Abstract
Purpose
PointBreak (A Study of Pemetrexed, Carboplatin and Bevacizumab in Patients With Nonsquamous Non-Small Cell Lung Cancer) compared the efficacy and safety of pemetrexed (Pem) plus carboplatin (C) plus bevacizumab (Bev) followed by pemetrexed plus bevacizumab (PemCBev) with paclitaxel (Pac) plus carboplatin (C) plus bevacizumab (Bev) followed by bevacizumab (PacCBev) in patients with advanced nonsquamous non–small-cell lung cancer (NSCLC).
Patients and Methods
Patients with previously untreated stage IIIB or IV nonsquamous NSCLC and Eastern Cooperative Oncology Group performance status of 0 to 1 were randomly assigned to receive pemetrexed 500 mg/m2 or paclitaxel 200 mg/m2 combined with carboplatin area under the curve 6 and bevacizumab 15 mg/kg every 3 weeks for up to four cycles. Eligible patients received maintenance until disease progression: pemetrexed plus bevacizumab (for the PemCBev group) or bevacizumab (for the PacCBev group). The primary end point of this superiority study was overall survival (OS).
Results
Patients were randomly assigned to PemCBev (n = 472) or PacCBev (n = 467). For PemCBev versus PacCBev, OS hazard ratio (HR) was 1.00 (median OS, 12.6 v 13.4 months; P = .949); progression-free survival (PFS) HR was 0.83 (median PFS, 6.0 v 5.6 months; P = .012); overall response rate was 34.1% versus 33.0%; and disease control rate was 65.9% versus 69.8%. Significantly more study drug–related grade 3 or 4 anemia (14.5% v 2.7%), thrombocytopenia (23.3% v 5.6%), and fatigue (10.9% v 5.0%) occurred with PemCBev; significantly more grade 3 or 4 neutropenia (40.6% v 25.8%), febrile neutropenia (4.1% v 1.4%), sensory neuropathy (4.1% v 0%), and alopecia (grade 1 or 2; 36.8% v 6.6%) occurred with PacCBev.
Conclusion
OS did not improve with the PemCBev regimen compared with the PacCBev regimen, although PFS was significantly improved with PemCBev. Toxicity profiles differed; both regimens demonstrated tolerability.
INTRODUCTION
Following a 2002 randomized phase III study that evaluated four platinum-based doublets, the Eastern Cooperative Oncology Group (ECOG) chose carboplatin plus paclitaxel as a reference regimen for future studies in patients with advanced non–small-cell lung cancer (NSCLC) because of its lower rate of toxic effects than the other regimens.1 In 2006, paclitaxel plus carboplatin plus bevacizumab induction followed by bevacizumab maintenance until progressive disease (PD) or unacceptable toxicity was approved as first-line therapy for patients with unresectable locally advanced recurrent or metastatic nonsquamous NSCLC on the basis of overall survival (OS).2 A large, randomized, first-line phase III study showed that pemetrexed plus cisplatin was noninferior to gemcitabine plus cisplatin in unselected patients with advanced-stage NSCLC. However, a prespecified subgroup analysis revealed significantly superior survival for patients with nonsquamous histology who were treated with pemetrexed plus cisplatin.3 Current guidelines recommend that patients with advanced NSCLC who had good performance status (PS) should receive four to six cycles of platinum-based induction therapy. For patients with nonsquamous NSCLC, options included a platinum doublet with bevacizumab or platinum with pemetrexed.4–6
Benefit has been observed for maintenance therapy in treating advanced NSCLC in patients who did not progress during initial induction therapy. The superior OS of pemetrexed in nonsquamous tumors has been demonstrated in the maintenance setting, with pemetrexed as switch or continuation maintenance therapy versus placebo.7–9
With the emerging role of pemetrexed in treatment of nonsquamous NSCLC, there was interest in evaluating pemetrexed in combination with bevacizumab. A single-arm phase II study of pemetrexed plus carboplatin plus bevacizumab followed by pemetrexed plus bevacizumab maintenance (PemCBev) demonstrated efficacy (OS, 14.1 months; progression-free survival [PFS], 7.8 months) and acceptable safety.10 These phase II results with PemCBev were the basis of the phase III PointBreak trial (A Study of Pemetrexed, Carboplatin and Bevacizumab in Patients With Nonsquamous Non-Small Cell Lung Cancer). The primary objective was comparison of OS for PemCBev with paclitaxel plus carboplatin plus bevacizumab followed by bevacizumab (PacCBev) for treatment of patients with advanced nonsquamous NSCLC.
PATIENTS AND METHODS
Eligibility
Patients were required to be at least 18 years old and have an ECOG PS of 0 or 1, histologically or cytologically confirmed nonsquamous NSCLC, stage IIIB with pleural effusion or stage IV disease (according to American Joint Committee on Cancer, version 611), adequate organ function, and no prior systemic therapy for lung cancer. Stable treated brain metastases were allowed. Exclusion criteria included a history of gastrointestinal fistula, perforation, abscess, inflammatory bowel disease, or diverticulitis; significant vascular disease; coagulopathy or use of full-dose anticoagulants at the time of random assignment; a serious cardiac condition; or a history of hemoptysis within 3 months of study entry. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines,12 and the protocol was approved by each participating center's ethics review board. All patients signed written informed consent before treatment.
