell types. We tested the hypothesis that species that rely on opportunistic passerine birds as the main effective pollinators lack papillate cells on the epidermal surface of petals.
2. Material and methods
We used published plant phylogenies to select 81 species and subspecies (electronic supplementary material, table S1 and figure S1) including 19 taxa from the Macaronesian bird-flowered elements (electronic supplementary material, table S2) and their closely related species from the mainland. We classified them according to three pollination systems: insect, mixed (insect and vertebrate) and bird pollination. Using microscopy analyses (light microscopy and SEM), we examined the petal surface of each taxa and classified them either as tabular or papillate. Then, we conducted two alternative statistical analyses to determine whether shifts in pollination systems (insects to birds or mixed) were associated with changes in petal epidermal surface (papillate versus tabular): (i) pairwise comparisons between closely related species according to the recommendation of Felsenstein [16] because a full phylogeny is not available and (ii) a test of correlated evolution for discrete characters [17] by using partial phylogenetic trees from available molecular phylogenies (electronic supplementary material, figures S2 and S3).
3. Results
We found six different epidermal cell types, three papillate and three tabular types. The primary structure of the papillate cells varied among conical (lemon-shaped) (PCS), round (dome-shaped) (PKR) or with an oblong shape (PO). Striations (secondary structure) were only observed in conical cells. Tabular cell types varied in shape from marked sinuations on the outer part of the cell (jigsaw puzzle-shaped) (tabular rugose cells with striations (TRS) and tabular rugose cells without striations (TR)) to rectangular (elongated shape) without marked sinuations (tabular flat cells with striations, TFS). Striations (secondary structure) were observed on all tabular cell types, except for TR (electronic supplementary material, table S3 and figure S4).
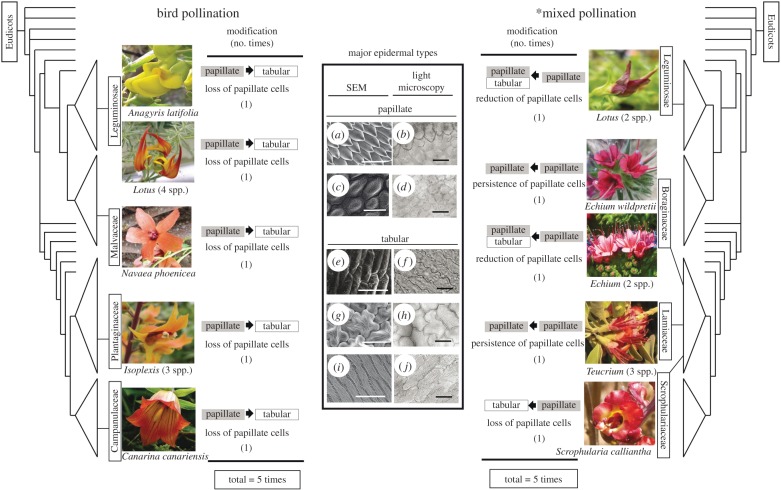
All five pairwise comparisons of the species with bird pollination have lost papillate cells when compared with their closely related species displaying insect pollination (figure 1). A similar trend was also observed on the other two bird-pollinated continental species of Canarina and Anagyris foetida. By contrast, only two of the eight pairwise comparisons (25%) with a mixed pollination have lost papillate cells (figure 1). By using McNemar's test among pairwise closest species, we detected a significant tendency to changes in epidermal surface in relation to pollinator type (McNemar's χ2 = 9.09, p < 0.001). This correlative tendency between petal epidermal type and pollination system was also confirmed when phylogenetic tree was incorporated by using Pagel's discrete character method (LR = 11.8, p < 0.001 by using 81 taxa, electronic supplementary material figure S2; and LR = 7.95, p < 0.001 with 16 species, electronic supplementary material, figure S3). These last results remain consistent despite the presence of two types of cells in some species (e.g. Lotus argyrodes, Scrophularia fontqueri; see electronic supplementary material, table S3).
Figure 1.
Transitions of epidermal types in the Macaronesian bird-flowered element. Boxes represent the modification of epidermal type in comparison with its closely related species. Papillate types: (a) papillate conical cells (PCS) in the dorsal petal of Lotus sessilifolius, (b) PCS in the upper lip of Digitalis purpurea, (c) papillate knobby cells (PKR) in Thermopsis macrophylla, (d) PKR cells in Teucrium abutiloides. Tabular types: (e,f) tabular rugose cells without striations (TR) in Navaea phoenicea, (g) tabular rugose cells with striations (TRS) in the lateral petals of Lotus sessilifolius, (h) TRS in the upper lip of Scrophularia calliantha, (i) tabular flat cells with striations (TFS) in the ventral petals of Lotus sessilifolius and (j) TFS in the ventral petals of Anagyris foetida. Scale bars, 20 µm. *Mixed vertebrate pollination also evolved at least three times in Mediterranean Scrophularia. (Online version in colour.)
