Abstract
Purpose
To examine the efficacy and safety of the combination of alemtuzumab and pentostatin in patients with T-cell neoplasms.
Patients and Methods
We treated 24 patients with a variety of T-cell leukemias and lymphomas with a combination of alemtuzumab 30 mg intravenously (IV) three times weekly for up to 3 months and pentostatin 4 mg/m2 IV weekly for 4 weeks followed by alternate weekly administration for up to 6 months. Prophylactic antibiotics including antiviral, antifungal, and antibacterial agents were administered during the treatment and for 2 months after its completion.
Results
The median age of patients was 57 years (range, 21 to 79 years). Eight patients were previously untreated, and 16 had a median of two prior therapies (range, one to six regimens). Thirteen patients responded to treatment (11 complete responses [CRs] and two partial responses), for an overall response rate of 54%. The median response duration was 19.5 months. Monoclonal T-cell receptor chain gene rearrangements were detected by polymerase chain reaction in bone marrow of 20 of 22 evaluable patients and became negative in five of seven evaluable patients in CR. Opportunistic infections caused by pathogens associated with severe T-cell dysfunction were common.
Conclusion
The combination of alemtuzumab and pentostatin is feasible and effective in T-cell neoplasms. Although infections, including cytomegalovirus reactivation, are a concern, they may be minimized with adequate prophylactic antibiotic therapy.
INTRODUCTION
T-cell malignancies are uncommon disorders representing approximately 10% to 15% of lymphoid neoplasms in adults.1 They include diverse entities such as T-cell acute lymphoblastic leukemia (T-ALL), mature leukemias such T-cell prolymphocytic leukemia (T-PLL) and T-cell large granular lymphocytic leukemia (T-LGL), extranodal tumors such as mycosis fungoides and hepatosplenic T-cell lymphoma (HSTCL), nodal disorders such as peripheral T-cell lymphoma (PTCL), and neoplasms with mixed patterns such as adult T-cell leukemia/lymphoma (ATLL). With the exception of anaplastic large-cell lymphoma and T-LGL, T-cell malignancies generally have an aggressive clinical course and a poor prognosis.
T-PLL is characterized by proliferation of small- to medium-size prolymphocytes with a mature post-thymic phenotype.2 It is a rare aggressive leukemia that responds poorly to chemotherapy, with a median survival time of 7.5 months.3,4 Nucleoside analogs such as pentostatin have been used with limited success, with a 46% response rate in one study.5 Dearden at al6 reported an overall response rate of 76%, with a 60% complete response (CR) rate and 16% partial response (PR) rate in 39 patients treated with alemtuzumab, including two previously untreated patients. These responses were durable, with a median disease-free survival time of 7 months. Survival was longer in patients achieving a CR, with nine patients remaining alive up to 29 months after the completion of therapy.6 Keating at al7 treated 76 patients with alemtuzumab and reported a 50% response rate, including a 37.5% CR rate. The median duration of CR was 8.7 months. Toxicity was acceptable, including grade 3 and 4 hematologic toxicity in 13% and infection events in 13% of patients.7
Peripheral and nodal T-cell lymphomas are a diverse group of diseases.8 Using purine analogs, such as pentostatin and gemcitabine, response rates of 25% to 60% have been reported in patients with relapsed disease.9,10 Enblad et al11 treated 14 patients with relapsed PTCL with alemtuzumab and reported an overall response rate of 36%, including 21% CRs. In another study of alemtuzumab in patients with relapsed PTCL, a CR rate of 33% was reported.12 Lundin et al13 also demonstrated the activity of alemtuzumab in advanced mycosis fungoides/Sézary syndrome. HSTCLs are extranodal neoplasms with an incomplete cytotoxic T-cell phenotype with sinusoidal liver, spleen, and low-level bone marrow infiltration.14,15 The course of the disease is highly aggressive, with poor prognosis and a median survival time of 16 months.15 Current therapies are ineffective in most patients.4
The incidence of ATLL is highest in areas where human T-cell lymphotropic virus I (HTLV-I) infection is endemic (ie, southern Japan and the Caribbean basin), but it also occurs sporadically in Africa, Central and South America, and the Southeast United States.16 ATLL occurs in only 2% to 4% of HTLV-I–infected individuals. It is an aggressive disease with poor prognosis; most patients survive for less than 1 year.16 Therapy with conventional chemotherapy has achieved CR rates of 20% to 45%, with a short duration usually lasting a few months.17,18 Regimens containing pentostatin have been associated with a significant response rate.19
The low incidence and lack of effective therapy for most patients with these disorders prompted us to develop a clinical trial combining two of the most effective agents in treating T-cell neoplasms. Studies in B-cell lymphoproliferative disorders have suggested that combinations of nucleoside analogs and monoclonal antibodies can effectively increase the response rate and improve the relapse-free and overall survival. Here, we report our experience with the combination of pentostatin and alemtuzumab in patients with T-cell neoplasms.
