Abstract
Because incubation by birds is energetically costly, parents frequently trade off investment in incubation against self-maintenance. This can be manifested by a reduction in incubation temperature, which comes at high somatic costs for nestlings. The extent to which these costs constrain fitness is poorly understood. We incubated wild blue tit clutches at three biologically relevant temperatures and subsequently recorded winter survival and survival to the breeding season. Fledglings from the coldest treatment (35.0°C) survived less well than other fledglings, but the proportion of winter and breeding survivors did not differ significantly between treatments. However, survival probability in both seasons increased with body mass at fledging in birds from low and mid incubation temperatures, but decreased with fledging body mass in the high-temperature treatment. Mid-temperature nestlings were heavier as adults, weighing 7% more than low- and high-temperature survivors. Thus, high incubation temperature can be beneficial in the short term, but costs of accelerated embryonic development may equal those of protracted development in the long term. Such hidden consequences of faster development could maintain natural selection for average incubation temperature.
Keywords: blue tit, Cyanistes caeruleus, development, maternal effect, recruitment, survival
1. Introduction
Incubation by birds is associated with a significant increase in parental energy expenditure, to levels comparable to those during chick rearing [1]. Accordingly, experimental changes in the demands of incubation either improve or hamper reproductive success, depending on the direction of manipulation [2–4]. Fitness costs of incubation are frequently also transferred to offspring, independently of effects acting on parents [5,6]. However, few attempts have been made to better understand how costs of incubation are transferred between generations.
There are indications of a causal relationship between the energetic demands of incubation and the thermal environment parents provide for their clutch: steady-state incubation temperature is reduced when costs of incubation increase, and elevated when demands are relieved [1]. The resultant variation in embryonic temperature may alter developmental trajectories [7] to ultimately constrain or improve phenotypic traits with links to fitness, such as physiological maturation, structural size, immunocompetence and thermoregulatory capacity [8]. Thus, variation in incubation temperature could be instrumental in explaining how costs of incubation operating on parents subsequently constrain chick performance in the short term. The long-term effects of variation in incubation temperature are poorly understood, but recent results indicate that a suboptimal thermal environment in the nest may have far-reaching consequences for both survival into adulthood and subsequent reproductive success [9,10].
To further our understanding of the link between incubation temperature and offspring performance, we manipulated incubation temperature in wild blue tit (Cyanistes caeruleus) clutches during 3 years. This showed that high incubation temperature accelerated the developmental period and decreased the incidence of embryo mortality, and produced structurally larger nestlings that maintained a lower energy turnover rate than low-temperature nestlings when leaving the nest [11]. In this study, we asked if these differences would persist into adulthood and be of a large enough magnitude to reduce long-term survival.
2. Methods
We manipulated incubation temperature (35.0°C, 36.5°C, or 38.0°C for two-thirds of the incubation period) in blue tit clutches from a population outside Lund in southernmost Sweden (55°42′ N, 13°28′ E) in 2008–2010 [11]. Clutch size (11 ± 0.1 eggs) and breeding start (23 April ± 0.3 days) did not differ between treatments (p > 0.3). Between 2009 and 2012, we systematically searched the study area for nestlings from this experiment that had survived and recruited into the local breeding population (breeding survival). Between 250 and 300 blue tit pairs bred each year during this time, of which we recaptured 90%. We also assessed survival to the first winter in January and February 2009–2010 (i.e. for nestlings hatched in 2008–2009) by searching all nest-boxes in the area for roosting birds at night. Each nest-box was visited twice during a period of one month, with two weeks between visits. Birds were considered winter survivors if they were resident during their first winter, or if they were observed in the population in the following spring. Biometric data (body mass, tarsus and wing lengths) were collected for all recaptured birds.
Statistics were performed using R v. 3.2.1. We analysed the probability of breeding survival in a generalized linear mixed model with a binomial error structure using survival/recruitment (0/1) as the dependent variable, temperature treatment as a factor, hatching date (standardized), brood size and body mass at fledging (and their interactions with the temperature treatment) as covariates. We included breeding attempt nested within treatment as a random effect. Winter survival was analysed in an identical model for the subset of nestlings that hatched in 2008–2009. Final models were derived using likelihood ratio tests. We compared mass, tarsus and wing lengths of recaptures in mixed effects models with the temperature treatment and season (winter/breeding) and their interaction as factors, hatching date as a covariate and bird identity as a random intercept.
