Abstract
Purpose
We conducted a phase II study of pegylated interferon alfa-2a (PEG-IFN-α-2a) in patients with essential thrombocythemia (ET) and polycythemia vera (PV).
Patients and Methods
Seventy-nine patients (40 with PV and 39 with ET) have been treated. Median time from diagnosis to PEG-IFN-α-2a was 54 months in patients with PV and 33 months in patients with ET. Eighty-one percent of patients had received prior therapy. The first three patients received PEG-IFN-α-2a at 450 μg weekly. As a result of poor tolerance, this dose was decreased in a stepwise manner to a current starting dose of 90 μg weekly. Seventy-seven patients are evaluable and have been observed for a median of 21 months.
Results
The overall hematologic response rate was 80% in PV and 81% in ET (complete in 70% and 76% of patients, respectively). The JAK2V617F mutation was detected in 18 patients with ET and 38 patients with PV; sequential measurements by a pyrosequencing assay were available in 16 patients with ET and 35 patients with PV. The molecular response rate was 38% in ET and 54% in PV, being complete (undetectable JAK2V617F) in 6% and 14%, respectively. The JAK2V617F mutant allele burden continued to decrease with no clear evidence for a plateau. The tolerability of PEG-IFN-α-2a at 90 μg weekly was excellent.
Conclusion
PEG-IFN-α-2a resulted in remarkable clinical activity, high rates of molecular response, and acceptable toxicity in patients with advanced ET or PV. The ability of PEG-IFN-α-2a to induce complete molecular responses suggests selective targeting of the malignant clone.
INTRODUCTION
Essential thrombocythemia (ET) and polycythemia vera (PV) are myeloproliferative neoplasms (MPNs) associated with increased risk of thrombotic and hemorrhagic complications. In addition, a subset of these MPNs will evolve into myelofibrosis or progress to acute myeloid leukemia. The V617F somatic mutation at exon 14 of the JAK2 gene, which causes the substitution of phenylalanine for valine at position 617, is frequently detected in these MPNs.1–5 JAK2V617F is present in 95% of patients with PV and in 50% to 60% of patients with ET,1–5 is linked to an increased risk of thrombosis, and has been proposed as a marker to monitor treatment response.6–8
Current therapy for ET or PV is predicated on the risk of major hemorrhagic or thrombotic events.9,10 Whereas patients with low-risk ET are managed with low-dose aspirin, patients with low-risk PV are treated with phlebotomy and aspirin. For most patients with high-risk ET or PV, hydroxyurea remains the agent of choice and is the only proven therapy to reduce life-threatening thrombotic events.11 The leukemogenic potential of hydroxyurea, particularly in association with other cytoreductive agents, remains a concern when treating young patients for extended periods of time.12,13
Interferon alfa (IFN-α) reduces significantly the colony-forming ability of erythroid, granulocytic, and megakaryocytic progenitors in patients with MPNs,14 but excessive toxicity and inconvenient dosing schedules have prevented the generalized use of this agent. Pegylated IFN-α (PEG-IFN-α) has a superior pharmacokinetic and toxicity profile compared with standard IFN-α.15 In chronic myeloid leukemia, PEG-IFN-α-2a resulted in superior survival compared with IFN-α-2a.16 In a recent phase II study of PEG-IFN-α-2a involving 40 patients with PV with limited or no exposure to cytoreductive therapy, most patients achieved a complete hematologic response (CHR), and in 24% of patients, the JAK2V617F mutation became undetectable, suggesting that PEG-IFN-α-2a could eradicate the malignant clone. We sought to evaluate the activity of PEG-IFN-α-2a in patients with advanced ET or PV. The main objectives of this trial were to establish the efficacy and tolerability of PEG-IFN-α-2a in patients with these MPNs and the impact of this therapy on the dynamics of JAK2V617F mutation.
PATIENTS AND METHODS
Inclusion and Exclusion Criteria
Patients with a diagnosis of ET or PV (according to the PV Study Group 2005 criteria), either newly diagnosed or previously treated, were eligible provided they had an Eastern Cooperative Oncology Group performance status ≤ 2, serum creatinine less than 2.0 mg/dL, serum bilirubin ≤ 2.0× the upper limit of the normal range, and normal cardiac function and were off chemotherapy for at least 1 week before entering the study (hydroxyurea or anagrelide was allowed for up to 1 month after the study entry if judged necessary). Exclusion criteria included standard contraindications to the use of PEG-IFN-α (eg, history of psychiatric disorder, particularly depression, autoimmune disorders, hypersensitivity to IFN-α, ischemic retinopathy, systemic infections such as hepatitis B or C or HIV), pregnant or lactating women, history of severe heart disease, renal disease on hemodialysis, or seizure disorder requiring anticonvulsant therapy. All patients signed an informed consent approved by The University of Texas M. D. Anderson Cancer Center Institutional Review Board.