PATIENTS AND METHODS
Study Objectives
The study was designed to examine the efficacy and safety of the concurrent administration of alemtuzumab and pentostatin in patients with T-cell malignancies. The study end points were the efficacy of alemtuzumab and pentostatin in achieving CR or PR and the toxicity of the combination.
Eligibility
Patients were eligible if they were 18 years or older and had newly diagnosed or relapsed or refectory T-PLL, PTCL, HSTCL, or extranodal natural killer/T-cell lymphoma. Patients with advanced mycosis fungoides, T-LGL, ATLL, and T-ALL were enrolled onto the study only if they had failed more standard treatments and had no other therapeutic options. Diagnoses were based on the revised WHO classification,20 using a combination of morphologic, flow cytometric, and immunohistochemical techniques along with serologic evaluation for HTLV-I to rule out ATLL in the appropriate patients. Patients had to have a performance status of 0 to 2; no active ongoing infections; and adequate renal, hepatic, and cardiac function, with a serum creatinine ≤ 2.0 mg/dL, calculated creatinine clearance (CC) ≥ 40 mL/min, bilirubin ≤ 3.0 mg/dL, serum transaminases ≤ 3× the upper limit of normal, and a cardiac left ventricular ejection fraction ≥ 30%. All patients provided written informed consent. The study was approved by the Institutional Review Board of The University of Texas M. D. Anderson Cancer Center.
Treatment Regimen
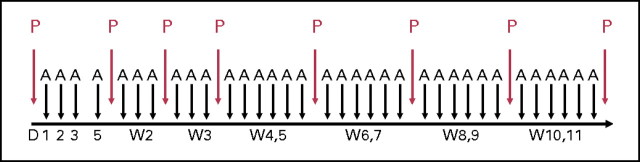
Patients received pentostatin 4 mg/m2 intravenously (IV) weekly for 4 weeks then every 2 weeks until they achieved a CR or best response or for up to 10 more doses (total of 14 doses). Hydration with 500 to 1,000 mL of normal saline before and after each dose of pentostatin was mandatory. One hour after completion of the first dose of pentostatin, alemtuzumab was started. In the first week of therapy, alemtuzumab was administered at a dose of 3 mg IV on the first day and, in the absence of intolerable adverse effects, 10 mg IV on the second day and 30 mg IV on the third day. Alemtuzumab was then administered at a dose of 30 mg IV three times weekly until achievement of CR or best response or for up to a total of 3 months (Fig 1). Pentostatin dosages were adjusted according to the CC; if the CC was ≥ 60, 40 to 59, or 35 to 39 mL/min, then the dose of pentostatin was adjusted to 4, 3, or 2 mg/m2, respectively. If the CC was less than 35 mL/min, pentostatin was held until the CC was ≥ 35 mL/min.
Fig 1.
Treatment regimen. Pentostatin (P) was administered weekly for 4 weeks, then every 2 weeks for up to 14 doses. After the initial 3-day dose escalation, alemtuzumab (A) was administered three times weekly for up to 3 months. D, day; W, week.
Prophylactic antibiotic therapy with antiviral agents (including acyclovir, valacyclovir, or valganciclovir),21 antifungal agents (including fluconazole, itraconazole, or voriconazole), and anti-Pneumocystis carinii pneumonia agents (trimethoprim/sulfamethoxazole or appropriate alternatives) was mandatory during the period of therapy and for a minimum of 2 months after its completion.
Response Criteria
CR was defined as the disappearance of all evidence of disease detectable by morphology of peripheral blood and bone marrow and computer tomography scanning at the end of therapy, if indicated. Hematologic parameters required an absolute neutrophil count ≥ 1.5 × 109/L, hemoglobin ≥ 12 g/dL for males and ≥ 11 g/dL for females, and platelets ≥ 100 × 109/L without growth factor or transfusion support. PR was defined as 50% or more reduction in detectable disease, but short of CR, maintained for 1 month or at least 50% reduction of sum of the products of the diameter of all lesions for 1 month. For patients with CTCL, a 50% reduction in the Sézary cell count and a 50% improvement in skin score were considered criteria for PR.
Assessment of Response
Upon enrollment onto the study, all patients had a complete history and physical, CBC count, complete metabolic panel, bone marrow aspirate and biopsy with cytogenetic and immunophenotypic studies including evaluation of CD52 expression, T-cell receptor (TCR) gene rearrangement analysis by polymerase chain reaction (PCR) using a multicolor capillary electrophoretic technique,22 skin biopsy if indicated, chest x-ray and computer tomography scans if indicated, assessment of left ventricular function, and assessment of CD4/CD8 lymphocyte subsets, immunoglobulin levels, and cytomegalovirus (CMV) status by antigenemia.