Study Design, End Points, and Treatment
In this multicenter, United States only, randomized, open-label, phase III study, the primary end point was to compare OS between treatment arms. Secondary end points included comparisons of PFS; overall response rate (ORR); disease control rate (DCR; complete response plus partial response plus stable disease); time to progressive disease (TTPD); toxicity; and supportive care, including hospitalizations, transfusions, and supportive therapies. Quality of life and biomarkers were also analyzed and are reported separately.13
Treatment consisted of up to four cycles of induction therapy followed by maintenance therapy until PD or treatment discontinuation. Eligible patients were randomly assigned (1:1) to either the experimental arm: pemetrexed (Pem; ALIMTA; Eli Lilly, Indianapolis, IN) 500 mg/m2 intravenously (IV) plus carboplatin (C) area under the serum concentration-time curve (AUC) 6 plus bevacizumab (Bev; Avastin; Genentech, South San Francisco, CA) 15 mg/kg on day 1 for up to four 21-day cycles, followed by pemetrexed 500 mg/m2 IV plus bevacizumab 15 mg/kg for maintenance (PemCBev); or the control arm: paclitaxel (Pac) 200 mg/m2 combined with carboplatin (C) AUC 6 and bevacizumab (Bev) 15 mg/kg on day 1 for up to four 21-day cycles, followed by bevacizumab 15 mg/kg for maintenance (PacCBev). Patients received premedications per pemetrexed and paclitaxel labels14,15; the pemetrexed arm also received folic acid and vitamin supplementation per the package label.14 Concomitant supportive therapies, such as erythropoietic agents or granulocyte colony-stimulating factors, were allowed according to the American Society of Clinical Oncology16 and National Comprehensive Cancer Network4 guidelines. After four cycles of induction treatment, patients with a complete response, partial response, or stable disease per Response Evaluation Criteria in Solid Tumors (RECIST) 1.017 received maintenance therapy. Dose reductions and discontinuations for toxicity were specified by the protocol.
Baseline and Treatment Assessments
The baseline tumor assessment method was repeated every other cycle and at 30 days after treatment discontinuation. Other follow-up assessments, including laboratory evaluations, were repeated before each therapy cycle. Complete blood counts were obtained weekly during induction therapy.
Efficacy analyses incorporated all randomly assigned patients on an intent-to-treat (ITT) basis. Patients receiving at least one dose of any study drug were assessable for safety (safety population). Toxicity was graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI-CTCAE) Version 3.0.18
Statistical Analysis
Approximately 900 randomly assigned patients (450 per arm) were needed for the OS analysis, which required 676 events to yield at least an 80% power and one-sided significance level of 0.025 to demonstrate superiority of the pemetrexed arm over the paclitaxel arm, assuming a hazard ratio (HR) of 0.80. All tests of treatment effects were conducted at a two-sided alpha level of .05 and all CIs were given at a two-sided 95% level, unless otherwise specified. OS, PFS, and TTPD analyses used Cox proportional hazard models and nonstratified log-rank tests19 for between-arm comparisons and Kaplan-Meier20 estimations for medians. Fisher's exact tests were used to compare ORR, DCR, and the incidence of toxicities, hospitalizations, and supportive care. Randomization was stratified according to disease stage (IIIB v IV), ECOG PS (0 v 1), sex (male v female), and measurable versus nonmeasurable disease.
Prespecified exploratory efficacy and safety analyses of the maintenance population, defined as patients receiving at least one dose of treatment at cycle 5, and PFS without grade 4 toxicity analyses (occurring at the time of PD, death, or first occurrence of any grade 4 adverse event [AE], whichever occurred first)21,22 were also conducted. The patients receiving maintenance therapy were a postrandomization population; thus, no statistical analyses comparing the two study arms, such as HRs or P values, may be appropriately applied to maintenance population data. The analyses of secondary end points and exploratory analyses were not adjusted to account for multiple comparisons. The sponsor performed the statistical analyses.
RESULTS
Patient Characteristics
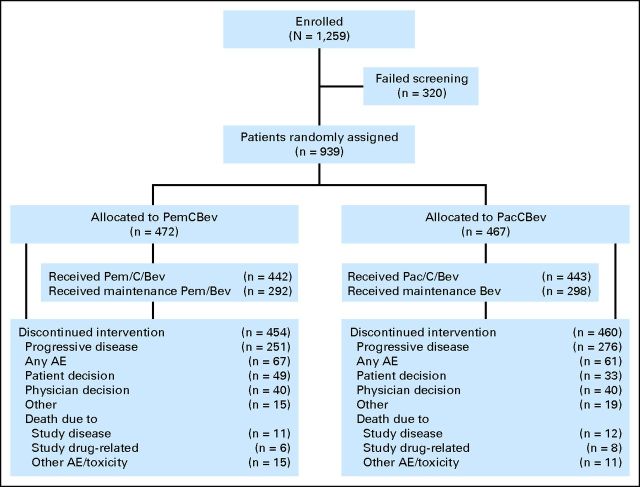
From December 30, 2008, to February 3, 2012, 939 patients were randomly assigned (472, PemCBev; 467, PacCBev). Fifty-four patients (30, PemCBev; 24, PacCBev) were randomly assigned but not treated, mostly because of patient-physician decision or unmet eligibility criteria. Efficacy analyses were performed on all 939 randomly assigned patients (ITT population), and 885 patients were eligible for safety analyses (442, PemCBev; 443, PacCBev). Overall, 292 patients were eligible for and received maintenance therapy with PemCBev and 298 were eligible for and received maintenance therapy with PacCBev. Figure 1 shows patient disposition. Baseline patient and disease-related characteristics were well balanced and similar between the two treatment arms for both the ITT and maintenance populations (Table 1).
Fig 1.
CONSORT diagram. PacCBev, paclitaxel (Pac), carboplatin (C), and bevacizumab (Bev) followed by bevacizumab; PemCBev, pemetrexed (Pem), carboplatin, and bevacizumab followed by pemetrexed and bevacizumab; AE, adverse event.
Table 1.