Treatment Schedule
The first three patients in the study received PEG-IFN-α-2a subcutaneously at 450 μg weekly. As a result of poor tolerance, the starting dose was decreased in a stepwise manner by 90-μg decrements based on tolerance (360 μg weekly, n = 3; 270 μg weekly, n = 19; 180 μg weekly, n = 26) to 90 μg weekly, which was subsequently used as a starting dose in the remainder of patients (n = 28) accrued to the study. PEG-IFN-α-2a was administered for as long as the patient obtained a clinical benefit. The dose was reduced based on toxicity or escalated in the absence of response and significant toxicity for 3 months. Therapy was maintained, if possible, in the event of grade 1 or 2 toxicity. In the event of persistent significant grade 2 toxicity or grade 3 or 4 toxicity, therapy was interrupted until resolution of the toxicity to grade 0 or 1 and resumed at the immediate lower dose level. Exceptionally, alternative dose schedules were allowed to achieve optimal benefit (eg, 90 μg every 2 weeks).
Patient Evaluation
Baseline studies included a complete physical examination, CBC count, comprehensive biochemistry panel (including liver function tests), pregnancy test, and bone marrow (BM) aspiration and biopsy with cytogenetics and molecular JAK2V617F testing. Follow-up evaluations included complete physical examination every 3 to 6 months; CBC and comprehensive biochemistry panel every other week for 4 weeks, then every 1 to 2 months for 12 months, and then every 3 months; thyroid function tests every 6 to 12 months; and BM aspiration and biopsy every 3 to 6 months, with JAK2V617F quantitation and cytogenetics when in complete remission. Toxicity was evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Response Criteria
CHR in patients with ET was defined as normalization of platelet counts (≤ 400 × 109/L) without the use of hydroxyurea or anagrelide and in the absence of thromboembolic events. Partial hematologic response required at least a 50% reduction in platelet counts (but still > 400 × 109/L). CHR in patients with PV was defined as normalization of hematocrit (< 45% in males and < 42% in females), WBC and platelet counts, and spleen size, without the use of phlebotomies, hydroxyurea, or anagrelide and in the absence of thromboembolic events. Partial hematologic response required the documentation of at least a 50% reduction in phlebotomy requirements or spleen size.
JAK2V617F was detected in DNA samples extracted from BM specimens by polymerase chain reaction and quantitated by a pyrosequencing assay (sensitivity 5%) developed at The University of Texas M. D. Anderson Cancer Center (Houston, TX). In the absence of mutation, patients were screened for exon 12 mutations by Sanger sequencing as previously described.17 Complete molecular response (CMR) required undetectable levels of JAK2V617F mutation; partial molecular response required ≥ 50% reduction of baseline JAK2V617F mutation level; and minor molecular response required 20% to 49% reduction of baseline JAK2V617F mutation level, as reported previously.7 Similar molecular response criteria have recently been proposed by the European LeukemiaNet.18
Statistical Considerations
This is a prospective, open-label, single-center, phase II trial of PEG-IFN-α-2a for patients with ET or PV. In each disease, a response rate ≥ 35% would be accepted as evidence of good efficacy to be further pursued in disease subsets in larger studies. Therefore, in each disease category, the study was to be stopped if responses were seen in less than one of 11 patients or less than two of 15 patients. Otherwise, it would accrue up to 80 patients (40 with ET and 40 with PV) to better define the response and toxicity profiles. In the total study group, a severe toxicity rate of ≥ 20% was not allowed. Therefore, the study would also stop if severe toxicity was observed in more than seven of 15, 11 of 30, or 15 of 45 patients. The statistical analysis for response rates was determined on an intent-to-treat basis.
