Abstract
Purpose
Progression-free survival (PFS) in metastatic castration-resistant prostate cancer (mCRPC) trials has been inconsistently defined and poorly associated with overall survival (OS). A reproducible quantitative definition of radiographic PFS (rPFS) was tested for association with a coprimary end point of OS in a randomized trial of abiraterone in patients with mCRPC.
Patients and Methods
rPFS was defined as ≥ two new lesions on an 8-week bone scan plus two additional lesions on a confirmatory scan, ≥ two new confirmed lesions on any scan ≥ 12 weeks after random assignment, and/or progression in nodes or viscera on cross-sectional imaging, or death. rPFS was assessed by independent review at 15% of deaths and by investigator review at 15% and 40% of deaths. rPFS and OS association was evaluated by Spearman's correlation.
Results
A total of 1,088 patients were randomly assigned to abiraterone plus prednisone or prednisone alone. At first interim analysis, the hazard ratio (HR) by independent review was 0.43 (95% CI, 0.35 to 0.52; P < .001; abiraterone plus prednisone: median rPFS, not estimable; prednisone: median rPFS, 8.3 months). Similar HRs were obtained by investigator review at the first two interim analyses (HR, 0.49; 95% CI, 0.41 to 0.60; P < .001 and HR, 0.53; 95% CI, 0.45 to 0.62; P < .001, respectively), validating the imaging data assay used. Spearman's correlation coefficient between rPFS and OS was 0.72.
Conclusion
rPFS was highly consistent and highly associated with OS, providing initial prospective evidence on further developing rPFS as an intermediate end point in mCRPC trials.
INTRODUCTION
Most men with metastatic castration-resistant prostate cancer (mCRPC) will suffer from severe symptoms and succumb to disease as a result of overwhelming osseous metastases. Indications for approved therapeutic agents for mCRPC include the control or relief of pain, and the delay or prevention of skeletal-related events or death.1
There has long been a need to develop additional time-to-event end points short of overall survival (OS) to accelerate drug development. For prostate cancer, that need has only grown more urgent with the approval of numerous life-prolonging therapies for mCRPC.2–7 These therapies can confound, blunt, or obscure the impact on OS of a drug under study when they are administered in the postprotocol setting. Historically, post-treatment changes in prostate-specific antigen (PSA) have not demonstrated robust associations with survival and not qualified as an end point to support regulatory approval. In addition, the limited degree of nodal and visceral disease in mCRPC has reduced the utility of standard imaging outcome measures,8 which also fail to accurately assess bone disease, the most common site of spread.
A particular unmet need in the assessment of bone disease with radionuclide bone scans is a reproducible assay that can be interpreted and reported consistently and quantitatively as a biomarker. As a result, reported associations of post-treatment changes on bone scans with clinical outcome have at best been modest.9,10
In 2005, a US Food and Drug Administration (FDA) advisory committee meeting was held on prostate cancer end points,11 spurring leaders in the field to develop the Prostate Cancer Working Group 2 (PCWG2) proposal to use a time-to-event progression end point for bone scan interpretation. Progression was defined as ≥ two new lesions on the initial post-treatment bone scan, followed by ≥ two additional lesions on the subsequent scan.1 This requirement (ie, 2 + 2) was designed to prevent mistaking new lesions that represented healing from a successful therapy, also known as flare or pseudoprogression, for true disease progression representing unsuccessful therapy.
PCWG2 also proposed that this clinical trial end point be studied prospectively once a validated means of capturing essential bone scan data was developed. Therefore, after the definition and proposal of the end point, a bone scan data capture assay was developed through the Prostate Cancer Clinical Consortium, iteratively modified, and tested to ensure that the interpretation of the scan and the recording of the results were consistent and reproducible.1,12 The assay itself is a series of forms that a trained local medical oncologist, radiologist, or nuclear medicine physician completes at each imaging time point.13,14
COU-AA-302 (Cougar–Abiraterone Acetate–Study 302) was a phase III randomized, double-blind, placebo-controlled study comparing the efficacy and safety of abiraterone acetate plus prednisone with placebo plus prednisone in asymptomatic or mildly symptomatic men with chemotherapy-naive mCRPC. In collaboration with US and European regulatory agencies, the COU-AA-302 study was designed with two coprimary end points—radiographic progression-free survival (rPFS) and OS—along with clinically relevant secondary end points (ie, time to cytotoxic chemotherapy initiation, opiate use for cancer-related pain, and performance status deterioration), which had been used previously as part of composite PFS or time-to-progression end points in earlier phase III studies of mCRPC.15,16
PATIENTS AND METHODS
Study Design
COU-AA-302 randomly assigned chemotherapy-naive patients with mCRPC to receive abiraterone acetate 1,000 mg daily plus prednisone 5 mg orally twice daily or placebo plus prednisone at a ratio of 1:1 (Fig 1). Full details of the study methodology have been reported.17 The review boards at all participating institutions approved the study, which was conducted according to the principles set forth in the Declaration of Helsinki. All patients provided written informed consent to participate in the study. Trial design discussion with the FDA specifically led to a special protocol assessment, with rPFS defined prospectively as a coprimary end point.