During the study, all patients had a CBC and complete metabolic panel weekly for 4 weeks and then every 2 to 4 weeks, CMV antigenemia weekly, and assessment of CD4/CD8 lymphocyte subsets and immunoglobulin level at best response. To assess tumor levels, bone marrow biopsy and aspirate with flow cytometric characterization was performed every 3 months, and TCR-γ PCR in blood or bone marrow was performed to assess best response.
Toxicity Assessment
Hematologic and nonhematologic toxicity was graded according to the National Cancer Institute Common Terminology Criteria of Adverse Events (version 3). In view of the prolonged lympholytic action of both agents, infectious complications were evaluated during and up to 6 months after the cessation of the regimen. We followed established institutional protocols for work-up and therapy of presumed or documented infections. CMV antigenemia was performed routinely in patients with a new febrile episode, but surveillance was conducted on a weekly basis in asymptomatic patients while on therapy.
Statistics
The trial was designed to have a maximum sample size of 60 patients. With 60 patients, the SE of the estimated overall response rate is 6%, assuming that the overall response rate is approximately 50%. However, because of the rarity of T-cell neoplasms, we accrued 24 patients in an approximately 4-year period, and the trial was terminated. With a sample size of 24 patients, the SE of the estimated overall response rate is 10%. Objective response and grade 3 and 4 opportunistic infections were monitored simultaneously using the Bayesian approach of Thall et al.23 The Kaplan-Meier method was used to estimate disease-free survival and overall survival.
RESULTS
Patient Characteristics
From October 2004 to May 2008, 24 patients were enrolled onto the study, including 13 patients with T-PLL, three patients with PTCL, three patients with HSTCL, two patients with T-ALL, two patients with T-LGL, and one patient with ATLL. The baseline characteristics of these patients are listed in Table 1. The median age of patients was 57 years (range, 21 to 79 years), median baseline WBC count was 24.8 × 109/L (range, 0.6 to 279.5 × 109/L), median platelet count was 120 × 109/dL (range, 9 to 285 × 109/dL), and median serum β2-microglobulin was 3.9 mg/L (range, 1.7 to 10.8 mg/L). Twenty-one patients had bone marrow involvement at presentation; nine patients had splenomegaly, and eight had lymphadenopathy. Presence of extramedullary disease was confirmed by biopsy/histopathology in nine patients and included lymph nodes (n = 5), skin (n = 5), liver (n = 1), gastric mucosa (n = 1), and spleen (n = 1). All of the tumors expressed CD52 by flow cytometric or immunohistochemistry evaluation of involved tissue with a median bone marrow expression of 98% (range, 37% to 100%). Monoclonal TCR-β or TCR-γ gene rearrangements were identified by PCR in bone marrow specimens of 20 of 22 evaluable patients.
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Characteristic | Patients (N = 24) |
|
|---|---|---|
| Median | Range | |
| Age, years | 57 | 21-79 |
| Sex, No. of patients | ||
| Male | 14 | |
| Female | 10 | |
| Performance status, No. of patients | ||
| 0-1 | 22 | |
| 2 | 2 | |
| Prior therapy | ||
| No. of patients | 16 | |
| No. of regimens | 2 | 1-6 |
| β2-microglobulin, mg/L | 3.9 | 1.7-10.8 |
| WBC ×109/dL | 24.8 | 0.6-279.5 |
| Platelets ×109/dL | 120 | 9-285 |
| Bone marrow involvement, No. of patients | 21 | |
| Lymphadenopathy, No. of patients | 8 | |
| Splenomegaly | ||
| No. of patients | 9 | |
| Size, cm | 1-10 | |
| Hepatomegaly | ||
| No. of patients | 3 | |
| Size, cm | 1-5 | |
Sixteen patients had received prior therapy (median, two prior regimens; range, one to six prior regimens). Three patients with PTCL and two patients with T-ALL had achieved prior responses to combination chemotherapy (range, 1.5 to 2.1 years) but were refractory to their immediate prior treatments. Among the 13 patients with T-PLL, eight patients had prior treatment with a median of two regimens (range, one to six regimens); the agents used included gemcitabine, fludarabine, single-agent pentostatin or alemtuzumab, and combination chemotherapy with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone. Two patients with T-PLL had received prior single-agent alemtuzumab with CRs lasting 1 and 2 years. None of the other prior regimens (including single-agent pentostatin in three patients) had produced responses of significant duration in the remaining six patients with T-PLL. Previously treated patients with other histologies (ATLL and T-LGL) were refractory to their immediate prior treatment.