4. Discussion
Bird pollination independently evolved at least five times in the Macaronesian Islands and in all cases involved a loss of papillate cell types (figure 1). This trend of lacking papillate cells was not only observed on those lineages endemic to oceanic environments (de novo origin), but also on two independent lineages (Anagyris and Canarina) from continental Europe and Africa, respectively. By contrast, only two lineages (two independent transitions in Scrophularia) with mixed pollination were associated with a loss of papillate cells (25%) and four independent transitions (50%) with a reduction of the area covered by papillate cells from insect to mixed pollination (figure 1; electronic supplementary material, table S4). Only three out of the 13 species pair comparisons (23%) showed no modifications in epidermal surface in relation to a shift in pollination system (electronic supplementary material, table S4).
Our results demonstrate that those species that rely on opportunistic birds as the most effective pollinators lack papillate cells. This modification might be associated with changes in feeding behaviours between insects and passerine birds. These birds do not land in the flowers (as most insects do), but rather they forage from the ground, a nearby branch or by hovering [12,18,19]. Thus, the main mechanical interaction occurs when the bird collects nectar from the flower. Loss of papillate cells on these species could be either an adaptation to deter less effective pollinators (as an anti-bee strategy) or as a bird-selected trait that increases attraction or pollination (pro-bird strategy) [3]. A similar trend in changes of petal surface has also been observed on those hummingbird-pollinated species in Antirrhineae [8] and Polemoniaceae [20]. Similar to those passerine birds in Macaronesia, hummingbirds do not usually land on flowers. These differences in feeding behaviour between insects and these two groups of birds might explain why papillate cells are absent. This pattern seems not to be unique to birds, as previous reports also indicate a loss of papillate cells in Solanaceae species pollinated by hovering insects (e.g. moth and buzz pollination), where insect handling is also reduced [4].
Loss of papillate cells in the Macaronesian bird-flowered element could also have been owing to several alternative explanations, e.g. neutral selection and differences in metabolic cost of each epidermal type. If selection no longer maintains the presence of papillate cells on these species that rely on passerine birds, it is, therefore, possible that loss of function can affect those genes responsible for the differentiation of papillate cells. Differences in the energetic cost of producing papillate versus tabular cells could also explain the loss of papillate cells on bird-pollinated species when selection for the former cell type has been released. More research is necessary to fully determine the mechanistic reasons for the loss of papillate cells.
This study represents the first comprehensive analysis of the characterization of a group of species collectively adapted to attract a set of pollinators for a particular biota on oceanic islands from a biogeographic region. Our data contribute to the emerging paradigm that papillate cells are lost on those lineages of angiosperms adapted to pollinators with reduced physical interaction, such as hovering and perching birds, and even in insects that do not land on the flower to collect rewards.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Linda Jennings (UBC Herbarium) for her help providing specimens, J. J. Aldasoro for providing the African Canarina samples and the Cabildos from the Canary Islands for sampling permits. F. J. Valtueña, T. Rodríguez-Riaño and M. L. Navarro collected some samples of Scrophularia. Special thanks to Sandra Cervantes for reviewing early versions of the manuscript. We thank three referees for insightful suggestions.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
D.I.O. conceived the experiment. D.I.O., A.V. and A.G.F.d.C. performed the microscopy and statistical analyses. All authors collected plant material. D.I.O. drafted the manuscript, all authors contributed to the manuscript. All authors approved the final version and are accountable for all aspects of the work.
Competing interests
We have no competing interests.
Funding
A.V. was supported by RYC-2007-00620 and SEV-2012-0262 (Ministerio de Economía y Competitividad, Spain), A.O.-O. was funded by project CGL2011-2414 (Ministerio de Ciencia e Innovación, Spain), co-financed by European Regional Developmental Fund. J.F.-A. and A.G.F.d.C. were financed by project CGL2010-16138 (Ministerio de Ciencia e Innovación, Spain).