RESULTS
Patient Characteristics
The patient characteristics of the study cohort are listed in Table 1. Seventy-nine patients (40 with PV and 39 with ET) have been accrued thus far. Eighty-one percent of patients with ET and PV had received some form of medical therapy (aspirin not accounted for) before enrollment, including standard IFN-α in 18% of patients with PV and 13% of patients with ET. More than 50% of patients with ET had received both hydroxyurea and anagrelide before enrollment. In accord with prior series, 95% of patients with PV and 46% of patients with ET carried JAK2V617F. The median allele burden was 64% (range, 18.5% to 94.6%) and 23% (range, 2.9% to 55.5%) for patients with PV and ET, respectively.
Table 1.
Baseline Demographics and Clinical Characteristics of Patients With PV or ET Receiving Therapy With PEG-IFN-α-2a
| Characteristic | PV (n = 40) |
ET (n = 39) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 53 | 50 | ||
| Range | 23-77 | 18-75 | ||
| Months from diagnosis | ||||
| Median | 54 | 33 | ||
| Range | 0-360 | 0-285 | ||
| JAK2V617F positive | 38 | 95 | 19 | 49 |
| JAK2V617F allele burden, % | ||||
| Median | 64 | 23 | ||
| Range | 18.5-94.6 | 2.9-55.5 | ||
| Abnormal karyotype | 2 | 5 | 7 | 18 |
| Splenomegaly | 5 | 13 | 0 | 0 |
| Spleen span below left costal margin for five patients, cm | ||||
| Median | 5 | |||
| Range | 4-12 | |||
| WBC, ×109/L | ||||
| Median | 12.1 | 7.2 | ||
| Range | 3.7-49.3 | 2.8-13.3 | ||
| Hemoglobin, g/dL | ||||
| Median | 14.4 | 13.2 | ||
| Range | 11.1-18.8 | 8.9-16.6 | ||
| Platelets, ×109/L | ||||
| Median | 491 | 698 | ||
| Range | 128-1,087 | 236-2,690 | ||
| No. of prior therapies | ||||
| Median | 1 | 2 | ||
| Range | 0-4 | 0-4 | ||
| Prior therapy | ||||
| Phlebotomy | 30 | 75 | 0 | 0 |
| Hydroxyurea | 18 | 45 | 27 | 69 |
| Anagrelide | 3 | 8 | 21 | 54 |
| Interferon alfa | 7 | 18 | 5 | 13 |
| Previously untreated | 6 | 15 | 9 | 23 |
Abbreviations: PV, polycythemia vera; ET, essential thrombocythemia; PEG-IFN-α-2a, pegylated interferon alfa-2a.
Hematologic Response to PEG-IFN-α-2a
The current median follow-up time is 21 months (range, 2 to 45 months). Seventy-seven of the 79 enrolled patients were evaluable for response (it is too soon to evaluate two patients). Similar rates of hematologic response were observed in patients with ET and PV (Fig 1A). The overall hematologic response was 80% for patients with PV and 81% for patients with ET, including a CHR rate of 70% and 76% for PV and ET, respectively (Fig 1B). Most responses were achieved within the first 3 months of therapy. The median time to CHR was 47 days (range, 3 to 350 days) for the whole cohort. Hematologic responses were durable. All responders were able to maintain their hematologic response.
Fig 1.
Response to pegylated interferon alfa-2a therapy. (A) Hematologic response in patients with essential thrombocythemia (ET) or polycythemia vera (PV). (B) Cumulative incidence of complete hematologic response (CHR) and partial hematologic response (PHR). (C) Cumulative incidence of complete molecular response (CMR), partial molecular response (PMR), minor molecular response (mMR), and overall molecular response. (D) Dynamics of molecular response for the six patients (two with ET and four with PV) who achieved a CMR.
Molecular Response and Dynamics of JAK2V617F Allele Burden
JAK2V617F was detected in 56 of the 79 patients treated (18 with ET and 38 with PV). In 51 of these patients (ET, n = 16; PV, n = 35), the JAK2V617F mutant allele burden was quantitated on at least two different occasions, and these patients were considered evaluable for molecular response (Table 2). The overall molecular response rate was 38% for ET and 54% for PV. The estimated cumulative incidence of molecular response with PEG-IFN-α-2a is shown in Figure 1C. Unlike hematologic responses, which were rapidly achieved in most patients, the achievement of meaningful molecular responses required exposure to PEG-IFN-α-2a for at least 6 months. In one (6%) of 16 evaluable patients with ET and five (14%) of 35 patients with PV, the JAK2V617F mutation became undetectable; these patients were considered to have achieved a CMR, which was confirmed in successive analyses in four of the six patients and has been ongoing for more than 12 months in three of the patients (Fig 1D). In addition, 13% of patients with ET and 29% of patients with PV achieved a partial molecular response, which was defined as a reduction of the mutant allele burden of at least 50% from the baseline.