Response
Thirteen patients (54%) achieved a response (11 CRs and two PRs), including nine of 13 patients with T-PLL, one of one patient with ATLL, two of three patients with HSTCL, and one of two patients with T-LGL (Table 2). None of the two patients with T-ALL and none of the three patients with PTCL achieved a response. The median duration of follow-up was 31 months (range, 2 to 43 months). The median overall survival and progression-free survival times for all patients were 9 months and 5.3 months, respectively. The median event-free survival duration for the responding patients was 19.5 months (Fig 2). The overall response rate for patients with T-PLL was 69%, including a CR rate of 62%. The median overall survival and progression-free survival times for patients with T-PLL were 10.2 months and 7.8 months, respectively. The median event-free survival time for the responding patients with T-PLL was 20 months (Fig 3). Of the three patients with HSTCL, two with no prior therapy achieved a CR, and one who was heavily pretreated did not respond. The single patient with ATLL had a CR.24 Monoclonal TCR chain rearrangements became negative in five of seven evaluable patients in CR. Only three patients had an allogeneic stem-cell transplantation after achieving a CR, including two patients with T-PLL and one patient with HSTCL.
Table 2.
Best Response by Tumor Type
| Diagnosis | No. of Patients | CR |
PR |
OR |
|||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| T-PLL | 13 | 8 | 62 | 1 | 8 | 9 | 69 |
| HSTCL | 3 | 2 | 67 | 0 | 0 | 2 | 67 |
| PTCL | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| T-LGL | 2 | 0 | 0 | 1 | 50 | 1 | 50 |
| T-ALL | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| ATLL | 1 | 1 | 100 | 0 | 0 | 1 | 100 |
| Total | 24 | 11 | 46 | 2 | 8 | 13 | 54 |
| Previously treated | 16 | 7 | 43 | 1 | 6 | 8 | 50 |
| Untreated | 8 | 4 | 50 | 1 | 12.5 | 5 | 62 |
Abbreviations: CR, complete response; PR, partial response; OR, overall response; T-PLL, T-cell prolymphocytic leukemia; HSTCL, hepatosplenic T-cell lymphoma; PTCL, nodal peripheral T-cell lymphoma; T-LGL, T-cell large granular lymphocytic leukemia; ATLL, adult T-cell leukemia/lymphoma; T-ALL, T-cell acute lymphoblastic leukemia.
Fig 2.
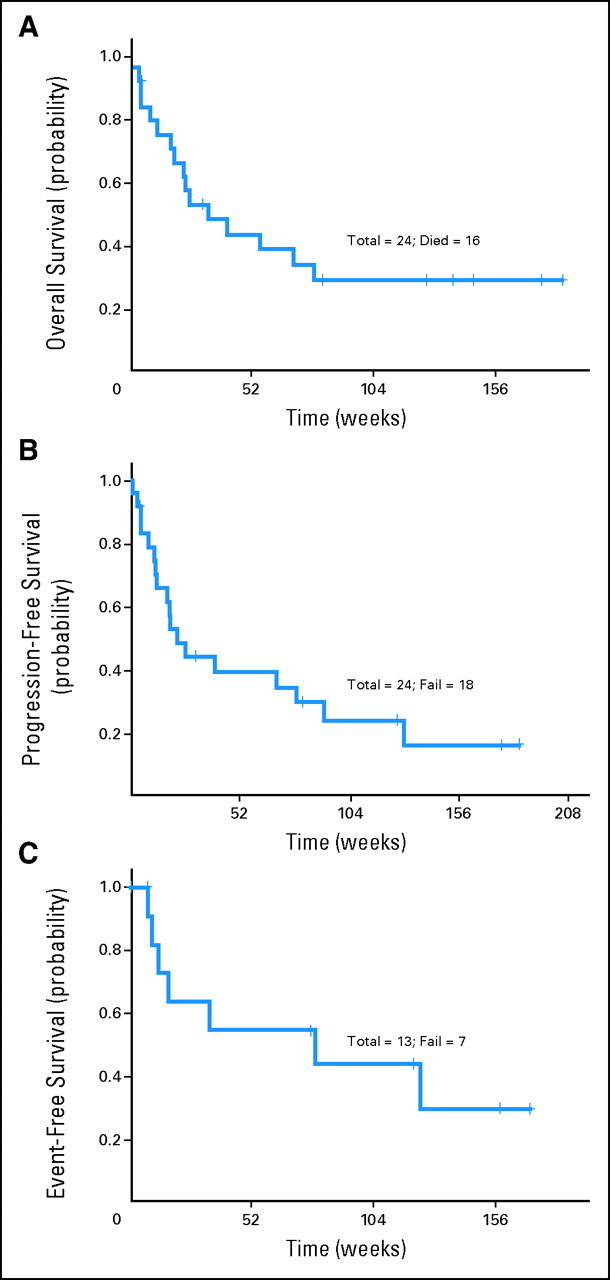
(A) Overall survival for the entire group. (B) Progression-free survival for the entire group. (C) Event-free survival for responding patients.
Fig 3.