Baseline Patient and Disease Characteristics
| Characteristic | ITT Population |
Maintenance Population |
||||||
|---|---|---|---|---|---|---|---|---|
| PemCBev (n = 472) |
PacCBev (n = 467) |
PemCBev (n = 292) |
PacCBev (n = 298) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Median age, years | 64.6 | 64.9 | 63.8 | 64.3 | ||||
| Sex | ||||||||
| Male | 251 | 53.2 | 249 | 53.3 | 148 | 50.7 | 159 | 53.4 |
| Female | 221 | 46.8 | 218 | 46.7 | 144 | 49.3 | 139 | 46.6 |
| ECOG PS* | ||||||||
| 0 | 207 | 43.9 | 207 | 44.4 | 138 | 47.3 | 142 | 47.7 |
| 1 | 265 | 56.1 | 259 | 55.6 | 154 | 52.7 | 156 | 52.3 |
| Disease stage* | ||||||||
| IIIB | 48 | 10.2 | 46 | 9.9 | 1 | 10.6 | 30 | 10.1 |
| IV | 424 | 89.8 | 420 | 90.1 | 261 | 89.4 | 268 | 89.9 |
| Histology* | ||||||||
| Adenocarcinoma | 378 | 80.1 | 365 | 78.3 | 237 | 81.2 | 230 | 77.2 |
| Large cell | 8 | 1.7 | 15 | 3.2 | 5 | 1.7 | 11 | 3.7 |
| Other or indeterminate | 86 | 18.2 | 86 | 18.5 | 50 | 17.1 | 57 | 19.1 |
| Race/ethnicity* | ||||||||
| White | 409 | 86.7 | 396 | 84.8 | 256 | 87.7 | 252 | 84.6 |
| African American | 42 | 8.9 | 52 | 11.1 | 23 | 7.9 | 35 | 11.7 |
| Asian | 15 | 3.2 | 14 | 3.0 | 10 | 3.4 | 9 | 3.0 |
| American Indian or Alaskan native | 1 | 0.2 | 1 | 0.2 | 1 | 0.3 | 0 | |
| Multiple | 2 | 0.4 | 3 | 0.6 | 2 | 0.7 | 2 | 0.7 |
| Smoking status* | ||||||||
| Never | 50 | 10.6 | 58 | 12.5 | 39 | 13.4 | 35 | 11.8 |
| Ever | 420 | 89.4 | 405 | 87.5 | 253 | 86.6 | 261 | 88.2 |
| Previously treated brain metastasis | ||||||||
| Yes | 52 | 11.0 | 52 | 11.1 | 24 | 8.2 | 29 | 9.7 |
| No | 420 | 89.0 | 415 | 88.9 | 268 | 91.8 | 269 | 90.3 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; ITT, intent-to-treat; PacCBev, paclitaxel, carboplatin, and bevacizumab followed by bevacizumab; PemCBev, pemetrexed, carboplatin, and bevacizumab followed by pemetrexed and bevacizumab.
Some patients have missing values for these characteristics; percentage was calculated accordingly.
Treatment
For the ITT population, the median number of cycles administered for PemCBev was seven (range, one to 41), and for PacCBev, the median was six (range, one to 39). For the maintenance population, the median number of cycles was 10 (range, four to 41) and nine (range, five to 39), respectively. Delivered mean dose intensities (mean actual dose/mean planned dose) were similar for both arms: pemetrexed 96.1%, carboplatin 95.9%, and bevacizumab 99.9% for PemCBev and paclitaxel 95.5%, carboplatin 95.7%, and bevacizumab 102.1% for PacCBev. Median follow-up was also similar for both arms (PemCBev v PacCBev): 11.7 versus 11.9 months for all patients and 21.2 versus 21.0 months for patients still alive at the data cutoff date (April 3, 2012).
Efficacy
OS.
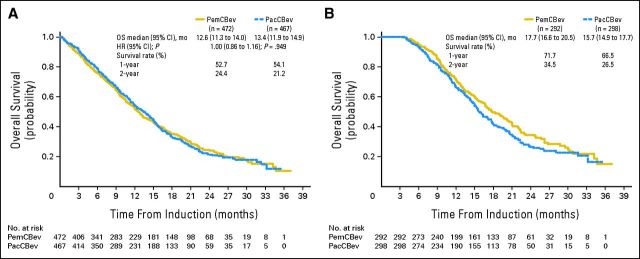
OS (Fig 2A) for patients randomly assigned to PemCBev was not superior to that of patients assigned to PacCBev (12.6 v 13.4 months; HR, 1.00; 95% CI, 0.86 to 1.16; P = .949). Survival rates at 12 and 24 months were 52.7% versus 54.1% and 24.4% versus 21.2% for PemCBev and PacCBev, respectively (no statistical differences).
Fig 2.
Kaplan-Meier overall survival (OS) from random assignment for (A) the intent-to-treat (ITT) population (censoring rates for PemCBev and PacCBev arms, 27.8% and 27.2%) and (B) the maintenance population (censoring rates for PemCBev and PacCBev, 36.0% and 30.2%). The duration of OS was measured from the date of random assignment to the date of death from any cause. If a patient had not died at the time of the data inclusion cutoff date for the analysis, OS was censored at the last date the patient was known by the treating physician to still be alive. HR, hazard ratio; mo, months; PacCBev, paclitaxel (Pac), carboplatin (C), and bevacizumab (Bev) followed by bevacizumab; PemCBev, pemetrexed (Pem), carboplatin, and bevacizumab followed by pemetrexed and bevacizumab.
Median OS for the exploratory analysis of the maintenance population was 17.7 months for PemCBev and 15.7 months for PacCBev (Fig 2B). Survival rates at 12 and 24 months for the maintenance population were 71.7% and 34.5% and 66.5% and 26.5% for PemCBev and PacCBev, respectively. Median OS for the exploratory analysis of patients not receiving maintenance treatment was 4.7 months (95% CI, 4.0 to 6.3 months) for PemCBev and 6.1 months (95% CI, 4.6 to 8.2 months) for PacCBev.
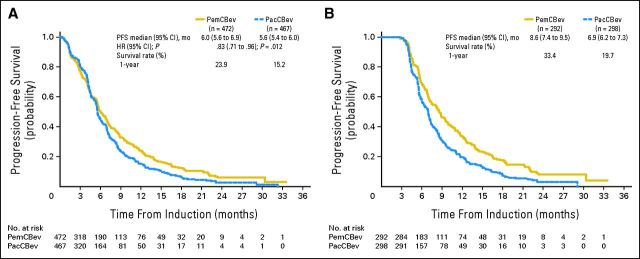
PFS.