Table 2.
Molecular Response Rates to PEG-IFN-α-2a Therapy
| Response | PV (n = 35) |
ET (n = 16) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| CMR (undetectable JAK2V617F) | 5 | 14 | 1 | 6 |
| PMR (≥ 50% JAK2V617F decrease) | 11 | 31 | 2 | 13 |
| Minor molecular response (20%-49% JAK2V617F decrease) | 3 | 9 | 3 | 19 |
| No response (0%-19% JAK2V617F decrease) | 16 | 46 | 10 | 62 |
Abbreviations: PEG-IFN-α-2a, pegylated interferon alfa-2a; PV, polycythemia vera; ET, essential thrombocythemia; CMR, complete molecular response; PMR, partial molecular response.
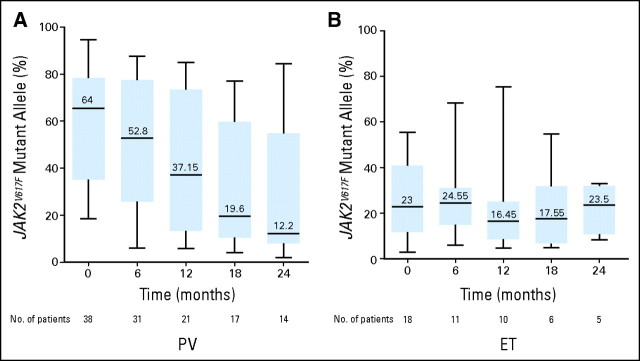
Twenty-three (66%) of 35 and two (13%) of 16 evaluable patients with PV and ET, respectively, were considered homozygous for the JAK2V617F mutation because they carried more than 50% mutant alleles. Notably, the dynamics of molecular response were different between the ET and PV cohorts (Fig 2). Whereas patients with PV experienced a marked decrease of the mutant allele burden from a median of 64% before start of PEG-IFN-α-2a therapy to 12% after 24 months (P = .0009), this decrement was not significant in patients with ET. The limited cohort size notwithstanding, these results suggest that PEG-IFN-α-2a may induce a deeper level of molecular response in patients with PV compared with ET and that the molecular responses induced by this agent are independent of the initial JAK2V617F mutant allele burden. Interestingly, the JAK2V617F mutant allele burden continues to decrease after a median follow-up of 21 months, with no clear evidence for a plateau.
Fig 2.
Dynamics of JAK2V617F mutant allele burden in patients with essential thrombocythemia (ET) or polycythemia vera (PV) who achieved molecular response (n = 25) during the first 24 months of pegylated interferon alfa-2a therapy. The JAK2V617F mutation was quantitated using a pyrosequencing assay. Black horizontal lines denote median values; black bars represent minimum and maximum values; and blue rectangular areas represent values included between the 25% and the 75% percentiles.
Toxicity
Ninety-six percent of patients developed some toxicity, but this was generally grade 1 or 2. The most frequent grade 3 or 4 toxicity was neutropenia, which occurred in 20% of patients (Table 3). Interestingly, the toxicity profile of PEG-IFN-α-2a was significantly more benign in the 28 patients who started therapy at 90 μg weekly (Table 4). This dosing schedule resulted in no grade 4 toxicities and a low rate of grade 3 toxicities, including neutropenia in two patients (7%) and infection, diarrhea, depression, and elevated liver function tests in one patient (4%) each. To date, 17 patients, accounting for 22% of the initial cohort, have been taken off study. Only eight patients (10%) experienced PEG-IFN-α-2a–related events necessitating discontinuation of therapy; events included retinal infiltrates (n = 1), autoimmune disease (n = 1), and multiple unacceptable grade 1 or 2 toxicities (n = 6). The remaining nine patients went off study for a variety of reasons, including relapse of a prior acute myeloid leukemia (n = 1), progression of disease to myelofibrosis (n = 1), inability to continue as a result of other comorbid conditions (n = 1), motor vehicle accident (n = 1), noncompliance (n = 2), withdrawal of consent (n = 1), and death as a result of unrelated causes (n = 2; heart disease and central pontine myelinolysis). Table 5 lists the current distribution of the 62 patients still on study according to the dose schedule of PEG-IFN-α-2a. Of note, 79% of patients are receiving PEG-IFN-α-2a at either 90 μg weekly or even less intense dose schedules with adequate response.