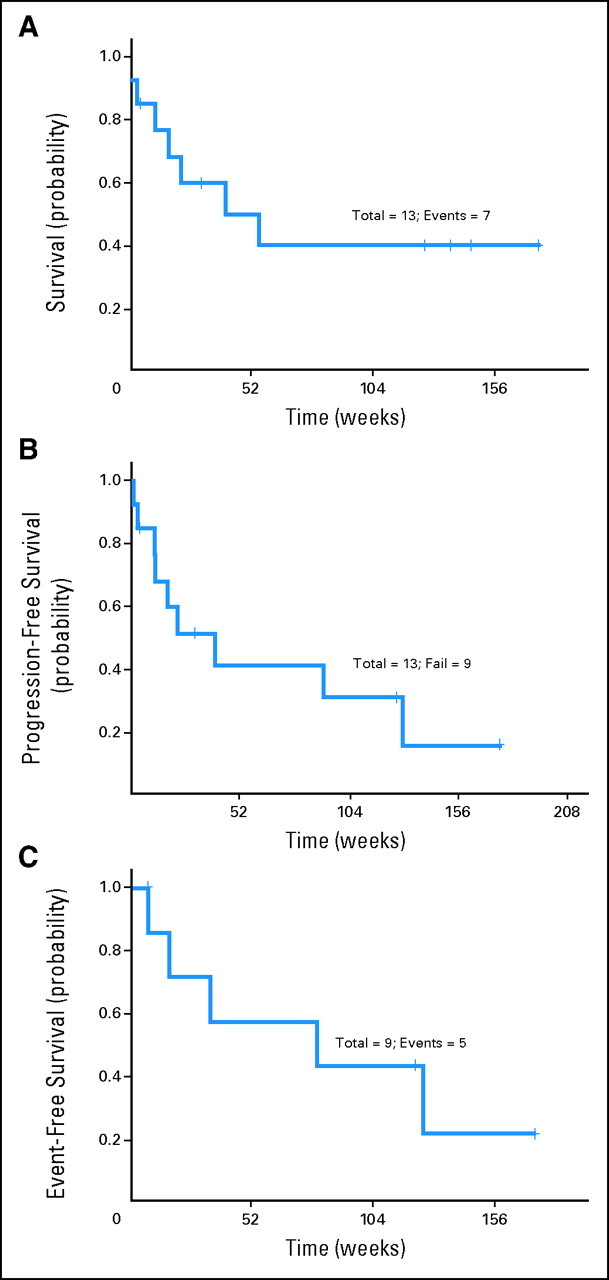
(A) Overall survival for patients with T-cell prolymphocytic leukemia (T-PLL). (B) Progression-free survival for patients with T-PLL. (C) Event-free survival for responding patients with T-PLL.
Toxicity
The main adverse effects of the pentostatin plus alemtuzumab combination were severe (grade 3 or 4) infections (Table 3). Only six patients (25%) had no infectious complications. The median peripheral-blood absolute CD4+ and CD8+ T-cell counts before treatment were 400 and 650/μL, respectively (range, 12 to 127,413/μL and 13 to 108,538/μL, respectively). Three to 6 months after therapy, the median CD4+ and CD8+ peripheral-blood T-cell counts decreased to 81 and 56/μL, respectively (range, 3 to 304/μL and 0 to 284/μL, respectively). Opportunistic infections caused by pathogens associated with severe T-cell dysfunction were common. CMV reactivation was particularly frequent, encountered in nine (38%) of 24 patients. One pancytopenic patient had human herpes virus type 6 detected by PCR in the bone marrow, and another patient developed cystitis with BK virus detected by PCR in the urine. Most patients (seven of nine patients) had rapid resolution of CMV antigenemia with pre-emptive ganciclovir or foscarnet. CMV disease (eg, colitis or pneumonia) was not documented. No patient developed varicella zoster infection. Viral pneumonia (influenza in two patients, respiratory syncytial virus in two patients, and parainfluenza and herpes simplex virus in one patient each) was seen in six patients. Opportunistic mycoses (invasive mold infection in four patients and fungemia in one patient), Mycobacterium avium complex pulmonary infection (two patients) and disseminated nocardiosis (one patient) were common. No patient developed Pneumocystis jiroveci pneumonia.
Table 3.
Toxicity
| Adverse Event | Grade 1 or 2 |
Grade 3 or 4 |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| CMV reactivation | 9 | 38 | ||
| HSV pneumonia | 1 | 4 | ||
| RSV pneumonia | 1 | 4 | ||
| Parainfluenza pneumonia | 2 | 8 | ||
| Bacterial pneumonia | 3 | 12 | ||
| Sepsis | 4 | 16 | ||
| Urinary tract infection | 1 | 4 | ||
| Epididymitis | 1 | 4 | ||
| Thrombocytopenia | 9 | 37 | ||
| Neutropenia | 11 | 45 | ||
| Anemia | 2 | 8 | ||
| Fever of unknown origin | 4 | 16 | ||
| Hepatic | 3 | 12 | ||
| Renal | 4 | 16 | ||
| Tumor lysis syndrome | 1 | 4 | ||
| Infusion reactions | 9 | 37 | 0 | 0 |
Abbreviations: CMV, cytomegalovirus; HSV, herpes simplex virus; RSV, respiratory syncytial virus.