PFS (Fig 3A) was statistically significantly longer for PemCBev than for PacCBev (6.0 v 5.6 months; HR, 0.83; 95% CI, 0.71 to .96; P = .012). Median PFS for the maintenance population was 8.6 months for PemCBev and 6.9 months for PacCBev (Fig 3B). Median PFS for patients not receiving maintenance treatment (discontinued study treatment after fewer than five cycles) was 2.3 months (95% CI, 1.7 to 2.6 months) for PemCBev and 2.5 months (95% CI, 1.7 to 2.9 months) for PacCBev (analysis not prespecified).
Fig 3.
Kaplan-Meier progression-free survival (PFS) from random assignment for (A) the intent-to-treat population (censoring rates for PemCBev and PacCBev arms, 26.9% and 23.3%) and (B) the maintenance population (censoring rates for PemCBev and PacCBev arms, 24.7% and 14.1%). The duration of PFS was measured from the date of random assignment to the date of objective progression of disease or the date of death from any cause, whichever was earlier. For patients who received subsequent systemic anticancer therapy (after discontinuation from the study chemotherapy) before objective progression or death, PFS was censored at the date of the last objective progression-free disease assessment before starting the subsequent systemic anticancer therapy. For patients not known to have died as of the data inclusion cutoff date and who did not have objective progressive disease, PFS was censored at the date of the last objective progression-free disease assessment before the cutoff date or the date of initiation of subsequent systemic anticancer therapy, whichever was earlier. HR, hazard ratio; mo, months; PacCBev, paclitaxel (Pac), carboplatin (C), and bevacizumab (Bev) followed by bevacizumab; PemCBev, pemetrexed (Pem), carboplatin (C), and bevacizumab (Bev) followed by pemetrexed and bevacizumab.
TTPD and ORR
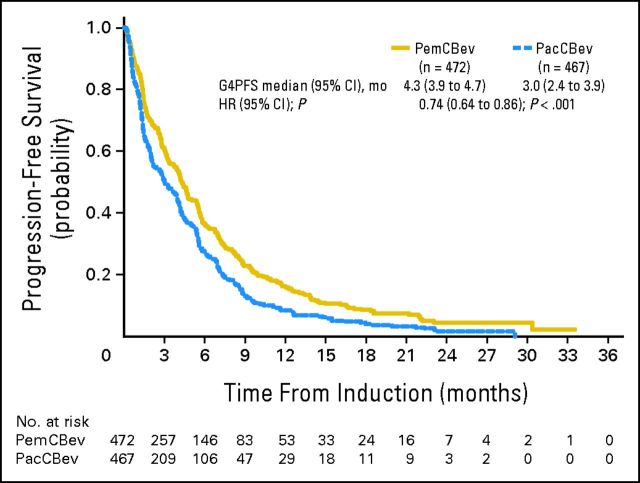
TTPD (ITT) was statistically significantly longer for PemCBev versus PacCBev (7.0 v 6.0 months; HR, 0.79; 95% CI, 0.67 to 0.94; P = .006) as was median PFS without grade 4 toxicity (4.3 v 3.0 months; HR, 0.74; 95% CI, 0.64 to 0.86; P < .001; Appendix Figure A1, online only). ORR (ITT) was comparable for the two arms: 34.1% for PemCBev and 33.0% for PacCBev; DCRs were 65.9% and 69.8%, respectively.
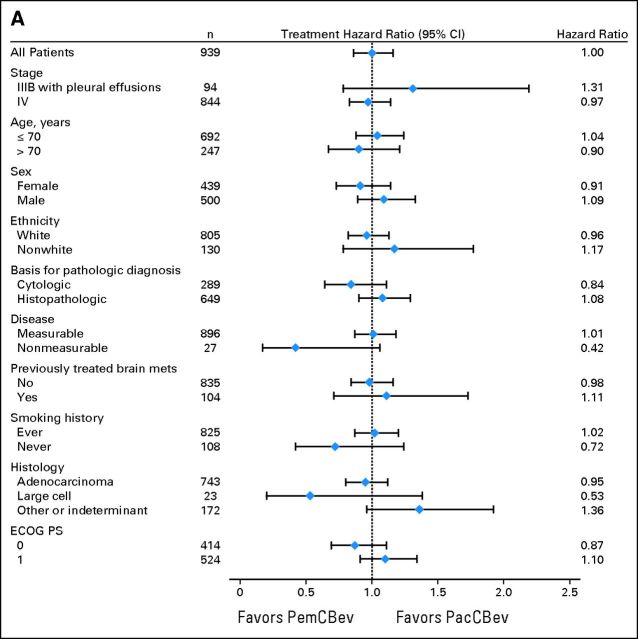
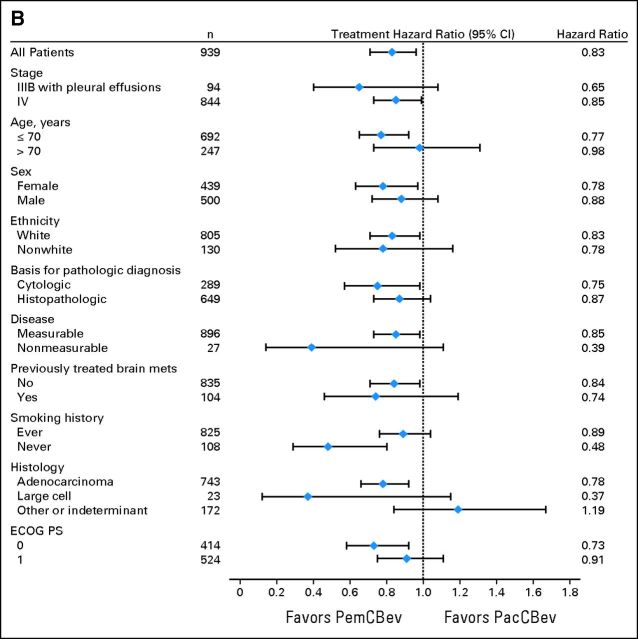
Subgroup and Sensitivity Analyses
Figure 4A shows the unadjusted HRs for the preplanned analyses evaluating differences in OS for baseline characteristic subgroups. Figure 4B shows unadjusted PFS HRs. The analyses of OS and PFS for subgroups produced results consistent with those for the ITT population. A sensitivity analysis (data not shown) that excluded 54 patients who were randomly assigned but were not treated indicated that OS and PFS for this group (safety population) were consistent with those for the ITT population.