Table 3.
Overall Grade 3 or 4 Toxicity Associated With PEG-IFN-α-2a Therapy
| Toxicity | PV (n = 40) |
ET (n = 39) |
Overall |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 3 |
Grade 4 |
Grade 3 |
Grade 4 |
Grade 3 or 4 |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Neutropenia | 3 | 8 | 0 | 0 | 12 | 31 | 1 | 3 | 16 | 20 |
| Elevated LFTs | 2 | 5 | 0 | 0 | 3 | 8 | 0 | 0 | 5 | 6 |
| Fatigue | 1 | 3 | 0 | 0 | 2 | 5 | 0 | 0 | 3 | 4 |
| Pain | 1 | 3 | 0 | 0 | 1 | 3 | 0 | 0 | 2 | 3 |
| Infection | 1 | 3 | 0 | 0 | 1 | 3 | 0 | 0 | 2 | 3 |
| Depression | 1 | 3 | 0 | 0 | 1 | 3 | 0 | 0 | 2 | 3 |
| Diarrhea | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Mucositis | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 1 |
| Blurred vision | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Dizziness | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Anemia | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 1 |
Abbreviations: PEG-IFN-α-2a, pegylated interferon alfa-2a; PV, polycythemia vera; ET, essential thrombocythemia; LFTs, liver function tests.
Table 4.
Grade 3 or 4 Toxicity Observed in Patients Who Started PEG-IFN-α-2a at 90 μg Weekly
| Toxicity | PV (n = 12) |
ET (n = 16) |
Overall |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 3 |
Grade 4 |
Grade 3 |
Grade 4 |
Grade 3 or 4 |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Neutropenia | 0 | 0 | 0 | 0 | 2 | 13 | 0 | 0 | 2 | 7 |
| Diarrhea | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 | 1 | 4 |
| Elevated LFTs | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 | 1 | 4 |
Abbreviations: PEG-IFN-α-2a, pegylated interferon alfa-2a; PV, polycythemia vera; ET, essential thrombocythemia; LFTs, liver function tests.
Table 5.
Current PEG-IFN-α-2a Dose and Schedule in Patients Still on Study
| PEG IFN-α-2a Dose (μg weekly) | Current No. of Patients on Dose Level |
|---|---|
| 450 | 1 |
| 360 | 1 |
| 270 | 3 |
| 180 | 6 |
| 135 | 2 |
| 90 | 18 |
| 45 | 18 |
| 90 (every 2-3 weeks) | 7 |
| 45 (every 2-4 weeks) | 6 |
Abbreviation: PEG-IFN-α-2a, pegylated interferon alfa-2a.
DISCUSSION
Patients with ET or PV have a clinical course characterized by a thrombophilic state, which results in arterial and venous thromboses that greatly impact on morbidity and mortality. Current treatment strategies for these MPNs are directed toward normalization of blood cell count, thus minimizing the risk of vascular events, which is estimated to occur at a rate of 1.5% to 6% per year.6 For patients with high-risk ET or PV, the agent of choice to manage hyperviscosity is hydroxyurea in combination with low-dose aspirin.6,19 Hydroxyurea provides effective control of the hematocrit in approximately 80% of patients and of the platelet count (< 400 × 109/L) in 55% of patients20 and has been shown to decrease the JAK2V617F allele burden by at least 50% in 33% of patients.21 Nonetheless, thrombosis and the potential oncogenicity of prolonged exposure to hydroxyurea in young patients are of concern. Mounting evidence suggests that patients carrying the JAK2V617F mutation have a higher incidence of thrombotic events compared with JAK2V617F–negative patients.22 Therefore, nonleukemogenic therapies that reduce the JAK2V617F allele burden are appealing.