3. Results
About 3% of birds (21 of 678) survived to their first winter, a number that had been reduced to 2% (22 of 965) by the breeding season. Birds from the 35.0°C treatment survived in slightly lower proportions during both seasons (winter: 2.8%; summer: 1.9%) compared with the other two treatments (38.0°C: winter: 3.1%; summer: 2.5%; 36.5°C: winter: 3.3%; summer: 2.5%; electronic supplementary material, table S1). However, differences between treatments were not significant (Fisher's exact test: p > 0.8).
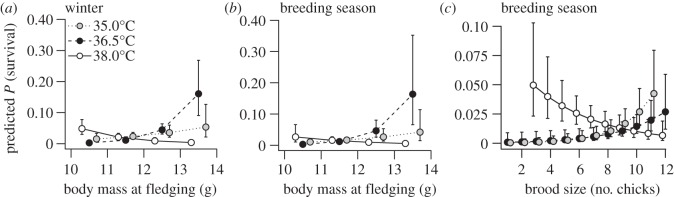
The probability of survival decreased with hatching date, similarly for all treatments in both seasons (table 1). Winter survival probability increased with increasing fledging body mass in both 35.0°C and 36.5°C nestlings, but decreased with body mass in the high-temperature treatment (figure 1a). Low (11.9 ± 0.3 g) and mid (12.6 ± 0.2 g) temperature nestlings that survived to the winter were 0.4 ± 0.3 (3%) and 1.0 ± 0.4 g (8%) heavier than those that did not whereas surviving high-temperature nestlings (11.0 ± 0.2 g) were 0.7 ± 0.2 g (6%) lighter than non-survivors. This was true also for breeding survival (figure 1b): nestlings from the low (11.8 ± 0.4 g) and mid (12.4 ± 0.2 g) -temperature treatments were 0.4 ± 0.3 (3%) and 1.1 ± 0.3 g (10%) heavier at fledging than were non-survivors, whereas high-temperature nestlings (11.1 ± 0.2 g) still alive at this time were 0.5 ± 0.2 g (4%) lighter at fledging than those that were not. The probability of breeding survival was also modulated by brood size: survival in low- and mid-temperature nestlings was close to 0 for broods of less than eight nestlings but increased nonlinearly thereafter, whereas survival probability in the high-temperature treatment decreased linearly with brood size and was the highest for nestlings from broods of less than eight (which contributed only 22% of fledglings; electronic supplementary material, figure S1).
Table 1.
Final models of winter and breeding season survival, and body mass at recapture. Estimates for survival models are presented as log odds and, for factors and interactions, are relative to the highest incubation temperature (i.e. 38.0°C). The test statistic for survival models is χ2; for the body mass model it is F. Significant (p <= 0.05) effects are given in bold type, and effects for which 0.05 < p <= 0.1 are given in italics.