Fig 4.
Forest plots for the intent-to-treat population for (A) overall survival and (B) progression-free survival. ECOG PS, Eastern Cooperative Oncology Group performance status; PacCBev, paclitaxel (Pac), carboplatin (C), and bevacizumab (Bev) followed by bevacizumab; PemCBev, pemetrexed (Pem), carboplatin (C), and bevacizumab (Bev) followed by pemetrexed and bevacizumab.
Safety
For the safety population, the two arms differed in the incidence of grade 3 or 4 drug-related toxicities. Grade 3 or 4 drug-related neutropenia (25.8% v 40.6%; P < .0001), febrile neutropenia (1.4% v 4.1%; P = .02), sensory neuropathy (SN; 0% v 4.1%; P < .0001), and alopecia (grade 1 or 2, 6.6% v 36.8%; P < .0001) were significantly lower for PemCBev compared with PacCBev (Table 2). Grade 4 SN was not seen in either arm; grade 2 SN was 1.6% for PemCBev versus 10.6% for PacCBev. Drug-related grade 3 or 4 thrombocytopenia (5.6% v 23.3%; P < .0001), anemia (2.7% v 14.5%; P < .0001), and fatigue (5.0% v 10.9%; P = .001) were significantly lower for PacCBev compared with PemCBev (Table 2). No CNS hemorrhage occurred in patients with stable treated brain metastases at the time of study enrollment. The pattern of significant differences in grade 3 or 4 toxicities between arms was consistent for the maintenance population (Table 3). Toxicities identified by the principal investigator and reported during the maintenance period, regardless of the AE starting date, are reported in Appendix Table A1 (online only).
Table 2.
CTCAE Grade 1 or 2 and Grade 3 or 4 Toxicities: Safety Population
| Toxicity | CTCAE Grade 1 or 2 |
P | CTCAE Grade 3 or 4 |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PemCBev (n = 442) |
PacCBev (n = 443) |
PemCBev (n = 442) |
PacCBev (n = 443) |
|||||||
| No. | % | No. | % | No. | % | No. | % | |||
| Thrombocytopenia | 79 | 17.9 | 76 | 17.2 | .79 | 103 | 23.3 | 25 | 5.6 | < .0001 |
| Neutropenia | 65 | 14.7 | 37 | 8.4 | .003 | 114 | 25.8 | 180 | 40.6 | < .0001 |
| Anemia | 137 | 31.0 | 108 | 24.4 | .03 | 64 | 14.5 | 12 | 2.7 | < .0001 |
| Fatigue | 186 | 42.1 | 175 | 39.5 | .45 | 48 | 10.9 | 22 | 5.0 | .0001 |
| Sensory neuropathy | 52 | 11.8 | 158 | 35.7 | < .0001 | 0 | 18 | 4.1 | < .0001 | |
| Febrile neutropenia | 1 | 0.2 | 1 | 0.2 | 1.00 | 6 | 1.4 | 18 | 4.1 | .02 |
| Thromboembolic event | 2 | 0.5 | 1 | 0.2 | .62 | 14 | 3.2 | 9 | 2.0 | .30 |
| GI or pulmonary hemorrhage | 16 | 3.6 | 17 | 3.8 | 1.00 | 8 | 1.8 | 2 | 0.5 | .06 |
| Hypertension | 49 | 11.1 | 29 | 6.5 | .02 | 15 | 3.4 | 24 | 5.4 | .19 |
| Alopecia* | 29 | 6.6 | 163 | 36.8 | < .0001 | — | — | — | ||
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; PacCBev, paclitaxel, carboplatin, and bevacizumab followed by bevacizumab; PemCBev, pemetrexed, carboplatin, and bevacizumab followed by pemetrexed and bevacizumab.
Maximum CTCAE grade for alopecia is 2.
Table 3.
Grade 1 or 2 and Grade 3 or 4 Toxicities: Maintenance Population From Random Assignment
| Toxicity | CTCAE Grade 1 or 2 |
P | CTCAE Grade 3 or 4 |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PemCBev (n = 292) |
PacCBev (n = 298) |
PemCBev (n = 292) |
PacCBev (n = 298) |
|||||||
| No. | % | No. | % | No. | % | No. | % | |||
| Thrombocytopenia | 58 | 19.9 | 59 | 19.8 | 1.00 | 70 | 24.0 | 13 | 4.4 | < .0001 |
| Neutropenia | 49 | 16.8 | 27 | 9.1 | .007 | 83 | 28.4 | 136 | 45.6 | < .0001 |
| Anemia | 99 | 33.9 | 75 | 25.2 | .02 | 46 | 15.8 | 5 | 1.7 | < .0001 |
| Fatigue | 146 | 50.0 | 137 | 46.0 | .36 | 35 | 12.0 | 8 | 2.7 | < .0001 |
| Sensory neuropathy | 47 | 16.1 | 127 | 42.6 | < .0001 | 0 | 15 | 5.0 | < .0001 | |
| Febrile neutropenia | 0 | 1 | 0.3 | — | 3 | 1.0 | 13 | 4.4 | .02 | |
| Thromboembolic event | 2 | 0.7 | 0 | — | 9 | 3.1 | 3 | 1.0 | .09 | |
| GI or pulmonary hemorrhage | 16 | 5.5 | 14 | 4.7 | .71 | 6 | 2.1 | 0 | .01 | |
| Hypertension | 42 | 14.4 | 26 | 8.7 | .04 | 11 | 3.8 | 21 | 7.0 | .10 |
| Alopecia* | 25 | 8.6 | 127 | 42.6 | < .0001 | — | — | — | ||
NOTE. Table reports the onset of adverse events at any time from induction to maintenance for the maintenance population.