IFN-α is a nonleukemogenic agent active in MPNs. In a study recently reported by French investigators, 37 patients with (mostly) newly diagnosed PV were treated with PEG-IFN-α-2a and observed for a median of 31 months.23 PEG-IFN-α-2a therapy was associated with a CHR rate of 78%, including 24% of patients in whom the JAK2V617F mutation, present in most patients at diagnosis, became undetectable. Our study complements the French study because it differs in two important aspects. First, we extended the scope of our investigation not only to patients with PV but also to patients with ET, an MPN generally associated with low disease-related mortality rates but subject to a similar vascular risk as patients with PV. Second, most patients had received MPN-directed therapy for prolonged periods of time before starting PEG-IFN-α-2a. The CHR rate was 76% for patients with ET and 70% for patients with PV; the molecular response rate was 38% for ET and 54% for PV. Both the French study and our study used a stringent set of response criteria compared with prior studies involving patients with ET or PV. A complete response not only required the normalization of hematocrit and platelet counts, but also the resolution of splenomegaly and the normalization of WBC count. The latter had frequently been overlooked as a response criterion but has been found to be an important risk factor for the development of thrombosis by several independent groups.24–26 It is critical for future studies to adopt stringent response criteria like the ones used herein or the recently proposed criteria by the European LeukemiaNet, which also include spleen size assessment by computed tomography or ultrasound and evaluation of disease-related symptoms,18 neither of which was available in our patients.
Among patients who achieved a molecular response, the JAK2V617F mutation became undetectable in 6% of those with ET and in 14% of those with PV. This level of response had never been reported with other forms of IFN-α. The exposure to PEG-IFN-α-2a required to achieve a molecular response was significantly longer than that necessary to attain a hematologic response, the latter being achieved almost invariably within 3 months from the start of therapy. A significant proportion of patients with PV (66%) were homozygous for the JAK2V617F mutation, likely as a consequence of mitotic recombination at chromosome 9p,1–5 but this phenomenon was more infrequent in patients with ET (13%).27 The dynamics of molecular response seemed to differ in ET and PV, with the latter patients experiencing, as a group, a statistically significant decrease in JAK2V617F allele burden. Although the limited number of patients precludes drawing definitive conclusions, it seems that a higher allele burden may render JAK2V617F–positive PV precursors more sensitive to PEG-IFN-α-2a than JAK2V617F–positive ET precursors. However, one should also note that our patients with ET seemed to have more aggressive disease than usual, with many patients carrying cytogenetic abnormalities and more that 50% being previously treated with both hydroxyurea and anagrelide.
Molecular responses, even after a median follow-up time of 21 months, continue to improve. Long-term follow-up of this cohort of patients will assess whether this trend is maintained beyond the 2-year landmark. None of the patients in whom the mutation became undetectable had a molecular relapse. Collectively, these results suggest that PEG-IFN-α-2a could eradicate JAK2V617F–positive BM progenitors in patients with ET or PV. This is supported by the recent report by Kiladjian et al23,28 in patients with PV in which seven patients achieved a CMR and five patients maintained such response even after PEG-IFN-α-2a discontinuation. In one of those five JAK2V617F–positive patients who achieved CMR, no evidence of JAK2V617F–positive marrow erythroid progenitor cells was found, either after erythropoietin stimulation or in endogenous erythroid colonies. In the remaining four patients, mutant progenitors were detected at low frequencies, suggesting the ability of some progenitor cells to generate endogenous erythroid colonies in a JAK2V617F–independent manner.28 The significance of these findings, both biologically and practically (possible testing for minimal residual disease and possible stopping of therapy in patients with negative result), is currently unknown. It is tempting to speculate that the results reported are a direct consequence of the ability of PEG-IFN-α-2a to suppress the proliferation of hematopoietic progenitors, inhibit BM fibroblast progenitor cells, and decrease the levels of profibrogenic cytokines in BM.29–32 In addition, several recently identified PV-associated antigens may represent targets against which PEG-IFN-α-2a may direct humoral and cellular immune responses.33
Therapy with PEG-IFN-α-2a, unlike standard IFN-α, is relatively well tolerated, with neutropenia being the most frequent grade 3 to 4 toxicity. The discontinuation rate caused by PEG-IFN-α-2a–related toxicity was only 10% and was mostly seen with the higher initial doses used in the study (180 to 450 μg weekly). An initial dose of 450 μg weekly was selected based on a dose-escalation study of PEG-IFN-α-2a in chronic myeloid leukemia.34 In our study, however, this dose resulted in an unacceptable rate of toxicity and was decreased by 90-μg decrements until the starting dose was set at 90 μg weekly. This dose resulted in minimal grade 3 to 4 toxicities and high activity, and most patients are currently receiving PEG-IFN-α-2a at 90 μg weekly or even less intense dose schedules with excellent efficacy.