| model | estimate (s.e.) | d.f. | χ2/F | p |
|---|---|---|---|---|
| winter survival | ||||
| incubation temperature (38.0°C) | 6.024 (5.084) | 2 | 11.050 | 0.0040 |
| incubation temperature (36.5°C) | −14.70 (7.83) | |||
| incubation temperature (35.0°C) | −26.72 (8.49) | |||
| hatching date | −0.56 (0.26) | 1 | 4.78 | 0.029 |
| body mass | −0.86 (0.46) | 1 | 1.32 | 0.25 |
| incubation temperature (36.5°C) × body mass | 1.29 (0.68) | 2 | 11.16 | 0.0038 |
| incubation temperature (35.0°C) × body mass | 2.27 (0.71) | |||
| breeding season survival | ||||
| incubation temperature (38.0°C) | 4.94 (5.39) | 2 | 13.00 | 0.00023 |
| incubation temperature (36.5°C) | −28.28 (8.074) | |||
| incubation temperature (35.0°C) | −17.99 (9.34) | |||
| hatching date | −0.52 (0.26) | 1 | 3.96 | 0.047 |
| body mass | −0.63 (0.44) | 1 | 1.96 | 0.16 |
| brood size | −0.23 (0.19) | 1 | 2.040 | 0.15 |
| incubation temperature (36.5°C) × body mass | 2.023 (0.62) | 2 | 11.030 | 0.0040 |
| incubation temperature (35.0°C) × body mass | 1.041 (0.75) | |||
| incubation temperature (36.5°C) × brood size | 0.55 (0.30) | 2 | 5.64 | 0.060 |
| incubation temperature (35.0°C) × brood size | 0.70 (0.34) | |||
| adult body mass | ||||
| incubation temperature (38.0°C) | 11.25 (0.17) | 2, 22 | 5.65 | 0.010 |
| incubation temperature (36.5°C) | 0.64 (0.19) | |||
| incubation temperature (35.0°C) | −0.071 (0.16) | |||
| season (summer) | 11.29 (0.17) | 1, 22 | 11.84 | 0.0023 |
| season (winter) | 0.31 (0.18) | |||
Figure 1.
Predicted (±s.e.) winter (a) and breeding season (b,c) survival in blue tits that had previously been incubated in one of three experimental temperatures, in relation to body mass at fledging (a,b) or brood size (c).
Mid-temperature nestlings were 6% (0.7 ± 0.2 g) and 8% (0.8 ± 0.2 g) heavier than low- and high-temperature nestlings upon recapture as adults (table 1 and figure 2). Body mass at recapture did not differ between high- and low-temperature nestlings. Structural size did not differ between treatments (tarsus and wing length: p > 0.27). Of the 10 recruiting females, only one was from the low incubation temperature treatment. This prevented evaluation of treatment-related effects on breeding performance.
Figure 2.
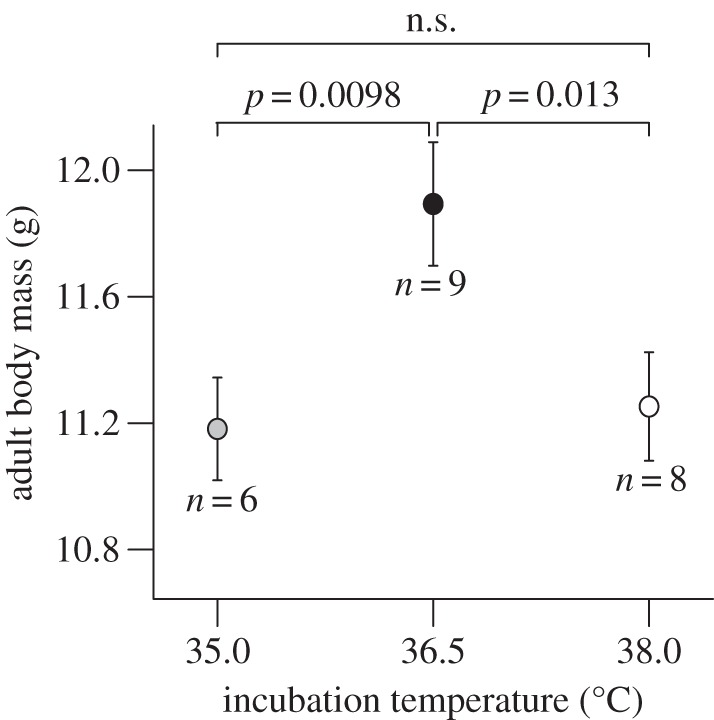
Predicted (±s.e.) body mass of experimentally incubated blue tits upon recapture as adults, averaged over sampling events when applicable. n-values denote recaptured birds.