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; PacCBev, paclitaxel, carboplatin, and bevacizumab followed by bevacizumab; PemCBev, pemetrexed, carboplatin, and bevacizumab followed by pemetrexed and bevacizumab.
Maximum CTCAE grade for alopecia is 2.
No statistically significant differences in hospital admissions due to study-drug related AEs (87 [19.7%] for PemCBev; 84 [19.0%] for PacCBev) were observed; however, the mean hospital days per patient were significantly longer for PemCBev (8.5 v 6.3 days; P = .003). More patients had at least one transfusion (26.2% v 9.9%), including RBCs (24.2% v 8.8%) and platelets (7.0% v 2.0%), administered with PemCBev compared with PacCBev. More ITT patients received erythropoietin factors (16.9% v 9.0%) with PemCBev, and fewer received granulocyte colony-stimulating factors (14.8% v 23.8%) compared with PacCBev.
Relatively few study drug–related deaths occurred (18 patients; 2.0%) in the safety population; numbers were similar between arms (eight, PemCBev; 10, PacCBev). Reasons for deaths occurring during study treatment were similar between PemCBev and PacCBev: hemorrhage (0.7% v 0.9%), cardiac disorders (0.2% v 0.7%), CNS ischemia (0.2% v 0.7%), infection (0.2% v 0%), and adult respiratory distress syndrome (0.5% v 0%).
Postdiscontinuation Therapies
Overall, 53.0% of ITT patients given PemCBev and 59.1% of ITT patients given PacCBev received subsequent systemic therapy after study discontinuation (ie, postdiscontinuation therapy [PDT]). Postdiscontinuation radiation therapy was given to 17.2% of patients receiving PemCBev and 13.9% of patients receiving PacCBev. Decisions regarding PDT were made by the investigators. The types of systemic therapy selected were balanced on the two arms, with the exception of more frequent pemetrexed use following discontinuation of PacCBev (36.2% v 14%; P < .001), and more frequent taxane use (docetaxel: 21.0% v 8.1%; P < .001; paclitaxel: 8.1% v 5.8%; P = .199) and cisplatin use (4.2% v 1.7%; P = .033) following discontinuation of PemCBev. Agents administered to ≥ 3% of patients included erlotinib (14.6% v 15.2%), carboplatin (14.4% v 12.0%), bevacizumab (13.6% v 11.8%), gemcitabine (8.3% v 5.1%), and vinorelbine (3.4% v 3.2%) for PemCBev and PacCBev.
DISCUSSION
PointBreak contributes to the published experience of bevacizumab in combination with platinum-based doublets in patients with advanced nonsquamous NSCLC. PointBreak did not meet its primary end point of improved OS in PemCBev. The median OS achieved for both arms in PointBreak (12.6 months, PemCBev; 13.4 months, PacCBev) was comparable to the median OS for the paclitaxel plus carboplatin plus bevacizumab arm in ECOG 4599 (12.3 months).2
In PointBreak, PemCBev showed a statistically significant PFS advantage. These results are consistent with PFS results from other phase III studies with bevacizumab-containing combinations, such as ECOG 45992 and AVAiL.23,24 Improvements in PFS (the primary end point of the AVAiL study) did not translate to an OS advantage.24
In prespecified exploratory analyses of the maintenance population, PointBreak had median OS of 17.7 months with PemCBev and 15.7 months with PacCBev; median PFS was 8.6 months with PemCBev and 6.9 months with PacCBev. Although comparison between trials is limited, these outcomes in patients who received maintenance therapy are similar to those reported in other recent pemetrexed maintenance studies in which randomization occurred postinduction.8,9,25 The median OS for the control arm of AVAPERL (AVAPERL1 Study: A Study of Avastin [Bevacizumab] With or Without Pemetrexed as Maintenance Therapy After Avastin in First Line in Patients With Non-Squamous Non-Small Cell Lung Cancer), which used pemetrexed plus cisplatin plus bevacizumab followed by random assignment to maintenance bevacizumab or bevacizumab plus pemetrexed, was 15.7 months,25 similar to that for the control arm of PointBreak. In AVAPERL, the median OS for the bevacizumab plus pemetrexed arm was not reached at the time of primary data analysis25 but was recently reported to be 19.8 months.26 For the PARAMOUNT study, in which pemetrexed continuation maintenance was administered following pemetrexed plus cisplatin induction therapy, the median OS from induction was 16.9 months.9 In AVAPERL, median PFS was 6.6 months for bevacizumab maintenance versus 10.2 months for bevacizumab plus pemetrexed maintenance (HR, 0.50; P < .001).25 However, comparisons of PointBreak with PARAMOUNT and AVAPERL should be made with caution, because the patients who received maintenance therapy in PointBreak were not randomly assigned postinduction, whereas those in the other recent pemetrexed maintenance studies were.8,9,25 Therefore, between-arm statistical comparisons in PointBreak were not appropriate, which is an additional limitation of our study.
In PointBreak, the between-arm rates of systemic PDT were 53.0% with PemCBev and 59.1% with PacCBev. Some patients received postdiscontinuation radiation therapy (17.2%, PemCBev; 13.9%, PacCBev). The apparently low rate of systemic PDT may be the result of patients being treated with maintenance therapy until PD. Thus, by the time patients were eligible to receive PDT, the patient or investigator may have felt that the patient was no longer a good candidate for PDT. In PointBreak, PDT was at patient and physician discretion. Nonetheless, the rate of PDT was the same in both arms of this study.
The grade 3 or 4 drug-related toxicities differed between the two study arms; observed toxicity was tolerable and was similar to that observed in other recent, randomized phase III studies of platinum doublets combined with bevacizumab.23,25 Toxicities reported in the two arms for grade 3 or 4 drug-related neutropenia, thrombocytopenia, anemia, febrile neutropenia, sensory neuropathy, fatigue, and grade 1 or 2 alopecia are consistent with those reported for platinum doublets combined with bevacizumab.27 In PointBreak, the pattern of significant differences between treatment arms for grade 3 or 4 toxicities in the maintenance population was consistent with that observed in the safety population. Toxicities seen for pemetrexed plus bevacizumab during maintenance appear to be higher than those previously reported for pemetrexed maintenance monotherapy9 but did not appear to increase during maintenance.