In conclusion, PEG-IFN-α-2a results in high rates of hematologic and molecular responses in patients with advanced, previously treated ET or PV, with an excellent toxicity profile when the drug is administered at 90 μg weekly. The JAK2V617F mutation becomes undetectable in a subset of patients receiving PEG-IFN-α-2a, suggesting a preferential effect on the malignant clone. Longer follow-up of this cohort of patients will further establish the long-term clinical implications of eliminating JAK2V617F–positive clones, such as the exciting possibility of safely discontinuing therapy in patients in complete molecular response.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00452023.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Srdan Verstovsek, Roche Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Alfonso Quintás-Cardama, Srdan Verstovsek
Provision of study materials or patients: Sherry Pierce, Mary Ann Richie, Srdan Verstovsek
Collection and assembly of data: Alfonso Quintás-Cardama, Srdan Verstovsek
Data analysis and interpretation: Alfonso Quintás-Cardama, Hagop Kantarjian, Taghi Manshouri, Rajyalakshmi Luthra, Zeev Estrov, Jorge Cortes, Srdan Verstovsek
Manuscript writing: Alfonso Quintás-Cardama, Srdan Verstovsek
Final approval of manuscript: Alfonso Quintás-Cardama, Hagop Kantarjian, Taghi Manshouri, Rajyalakshmi Luthra, Zeev Estrov, Sherry Pierce, Mary Ann Richie, Gautam Borthakur, Marina Konopleva, Jorge Cortes, Srdan Verstovsek
REFERENCES
- 1.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Zhao R, Xing S, Li Z, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landolfi R, Di Gennaro L, Falanga A. Thrombosis in myeloproliferative disorders: Pathogenetic facts and speculation. Leukemia. 2008;22:2020–2028. doi: 10.1038/leu.2008.253. [DOI] [PubMed] [Google Scholar]
- 7.Kiladjian JJ, Cassinat B, Turlure P, et al. High molecular response rate of polycythemia vera patients treated with pegylated interferon alpha-2a. Blood. 2006;108:2037–2040. doi: 10.1182/blood-2006-03-009860. [DOI] [PubMed] [Google Scholar]
- 8.Jones AV, Silver RT, Waghorn K, et al. Minimal molecular response in polycythemia vera patients treated with imatinib or interferon alpha. Blood. 2006;107:3339–3341. doi: 10.1182/blood-2005-09-3917. [DOI] [PubMed] [Google Scholar]
- 9.Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224–2232. doi: 10.1200/JCO.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 10.Cortelazzo S, Viero P, Finazzi G, et al. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J Clin Oncol. 1990;8:556–562. doi: 10.1200/JCO.1990.8.3.556. [DOI] [PubMed] [Google Scholar]
- 11.Finazzi G, Barbui T. Evidence and expertise in the management of polycythemia vera and essential thrombocythemia. Leukemia. 2008;22:1494–1502. doi: 10.1038/leu.2008.177. [DOI] [PubMed] [Google Scholar]
- 12.Sterkers Y, Preudhomme C, Lai JL, et al. Acute myeloid leukemia and myelodysplastic syndromes following essential thrombocythemia treated with hydroxyurea: High proportion of cases with 17p deletion. Blood. 1998;91:616–622. [PubMed] [Google Scholar]
- 13.Finazzi G, Ruggeri M, Rodeghiero F, et al. Second malignancies in patients with essential thrombocythaemia treated with busulphan and hydroxyurea: Long-term follow-up of a randomized clinical trial. Br J Haematol. 2000;110:577–583. doi: 10.1046/j.1365-2141.2000.02188.x. [DOI] [PubMed] [Google Scholar]
- 14.Kiladjian JJ, Chomienne C, Fenaux P. Interferon-alpha therapy in bcr-abl-negative myeloproliferative neoplasms. Leukemia. 2008;22:1990–1998. doi: 10.1038/leu.2008.280. [DOI] [PubMed] [Google Scholar]
- 15.Quintás-Cardama A, Kantarjian HM, Giles F, et al. Pegylated interferon therapy for patients with Philadelphia chromosome-negative myeloproliferative disorders. Semin Thromb Hemost. 2006;32:409–416. doi: 10.1055/s-2006-942761. [DOI] [PubMed] [Google Scholar]
- 16.Lipton JH, Khoroshko N, Golenkov A, et al. Phase II, randomized, multicenter, comparative study of peginterferon-alpha-2a (40 kD) (Pegasys) versus interferon alpha-2a (Roferon-A) in patients with treatment-naive, chronic-phase chronic myelogenous leukemia. Leuk Lymphoma. 2007;48:497–505. doi: 10.1080/10428190601175393. [DOI] [PubMed] [Google Scholar]