4. Discussion
About 2% of nestlings survived to breed in their first year, but we found no indication that overall survival probability differed between treatments. Although based on a small sample, this contrasts with laboratory findings [10] and observations made in the field [9], where low incubation temperature hampered actual or apparent survival, respectively. It is possible that in our study early developmental conditions were less important for long-term survival than post-fledging ecological costs such as predation, which is underscored by low overall juvenile survival (electronic supplementary material, table S1). This would not be important in the laboratory (cf. [10]), and probably would be less important in natural systems where juvenile survival is higher (e.g. in wood ducks [12]). Alternatively, fitness costs of low incubation temperature in blue tits operate only at the embryonic stage (where low-temperature increases mortality). In that case temperature-dependent effects on the pre-fledging phenotype [11] are less important determinants of subsequent performance.
However, variation in incubation temperature affected survival also in the blue tits when taking body mass and brood size into account. High-temperature nestlings that survived were lighter at fledging than those that did not. Selective mortality of heavy high-temperature nestlings could occur via accumulation of oxidative damage and subsequent telomere attrition [13] if any survival costs from accelerated embryonic development alone [14] acted in synergy with potential consequences of higher post-natal growth rate in heavier fledglings. This could have been further exacerbated in nestlings from the more competitive environment of a large brood [14,15], consistent with the decline in breeding survival probability with brood size in this treatment. By contrast, surviving low- and mid-temperature birds were heavier at fledging than non-survivors, in line with commonly observed selection on fledgling condition [16]. Breeding survival was also the highest among larger broods in these treatments, as expected from the frequency distribution of fledglings (electronic supplementary material, figure S1). This could indicate that, at least in blue tits, there may be survival benefits of slower development for the individual nestling.
Variation in incubation temperature typically alters the juvenile phenotype [8], but it is poorly understood if developmental changes persist into adulthood. Of the two studies that manipulated incubation temperature and subsequently measured birds as adults, one found no effects of incubation temperature on either the juvenile or the adult phenotype [10], and the other does not report data on adult biometry [9]. We found temperature-related effects on adult morphology (figure 2), but contrary to expectation this manifested as higher body mass in mid-temperature birds. This could result in stabilizing selection for incubation temperature over longer time periods, e.g. if higher adult body mass renders birds better prepared for winter conditions [17] or put birds at a competitive advantage.
A bad start in life may impact later life-history stages or even coming generations [18]. We show that such patterns can be far from straightforward: a seemingly optimal start in life [11] may alter survival trajectories (figure 1) and subsequent morphological development (figure 2), to the point where the adult phenotypes converge with those of individuals that suffered a bad start. This highlights the need to extend measurements of incubation temperature-dependent phenotypes beyond the juvenile stage, and pleads for caution in the interpretation of experimental effects wherein larger size of high-temperature individuals is implicitly considered permanent, and of subsequent fitness advantage.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Sandra Sköld-Chiriac, Johan Nilsson, Sieglinde Kundisch and Martin Stjernman for assistance in the field.
Ethics
The experiment adheres to national legislation and was approved by the Malmö/Lund Animal Ethics Committee (permit no. M94-07). Capture and ringing of birds was permitted by the Swedish Bird Ringing Centre (licence no. 475).
Data accessibility
Data are deposited at Dryad: http://dx.doi.org/10.5061/dryad.f48f5.
Authors' contributions
A.N. and J.-Å.N. jointly conceived the study, designed the experiment, and performed the fieldwork. A.N. analysed the data and drafted the manuscript, which was then critically revised by A.N. and J.-Å.N. Both authors approved the final version of the manuscript and agree to be accountable for all contents.
Competing interests
The authors have no competing or financial interests.
Funding
A.N. and J.-Å.N. were supported by the Swedish Research Council (grant nos 637-2013-7442 and 621-2013-4386, respectively). Fieldwork was supported by the Swedish Research Council (to J.-Å.N.), the Royal Physiographic Society in Lund, the Lund Animal Protection Foundation, the Helge Ax:son Johnson Foundation, and the Längman Cultural Foundation (to A.N.).