Bacterial pneumonia (typically caused by a Gram-negative rod; n = 3), septicemia (n = 2), colitis (n = 2; documented as caused by Clostridium difficile in one patient), and perirectal cellulitis (n = 1) were seen, typically associated with pancytopenia. Mixed infections (> one pathogen) and multiple sequential infections were common. Polymicrobial pneumonia or sepsis with multiple organ failure in the setting of refractory leukemia and cytopenia was a common terminal event. However, only five patients died during or within a month of completion of treatment from infections (hence thought to be directly related to the treatment).
Grade 3 or 4 thrombocytopenia occurred in nine patients, grade 3 or 4 neutropenia occurred in 11 patients, and two patients developed grade 4 anemia. Grade 4 renal toxicity occurred in four patients. Other toxicities were mainly grade 1 or 2, including infusion reactions in nine patients, GI adverse events in 15 patients, fatigue in six patients, edema in three patients, and elevation of hepatic transaminases in four patients.
Dose reductions were not necessary in any patients. However, in five patients, both agents were held during the course of treatment as a result of persistent cytopenias (n = 3) and persistent fever or pneumonia (n = 2). In these five patients, neither drug was resumed, and all five patients died.
DISCUSSION
T-cell malignancies are rare and are often resistant to conventional chemotherapy. Pentostatin and alemtuzumab are two of the most effective agents for the treatment of T-lymphoid neoplasms. Alemtuzumab was evaluated for the treatment of patients with relapsed T-PLL in two clinical trials; the reported CR rates were 37.5% and 60%, and the median survival times were 13 and 7.5 months.6,7 In both studies, survival was significantly longer for patients achieving a CR (median of 24 and 14.8 months for patients achieving CR compared with 9 and 7.5 months for patients achieving PR).6,7 Dearden et al25 reported a 100% CR rate in 11 previously untreated patients with T-PLL. Hopfinger et al26 treated 16 patients with T-PLL with a combination of fludarabine, cyclophosphamide, and mitoxantrone followed by alemtuzumab. The response rate was 68% after fludarabine, cyclophosphamide, and mitoxantrone and improved to 90% after consolidation with alemtuzumab. In this trial, we treated 13 patients with T-PLL, including eight patients who had a median of two prior regimens (range, one to six regimens). The overall response and CR rates were 69% and 62%, respectively, with a median response duration of 20 months and median overall survival time of 10.2 months. The results compared favorably with those reported with alemtuzumab or pentostatin alone. The responses lasted longer than the responses in two previously reported trials of alemtuzumab in patients with T-PLL (20 months v 4.5 and 10 months).6,7 Our trial, compared with the report by Keating et al,7 also achieved a higher overall response rate (69% v 50%, respectively), CR rate (62% v 37.5%, respectively), and median overall survival time (10.2 v 7.5 months, respectively). The regimen was also effective in patients with other T-cell neoplasms except patients with relapsed T-ALL or PTCL. However, because of the small numbers of patients treated, no definitive conclusions can be made.
Combination of alemtuzumab with chemotherapy has been previously evaluated in patients with chronic lymphocytic leukemia (CLL). Alemtuzumab was administered in combination with fludarabine, cyclophosphamide, and rituximab in 31 heavily pretreated patients with CLL.27 The overall response rate was 55%, with 23% of patients achieving CR. Despite the increase in the incidence of myelosuppression, no significant increase in the incidence of major infections over that seen with the fludarabine, cyclophosphamide, and rituximab combination alone was observed.27
Pentostatin has been combined with cyclophosphamide and rituximab for chemotherapy-naïve and previously treated patients with CLL.28,29 In the salvage study, the overall response rate was 75%, with 25% of the patients achieving CR. The regimen was well tolerated with myelosuppression as the main toxicity. In the chemotherapy-naïve patients using pentostatin, cyclophosphamide, and rituximab, an overall response of 91%, including 41% CR, was reported.30 Whether the addition of alemtuzumab to this regimen would be beneficial remains unclear; our study suggests that although feasible, this has to be carefully balanced for the potential for increased risk of opportunistic infections.