This study is limited by the possibility that induction therapy influenced the outcome of the maintenance regimens and that the study design did not allow separate evaluation of the contribution of either induction therapy or maintenance therapy to the efficacy outcomes. Study design also did not allow for comparison of single-agent pemetrexed versus paclitaxel in induction therapy or single-agent pemetrexed versus bevacizumab in maintenance therapy.
In conclusion, there was no improvement in OS with the pemetrexed regimen compared with the paclitaxel regimen. The two Kaplan-Meier OS curves are superimposable and, from an efficacy perspective, patients could benefit equally from either treatment arm. However PointBreak was not designed or powered to demonstrate equivalence of the treatment regimens. The efficacy results are consistent with other phase III first-line studies of platinum doublets for induction followed by continuation maintenance for patients who do not progress.9,25 The significant difference in PFS suggests that the PemCBev combination had a positive effect in this trial, although this did not translate into an OS advantage. Although the toxicity profiles for the regimens differed, both demonstrated tolerability. The similar efficacy seen in this study between treatment arms and compared with other platinum doublet therapy allows clinicians to choose a therapy most appropriate for a given patient on the basis of that specific patient's clinical situation and tolerance to toxicities.
Supplementary Material
Acknowledgment
We thank all of the patients and institutions involved in this study. We also thank Patti Moore and Noelle Gasco of Eli Lilly and Lori Kornberg of inVentiv Health Clinical, for assistance with writing and editing.
Appendix
Table A1.
Grade 1 or 2 and Grade 3 or 4 Toxicities: Maintenance Period Excluding the Induction Period
| Toxicity | CTCAE Grade 1 or 2 |
P | CTCAE Grade 3 or 4 |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PemCBev (n = 292) |
PacCBev (n = 298) |
PemCBev (n = 292) |
PacCBev (n = 298) |
|||||||
| No. | % | No. | % | No. | % | No. | % | |||
| Thrombocytopenia | 46 | 15.8 | 31 | 10.4 | .07 | 21 | 7.2 | 7 | 2.3 | .006 |
| Neutropenia | 27 | 9.2 | 12 | 4.0 | .01 | 41 | 14.0 | 34 | 11.4 | .39 |
| Anemia | 94 | 32.2 | 57 | 19.1 | .0003 | 32 | 11.0 | 1 | 0.3 | < .0001 |
| Fatigue | 137 | 46.9 | 114 | 38.3 | .037 | 28 | 9.6 | 5 | 1.7 | < .0001 |
| Sensory neuropathy | 40 | 13.7 | 117 | 39.3 | < .0001 | 0 | 14 | 4.7 | .001 | |
| Febrile neutropenia | 0 | 1 | 0.3 | — | 3 | 1.0 | 0 | .12 | ||
| Thromboembolic event | 2 | 0.7 | 0 | — | 7 | 2.4 | 2 | 0.7 | .10 | |
| GI or pulmonary hemorrhage | 12 | 4.1 | 9 | 3.0 | .51 | 4 | 1.4 | 0 | .06 | |
| Hypertension | 43 | 14.7 | 23 | 7.7 | .01 | 9 | 3.1 | 18 | 6.0 | .11 |
| Alopecia* | 25 | 8.6 | 125 | 41.9 | < .0001 | — | — | — | ||
NOTE. Table shows adverse events identified by the principal investigator and reported during the maintenance period, regardless of the adverse event starting date.
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; PacCBev, paclitaxel, carboplatin, and bevacizumab followed by bevacizumab; PemCBev, pemetrexed, carboplatin, and bevacizumab followed by pemetrexed and bevacizumab.
Maximum CTCAE grade for alopecia is 2.
Fig A1.
Kaplan-Meier progression-free survival (PFS) from random assignment for the progression-free survival without grade 4 toxicity (G4PFS) population (censoring rates for PemCBev and PacCBev arms, 19.3% and 15.2%, respectively). HR, hazard ratio; mo, months; PacCBev, paclitaxel (Pac), carboplatin (C), and bevacizumab (Bev) followed by bevacizumab; PemCBev, pemetrexed (Pem), carboplatin (C), and bevacizumab (Bev) followed by pemetrexed and bevacizumab.
Footnotes
Supported by Eli Lilly, Indianapolis, IN, and Genentech, South San Francisco, CA.