References
- 1.Johnson S. 2006. Pollinator-driven speciation in plants. In Ecology and evolution of flowers (eds Harder S, Barret LD), pp. 295–310. New York, NY: Oxford University Press. [Google Scholar]
- 2.Whitney HM, Chittka L, Bruce TJ, Glover BJ. 2009. Conical epidermal cells allow bees to grip flowers and increase foraging efficiency. Curr. Biol. 19, 948–953. ( 10.1016/j.cub.2009.04.051) [DOI] [PubMed] [Google Scholar]
- 3.Ojeda I, Santos-Guerra A, Oliva-Tejera F, Jaén-Molina R, Caujapé-Castilles J, Marrero A, Cronk QCB. 2012. Comparative micromorphology of petals in Macaronesian Lotus (Leguminosae) reveals a loss of papillose conical cells during the evolution of bird pollination. Int. J. Plant Sci. 173, 365–374. ( 10.1086/664713) [DOI] [Google Scholar]
- 4.Glover B. 2014. Understanding flowers and flowering, 2nd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Whitney HM, Bennett KMV, Dorling M, Sandbach L, Prince D, Chittka L, Glover BJ. 2011. Why do so many petals have conical epidermal cells? Ann. Bot. 108, 609–616. ( 10.1093/aob/mcr065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rands S, Glover BJ, Whitney H. 2011. Floral epidermal structure and flower orientation: getting to grips with awkward flowers. Arthropod Plant Interact. 5, 279–285. ( 10.1007/s11829-011-9146-3) [DOI] [Google Scholar]
- 7.Kevan PG, Lane MA. 1985. Flower petal microtexture is a tactile cue for bees. Proc. Natl Acad. Sci. USA 82, 4750–4752. ( 10.1073/pnas.82.14.4750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landis JB. 2011. Pollinator attractors: petaloidy and epidermal cell shape in close relatives of snapdragon, p. 62. MSc thesis, Kansas University, USA.
- 9.Ojeda DI. 2013. The Macaronesian bird-flowered element as a model system to study the evolution of ornithophilous floral traits. Vieraea 41, 67–83. [Google Scholar]
- 10.Valido A, Dupont YL, Olesen JM. 2004. Bird–flower interactions in the Macaronesian Islands. J. Biogeogr. 31, 1945–1953. ( 10.1111/j.1365-2699.2004.01116.x) [DOI] [Google Scholar]
- 11.Valido A, Olesen JM. 2010. Pollination on islands: examples from the Macaronesian archipelagos. In Terrestrial arthropods of Macaronesia. Biodiversity, ecology and evolution (eds Serrano ARM, Borges PAV, Boieiro M, Oromí P), pp. 249–283. Lisboa, Portugal: Sociedade Portuguesa de Entomologia. [Google Scholar]
- 12.Ollerton J, Cranmer L, Stelzer RJ, Sullivan S, Chittka L. 2009. Bird pollination of Canary Island endemic plants. Naturwissenschaften 96, 221–232. ( 10.1007/s00114-008-0467-8) [DOI] [PubMed] [Google Scholar]
- 13.Dupont YL, Hansen DM, Rasmussen JT, Olesen JM. 2004. Evolutionary changes in nectar sugar composition associated with switches between bird and insect pollination: the Canarian bird-flower element revisited. Funct. Ecol. 18, 670–676. ( 10.1111/j.0269-8463.2004.00891.x) [DOI] [Google Scholar]
- 14.Olesen JM. 1985. The Macaronesian bird flower element and its relation to bird and bee opportunists. Bot. J. Linn. Soc. 91, 395–414. ( 10.1111/j.1095-8339.1985.tb01010.x) [DOI] [Google Scholar]
- 15.Ojeda DI, Santos-Guerra A, Oliva-Tejera F, Valido A, Xue X, Marrero A, Caujapé-Castells J, Cronk QCB. 2013. Bird-pollinated Macaronesian Lotus (Leguminosae) evolved within a group of entomophilous ancestors with post-anthesis flower colour change. Perspect. Plant Ecol. Evol. Syst. 15, 193–204. ( 10.1016/j.ppees.2013.05.002) [DOI] [Google Scholar]
- 16.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 17.Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45. ( 10.1098/rspb.1994.0006) [DOI] [Google Scholar]
- 18.Rodríguez-Rodríguez MC, Valido A. 2008. Opportunistic nectar-feeding birds are effective pollinators of bird-flowers from Canary Islands: experimental evidence from Isoplexis canariensis (Scrophulariaceae). Am. J. Bot. 95, 1408–1415. ( 10.3732/ajb.0800055) [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Rodríguez MC, Valido A. 2011. Consequences of plant–pollinator and floral–herbivore interactions on the reproductive success of the Canary Islands endemic Canarina canariensis (Campanulaceae). Am. J. Bot. 98, 1465–1474. ( 10.3732/ajb.1100146) [DOI] [PubMed] [Google Scholar]
- 20.Kay QON, Daoud HS, Stirton CH. 1981. Pigment distribution, light reflection and cell structure in petals. Bot. J. Linn. Soc. 83, 57–84. ( 10.1111/j.1095-8339.1981.tb00129.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.