We conclude that the combination of alemtuzumab and pentostatin in treating patients with T-cell neoplasms is relatively safe and feasible with manageable toxicity. In patients with T-PLL, the response rates and duration seem to be superior to single-agent therapy.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00453193.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Susan O'Brien, Genta (C), sanofi-aventis (C), Celgene (C), Genmab (C), GlaxoSmithKline (C), Gemin X (C), Biogen Idec (C), Eli Lilly (C); William Wierda, Bayer Healthcare (C); Dimitrios Kontoyiannis, Merck (C), Schering-Plough (C) Stock Ownership: None Honoraria: Farhad Ravandi, SuperGen, Bayer Healthcare; Dimitrios Kontoyiannis, Merck, Enzon Pharmaceuticals, Astellas Pharma Research Funding: Farhad Ravandi, SuperGen, Bayer Healthcare; Susan O'Brien, Genentech, Berlex Laboratories, Biogen Idec, Eli Lilly, Novartis, Bristol-Myers Squibb, Gemin X, Genta, Hana BioSciences; William Wierda, Bayer Healthcare; Dimitrios Kontoyiannis, Merck, Enzon Pharmaceuticals, Astellas Pharma Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Farhad Ravandi
Administrative support: Sergernne York
Provision of study materials or patients: Farhad Ravandi, Susan O'Brien, Stefan Faderl, Dan Jones, Alessandra Ferrajoli, William Wierda, Srdan Verstovsek, Barbara Pro, Luis Fayad, Michael Keating, Hagop Kantarjian
Collection and assembly of data: Farhad Ravandi, Ahmed Aribi, Sergernne York, Sherry Pierce
Data analysis and interpretation: Farhad Ravandi, Ahmed Aribi, Xuelin Huang, Sherry Pierce, Dimitrios Kontoyiannis
Manuscript writing: Farhad Ravandi, Ahmed Aribi, Dan Jones, Dimitrios Kontoyiannis
Final approval of manuscript: Farhad Ravandi, Dan Jones
REFERENCES
- 1.Ravandi F, Kantarjian H, Jones D, et al. Mature T-cell leukemias. Cancer. 2005;104:1808–1818. doi: 10.1002/cncr.21405. [DOI] [PubMed] [Google Scholar]
- 2.Catovsky D, Galetto J, Okos A, et al. Prolymphocytic leukaemia of B and T cell type. Lancet. 1973;2:232–234. doi: 10.1016/s0140-6736(73)93135-8. [DOI] [PubMed] [Google Scholar]
- 3.Matutes E, Brito-Babapulle V, Swansbury J, et al. Clinical and laboratory features of 78 cases of T-prolymphocytic leukemia. Blood. 1991;78:3269–3274. [PubMed] [Google Scholar]
- 4.Herling M, Khoury JD, Washington LT, et al. A systematic approach to diagnosis of mature T-cell leukemias reveals heterogeneity among WHO categories. Blood. 2004;104:328–335. doi: 10.1182/blood-2004-01-0002. [DOI] [PubMed] [Google Scholar]
- 5.Dearden C, Matutes E, Catovsky D. Deoxycoformycin in the treatment of mature T-cell leukaemias. Br J Cancer. 1991;64:903–906. doi: 10.1038/bjc.1991.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dearden CE, Matutes E, Cazin B, et al. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood. 2001;98:1721–1726. doi: 10.1182/blood.v98.6.1721. [DOI] [PubMed] [Google Scholar]
- 7.Keating MJ, Cazin B, Coutre S, et al. Campath-1H treatment of T-cell prolymphocytic leukemia in patients for whom at least one prior chemotherapy regimen has failed. J Clin Oncol. 2002;20:205–213. doi: 10.1200/JCO.2002.20.1.205. [DOI] [PubMed] [Google Scholar]
- 8.Dearden CE, Foss FM. Peripheral T-cell lymphomas: Diagnosis and management. Hematol Oncol Clin North Am. 2003;17:1351–1366. doi: 10.1016/s0889-8588(03)00119-9. [DOI] [PubMed] [Google Scholar]
- 9.Zinzani PL, Magagnoli M, Bendandi M, et al. Therapy with gemcitabine in pretreated peripheral T-cell lymphoma patients. Ann Oncol. 1998;9:1351–1353. doi: 10.1023/a:1008409601731. [DOI] [PubMed] [Google Scholar]
- 10.Tsimberidou AM, Giles F, Duvic M, et al. Phase II study of pentostatin in advanced T-cell lymphoid malignancies: Update of an M.D. Anderson Cancer Center series. Cancer. 2004;100:342–349. doi: 10.1002/cncr.11899. [DOI] [PubMed] [Google Scholar]
- 11.Enblad G, Hagberg H, Erlanson M, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103:2920–2924. doi: 10.1182/blood-2003-10-3389. [DOI] [PubMed] [Google Scholar]
- 12.Zinzani PL, Alinari L, Tani M, et al. Preliminary observations of a phase II study of reduced-dose alemtuzumab treatment in patients with pretreated T-cell lymphoma. Haematologica. 2005;90:702–703. [PubMed] [Google Scholar]