Presented in part at the Chicago Multidisciplinary Symposium in Thoracic Oncology, Chicago, IL, September 6-8, 2012, and at the American Society of Clinical Oncology's Quality Care Symposium, San Diego, CA, November 30-December 1, 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00762034.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Susan C. Guba, Eli Lilly (C); Jingyi Liu, Eli Lilly (C); Bente Frimodt-Moller, Eli Lilly (C); William J. John, Eli Lilly (C); Coleman K. Obasaju, Eli Lilly (C); Eduardo J. Pennella, Eli Lilly (C) Consultant or Advisory Role: Craig H. Reynolds, Eli Lilly (C), Genentech (C); Philip Bonomi, Eli Lilly (C); Ramaswamy Govindan, Bristol-Myers Squibb (C), Merck (C), Covidien (C) Stock Ownership: Susan C. Guba, Eli Lilly; Jingyi Liu, Eli Lilly; Bente Frimodt-Moller, Eli Lilly; William J. John, Eli Lilly; Coleman K. Obasaju, Eli Lilly; Eduardo J. Pennella, Eli Lilly Honoraria: Craig H. Reynolds, Eli Lilly, Genentech; Robert M. Jotte, Genentech, Eli Lilly; Philip Bonomi, Eli Lilly; Ramaswamy Govindan, Bristol-Myers Squibb, Merck, Covidien, Boehringer Ingelheim Research Funding: Edward B. Garon, Eli Lilly, Genentech; Thaddeus Beck, Eli Lilly; Philip Bonomi, Eli Lilly, Genentech, ImClone Systems Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jyoti D. Patel, Mark A. Socinski, Edward B. Garon, Craig H. Reynolds, Susan C. Guba, Jingyi Liu, William J. John, Coleman K. Obasaju, Eduardo J. Pennella, Philip Bonomi, Ramaswamy Govindan
Administrative support: Craig H. Reynolds
Provision of study materials or patients: Mark A. Socinski, Edward B. Garon, Craig H. Reynolds, David R. Spigel, Mark R. Olsen, Robert C. Hermann, Thaddeus Beck, Donald A. Richards
Collection and assembly of data: Mark A. Socinski, Edward B. Garon, Craig H. Reynolds, Mark R. Olsen, Robert C. Hermann, Robert M. Jotte, Thaddeus Beck, Susan C. Guba, Jingyi Liu, Bente Frimodt-Moller, Eduardo J. Pennella, Ramaswamy Govindan
Data analysis and interpretation: Mark A. Socinski, Edward B. Garon, Craig H. Reynolds, David R. Spigel, Donald A. Richards, Susan C. Guba, Jingyi Liu, William J. John, Coleman K. Obasaju, Eduardo J. Pennella, Philip Bonomi, Ramaswamy Govindan
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 2.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 3.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer, Version 3, 2012. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 5.D'Addario G, Früh M, Reck M, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v116–v119. doi: 10.1093/annonc/mdq189. [DOI] [PubMed] [Google Scholar]
- 6.Azzoli CG, Temin S, Aliff T, et al. 2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J Clin Oncol. 2011;29:3825–3831. doi: 10.1200/JCO.2010.34.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 8.Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): A double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–255. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival (OS) results of the phase III study of maintenance pemetrexed (pem) plus best supportive care (BSC) versus placebo (plb) plus BSC immediately following induction treatment with pem plus cisplatin (cis) for advanced nonsquamous (NS) non-small cell lung cancer (NSCLC) J Clin Oncol. 2012;(suppl 15s):30. abstr LBA7507. [Google Scholar]
- 10.Patel JD, Hensing TA, Rademaker A, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol. 2009;27:3284–3289. doi: 10.1200/JCO.2008.20.8181. [DOI] [PubMed] [Google Scholar]
- 11.Greene FL, Compton CC, Fritz AG, et al., editors. American Joint Committee on Cancer Staging Atlas. ed 6. Chicago, IL: Springer; 2006. pp. 167–176. [Google Scholar]
- 12.ICH: ICH Efficacy Guidelines E6(R1) Good Clinical Practice—Consolidated Guideline, Finalized May 1996. http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html.
- 13.Spigel DR, Patel JD, Reynolds CH, et al. Patient-reported outcomes from POINTBREAK: The randomized, open-label, phase III study of pemetrexed (pem) + carboplatin (cb) + bevacizumab (bev) followed by maintenance pem + bev versus paclitaxel (pac) + cb + bev followed by maintenance bev in patients with stage IIIB or IV nonsquamous non-small cell lung cancer (NS-NSCLC). American Society of Clinical Oncology Quality Care Symposium; November 30-December 1, 2012; San Diego, CA. abstr 53. [Google Scholar]
- 14.Eli Lilly. Indianapolis, IN: Eli Lilly; 2012. Nov 17, ALIMTA (pemetrexed) US prescribing information. http://pi.lilly.com/us/alimta-pi.pdf. [Google Scholar]
- 15.Bristol-Myers Squibb. Princeton, NJ: Bristol-Myers Squibb; 2011. Apr, Taxol (paclitaxel) US prescribing information. http://packageinserts.bms.com/pi/pi_taxol.pdf. [Google Scholar]
- 16.Ozer H, Armitage JO, Bennett CL, et al. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: Evidence-based, clinical practice guidelines—American Society of Clinical Oncology Growth Factors Expert Panel. J Clin Oncol. 2000;18:3558–3585. doi: 10.1200/JCO.2000.18.20.3558. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute, Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, v3.0 (CTCAE), published August 9, 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 19.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1:121–129. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Pujol JL, Paul S, Chouaki N, et al. Survival without Common Toxicity Criteria grade 3/4 toxicity for pemetrexed compared with docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC): A risk-benefit analysis. J Thorac Oncol. 2007;2:397–401. doi: 10.1097/01.JTO.0000268672.57002.69. [DOI] [PubMed] [Google Scholar]
- 22.Scagliotti GV, Park K, Patil S, et al. Survival without toxicity for cisplatin plus pemetrexed versus cisplatin plus gemcitabine in chemonaïve patients with advanced non-small cell lung cancer: A risk-benefit analysis of a large phase III study. Eur J Cancer. 2009;45:2298–2303. doi: 10.1016/j.ejca.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 23.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 24.Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: Results from a randomized phase III trial (AVAiL) Ann Oncol. 2010;21:1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlesi F, de Castro J, Dvornichenko V, et al. AVAPERL (MO22089): Final efficacy outcomes for patients (pts) with advanced non-squamous non-small cell lung cancer (nsNSCLC) randomised to continuation maintenance (mtc) with bevacizumab (bev) or bev + pemetrexed (pem) after first-line (1L) bev-cisplatin (cis)-pem treatment (Tx) Eur J Cancer. 2011;47(suppl 2):16. abstr 34LBA. [Google Scholar]
- 26.Rittmeyer A, Scherpereel A, Gorbunova VA, et al. Effect of maintenance bevacizumab (Bev) plus pemetrexed (Pem) after first-line cisplatin/Pem/Bev in advanced nonsquamous non-small cell lung cancer (nsNSCLC) on overall survival (OS) of patients (pts) on the AVAPERL (MO22089) phase III randomized trial. J Clin Oncol. 2013;(suppl 15s):31. doi: 10.1200/JCO.2012.42.3749. abstr 8014. [DOI] [PubMed] [Google Scholar]
- 27.Sandomenico C, Costanzo R, Carillio G, et al. Bevacizumab in non small cell lung cancer: Development, current status and issues. Curr Med Chem. 2012;19:961–971. doi: 10.2174/092986712799320673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.