References
- 1.Nord A, Williams JB. 2015. The energetic costs of incubation. In Nests, eggs, and incubation (eds Deeming DC, Reynolds SJ), pp. 152–170. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.de Heij ME, van den Hout PJ, Tinbergen JM. 2006. Fitness cost of incubation in great tits (Parus major) is related to clutch size. Proc. R. Soc. B 273, 2353–2361. ( 10.1098/rspb.2006.3584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visser ME, Lessells CM. 2001. The costs of egg production and incubation in great tits (Parus major). Proc. R. Soc. Lond. B 268, 1271–1277. ( 10.1098/rspb.2001.1661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ardia DR, Pérez JH, Chad EK, Voss MA, Clotfelter ED. 2009. Temperature and life history: experimental heating leads female tree swallows to modulate egg temperature and incubation behaviour. J. Anim. Ecol. 78, 4–13. ( 10.1111/j.1365-2656.2008.01453.x) [DOI] [PubMed] [Google Scholar]
- 5.Heaney V, Monaghan P. 1996. Optimal allocation of effort between reproductive phases: the trade-off between incubation costs and subsequent brood rearing capacity. Proc. R. Soc. Lond. B 263, 1719–1724. ( 10.1098/rspb.1996.0251) [DOI] [Google Scholar]
- 6.Ardia DR, Pérez JH, Clotfelter ED. 2010. Experimental cooling during incubation leads to reduced innate immunity and body condition in nestling tree swallows. Proc. R. Soc. B 277, 1881–1888. ( 10.1098/rspb.2009.2138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson CR, Vleck CM, Adams DC. 2008. Decoupling morphological development from growth in periodically cooled zebra finch embryos. J. Morphol. 269, 875–883. ( 10.1002/jmor.10635) [DOI] [PubMed] [Google Scholar]
- 8.DuRant SE, Hopkins WA, Hepp GR, Walters JR. 2013. Ecological, evolutionary, and conservation implications of incubation temperature-dependent phenotypes in birds. Biol. Rev. 88, 499–509. ( 10.1111/brv.12015) [DOI] [PubMed] [Google Scholar]
- 9.Hepp GR, Kennamer RA. 2012. Warm is better: incubation temperature influences apparent survival and recruitment of wood ducks (Aix sponsa). PLoS ONE 7, e47777 ( 10.1371/journal.pone.0047777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berntsen HH, Bech C. 2016. Incubation temperature influences survival in a small passerine bird. J. Avian Biol. 47, 141–145. ( 10.1111/jav.00688) [DOI] [Google Scholar]
- 11.Nord A, Nilsson J-Å. 2011. Incubation temperature affects growth and energy metabolism in blue tit nestlings. Am. Nat. 178, 639–651. ( 10.1086/662172) [DOI] [PubMed] [Google Scholar]
- 12.Hepp GR, Kennamer RA, Harvey WF. 1989. Recruitment and natal philopatry in wood ducks. Ecology 70, 897–903. ( 10.2307/1941357) [DOI] [Google Scholar]
- 13.Reichert S, Criscuolo F, Zahn S, Arrivé M, Bize P, Massemin S. 2015. Immediate and delayed effects of growth conditions on ageing parameters in nestling zebra finches. J. Exp. Biol. 218, 491–499. ( 10.1242/jeb.109942) [DOI] [PubMed] [Google Scholar]
- 14.Ricklefs RE. 2006. Embryo development and ageing in birds and mammals. Proc. R. Soc. B 273, 2077–2082. ( 10.1098/rspb.2006.3544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonso-Alvarez C, Bertrand S, Faivre B, Sorci G. 2007. Increased susceptibility to oxidative damage as a cost of accelerated somatic growth in zebra finches. Funct. Ecol. 21, 873–879. ( 10.1111/j.1365-2435.2007.01300.x) [DOI] [Google Scholar]
- 16.Lindén M, Gustafsson L, Pärt T. 1992. Selection on fledging mass in the collared flycatcher and the great tit. Ecology 73, 336–343. ( 10.2307/1938745) [DOI] [Google Scholar]
- 17.Macdonald CA, McKinnon EA, Gilchrist HG, Love OP. 2016. Cold tolerance, and not earlier arrival on breeding grounds, explains why males winter further north in an Arctic-breeding songbird. J. Avian Biol. 47, 7–15. ( 10.1111/jav.00689) [DOI] [Google Scholar]
- 18.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later?. Trends Ecol. Evol. 16, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are deposited at Dryad: http://dx.doi.org/10.5061/dryad.f48f5.