- 13.Lundin J, Hagberg H, Repp R, et al. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sézary syndrome. Blood. 2003;101:4267–4272. doi: 10.1182/blood-2002-09-2802. [DOI] [PubMed] [Google Scholar]
- 14.Vega F, Medeiros LJ, Bueso-Ramos C, et al. Hepatosplenic gamma/delta T-cell lymphoma in bone marrow: A sinusoidal neoplasm with blastic cytologic features. Am J Clin Pathol. 2001;116:410–419. doi: 10.1309/BM40-YM6J-9T3X-MH8H. [DOI] [PubMed] [Google Scholar]
- 15.Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: Report on a series of 21 patients. Blood. 2003;102:4261–4269. doi: 10.1182/blood-2003-05-1675. [DOI] [PubMed] [Google Scholar]
- 16.Siegel RS, Gartenhaus RB, Kuzel TM. Human T-cell lymphotropic-I-associated leukemia/lymphoma. Curr Treat Options Oncol. 2001;2:291–300. doi: 10.1007/s11864-001-0022-8. [DOI] [PubMed] [Google Scholar]
- 17.Pawson R, Richardson DS, Pagliuca A, et al. Adult T-cell leukemia/lymphoma in London: Clinical experience of 21 cases. Leuk Lymphoma. 1998;31:177–185. doi: 10.3109/10428199809057597. [DOI] [PubMed] [Google Scholar]
- 18.Taguchi H, Kinoshita KI, Takatsuki K, et al. An intensive chemotherapy of adult T-cell leukemia/lymphoma: CHOP followed by etoposide, vindesine, ranimustine, and mitoxantrone with granulocyte colony-stimulating factor support. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:182–186. doi: 10.1097/00042560-199606010-00012. [DOI] [PubMed] [Google Scholar]
- 19.Tsukasaki K, Tobinai K, Shimoyama M, et al. Deoxycoformycin-containing combination chemotherapy for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study (JCOG9109) Int J Hematol. 2003;77:164–170. doi: 10.1007/BF02983215. [DOI] [PubMed] [Google Scholar]
- 20.Catovsky D, Ralfkiaer E, Muller-Hermelink HK. T-cell prolymphocytic leukaemia. In: Jaffe ES, Harris NL, Stein H, et al., editors. World Health Organization Classification of Tumours: Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. pp. 195–196. [Google Scholar]
- 21.O'Brien S, Ravandi F, Riehl T, et al. Valganciclovir prevents cytomegalovirus reactivation in patients receiving alemtuzumab-based therapy. Blood. 2008;111:1816–1819. doi: 10.1182/blood-2007-03-080010. [DOI] [PubMed] [Google Scholar]
- 22.Vega F, Medeiros LJ, Jones D, et al. A novel four-color PCR assay to assess T-cell receptor gamma gene rearrangements in lymphoproliferative lesions. Am J Clin Pathol. 2001;116:17–24. doi: 10.1309/5WFQ-N12E-DT05-UX1T. [DOI] [PubMed] [Google Scholar]
- 23.Thall PF, Simon RM, Estey EH. New statistical strategy for monitoring safety and efficacy in single-arm clinical trials. J Clin Oncol. 1996;14:296–303. doi: 10.1200/JCO.1996.14.1.296. [DOI] [PubMed] [Google Scholar]
- 24.Ravandi F, Faderl S. Complete response in a patient with adult T-cell leukemia (ATL) treated with combination of alemtuzumab and pentostatin. Leuk Res. 2006;30:103–105. doi: 10.1016/j.leukres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Dearden CE, Matutes E, Cazin B, et al. Very high response rates in previously untreated T-cell prolymphocytic leukaemia patients receiving alemtuzumab (Campath-1H) therapy. Blood. 2003;102:644a. abstr. [Google Scholar]
- 26.Hopfinger G, Busch R, Eichhorst B, et al. T-PLL-1 protocol of the German CLL Study Group (GCLLSG): A prospective phase II trial of fludarabine phosphate, mitoxantrone and cyclophosphamide (FMC) followed by alemtuzumab consolidation in T-PLL. Blood. 2005;106:2130. abstr. [Google Scholar]
- 27.Wierda W, Faderl S, O'Brien S, et al. Combined cyclophosphamide, fludarabine, alemtuzumab, and rituximab (CFAR) is active for relapsed and refractory patients with CLL. Blood. 2004;104:340. abstr. [Google Scholar]
- 28.Weiss MA, Maslak PG, Jurcic JG, et al. Pentostatin and cyclophosphamide: An effective new regimen in previously treated patients with chronic lymphocytic leukemia. J Clin Oncol. 2003;21:1278–1284. doi: 10.1200/JCO.2003.08.100. [DOI] [PubMed] [Google Scholar]
- 29.Lamanna N, Kalaycio M, Maslak P, et al. Pentostatin, cyclophosphamide, and rituximab is an active, well-tolerated regimen for patients with previously treated chronic lymphocytic leukemia. J Clin Oncol. 2006;24:1575–1581. doi: 10.1200/JCO.2005.04.3836. [DOI] [PubMed] [Google Scholar]
- 30.Kay NE, Geyer SM, Call TG, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood. 2007;109:405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]