- 17.Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barosi G, Birgegard G, Finazzi G, et al. Response criteria for essential thrombocythemia and polycythemia vera: Result of a European LeukemiaNet (ELN) consensus conference. Blood. 2009;113:4829–4833. doi: 10.1182/blood-2008-09-176818. [DOI] [PubMed] [Google Scholar]
- 19.Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353:33–45. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]
- 20.Najean Y, Rain JD. Treatment of polycythemia vera: The use of hydroxyurea and pipobroman in 292 patients under the age of 65 years. Blood. 1997;90:3370–3377. [PubMed] [Google Scholar]
- 21.Besses C, Alvarez-Larran A, Martinez-Aviles L, et al. Major hematological response is the main factor for achieving a major molecular response in JAK2V617F-positive essential thrombocythemia and polycythemia vera patients treated with hydroxyurea. Blood. 2008;112:660. abstr. [Google Scholar]
- 22.Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: A prospective study. Lancet. 2005;366:1945–1953. doi: 10.1016/S0140-6736(05)67785-9. [DOI] [PubMed] [Google Scholar]
- 23.Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112:3065–3072. doi: 10.1182/blood-2008-03-143537. [DOI] [PubMed] [Google Scholar]
- 24.Gangat N, Wolanskyj AP, McClure RF, et al. Risk stratification for survival and leukemic transformation in essential thrombocythemia: A single institutional study of 605 patients. Leukemia. 2007;21:270–276. doi: 10.1038/sj.leu.2404500. [DOI] [PubMed] [Google Scholar]
- 25.Kiladjian JJ, Gardin C, Renoux M, et al. Long-term outcomes of polycythemia vera patients treated with pipobroman as initial therapy. Hematol J. 2003;4:198–207. doi: 10.1038/sj.thj.6200250. [DOI] [PubMed] [Google Scholar]
- 26.Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109:2446–2452. doi: 10.1182/blood-2006-08-042515. [DOI] [PubMed] [Google Scholar]
- 27.Jones AV, Kreil S, Zoi K, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–2168. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 28.Kiladjian J, Dupont S, Cassinat B, et al. Can peg-interferon (IFN) α-2a eradicate JAK2V617F–positive bone marrow progenitors in polycythemia vera (PV)? Blood. 2008;112 abstr 659. [Google Scholar]
- 29.Carlo-Stella C, Cazzola M, Gasner A, et al. Effects of recombinant alpha and gamma interferons on the in vitro growth of circulating hematopoietic progenitor cells (CFU-GEMM, CFU-Mk, BFU-E, and CFU-GM) from patients with myelofibrosis with myeloid metaplasia. Blood. 1987;70:1014–1019. [PubMed] [Google Scholar]
- 30.Castello G, Lerza R, Cerruti A, et al. The in vitro and in vivo effect of recombinant interferon alpha-2a on circulating haemopoietic progenitors in polycythaemia vera. Br J Haematol. 1994;87:621–623. doi: 10.1111/j.1365-2141.1994.tb08324.x. [DOI] [PubMed] [Google Scholar]
- 31.Dudley JM, Westwood N, Leonard S, et al. Primary polycythaemia: Positive diagnosis using the differential response of primitive and mature erythroid progenitors to erythropoietin, interleukin 3 and alpha-interferon. Br J Haematol. 1990;75:188–194. doi: 10.1111/j.1365-2141.1990.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 32.Hino M, Futami E, Okuno S, et al. Possible selective effects of interferon alpha-2b on a malignant clone in a case of polycythemia vera. Ann Hematol. 1993;66:161–162. doi: 10.1007/BF01697629. [DOI] [PubMed] [Google Scholar]
- 33.Xiong Z, Yan Y, Liu E, et al. Novel tumor antigens elicit anti-tumor humoral immune reactions in a subset of patients with polycythemia vera. Clin Immunol. 2007;122:279–287. doi: 10.1016/j.clim.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talpaz M, Rakhit A, Rittweger K, et al. Phase I evaluation of a 40-kDa branched-chain long-acting pegylated IFN-alpha-2a with and without cytarabine in patients with chronic myelogenous leukemia. Clin Cancer Res. 2005;11:6247–6255. doi: 10.1158/1078-0432.CCR-05-0882. [DOI] [PubMed] [Google Scholar]