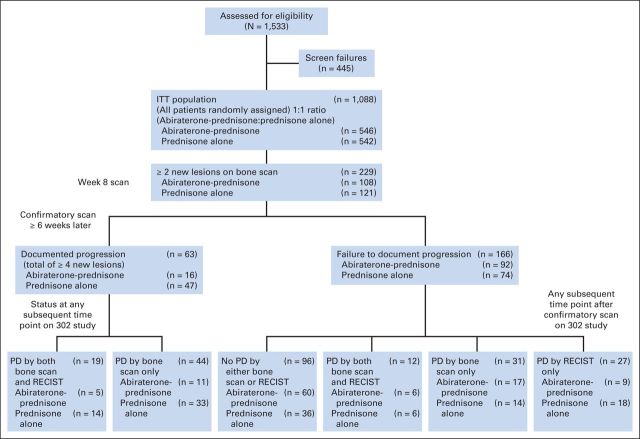
Fig 1.
Study profile. Adapted Prostate Cancer Working Group criteria identified a substantial number of patients with ≥ two new lesions at 8 weeks compared with baseline for whom progression was not confirmed by having ≥ two additional new lesions on subsequent scan (total, 166 [72%] of 229; abiraterone, 92 [85%] of 108; prednisone, 74 [61%] of 121). ITT, intent to treat; PD, progressive disease.
rPFS
rPFS, the coprimary end point, was defined as the time from random assignment to the first occurrence of either progression by bone scan, progression by computed tomography (CT) or magnetic resonance imaging as defined by modified RECIST (version 1.0), or death resulting from any cause. Bone and CT scans were obtained every 8 weeks during the first 24 weeks and every 12 weeks thereafter. The imaging schedule was based on sponsor and FDA discussions leading to the special protocol assessment. Progression by bone scan was adapted from PCWG2 consensus criteria1 and defined as follows:
If the first bone scan with ≥ two new lesions compared with baseline was observed < 12 weeks from random assignment, a second bone scan was required, taken ≥ 6 weeks later, which was required to demonstrate ≥ two additional new lesions (total of ≥ four new lesions from baseline).1
If the first bone scan with ≥ two new lesions compared with baseline was observed ≥ 12 weeks from random assignment (ie, outside of flare window), a confirmatory second bone scan was required, ≥ 6 weeks later, to verify the continued presence of the new lesions, but two additional new lesions were not required (total of ≥ two new lesions v baseline).
In the protocol-defined modified RECIST (version 1.0) criteria, baseline lymph nodes ≥ 2 cm and visceral or extranodal lesions ≥ 1 cm using spiral CT were defined as target lesions. Progression by CT or magnetic resonance imaging was defined as a ≥ 20% increase from nadir in target lesion sum of long diameters, appearance of new soft tissue or visceral lesions, and/or unequivocal progression of baseline nontarget lesions. Images were assessed by a blinded independent central radiologist review.
In addition to an independent central radiology review, an investigator-reviewed assessment of bone scans was also conducted. At each site, a principal oncology- or nuclear medicine–trained bone scan reviewer was trained on the protocol definitions of progression and assigned to consistently provide the investigator assessment and complete the bone scan assessment worksheet adapted from the Prostate Cancer Clinical Trials Consortium.13,14
OS and Secondary End Points
OS was a coprimary end point and was defined as the time from random assignment to the date of death resulting from any cause. Secondary end points included time to cytotoxic chemotherapy initiation, opiate use for cancer-related pain, PSA progression, and performance status deterioration. These data have been reported.17
Statistical Design and Data Analysis
The statistical plan for COU-AA-302 is summarized in Appendix Table 1 (online only). The planned final analysis of 378 rPFS events by independent review provided 91% power to detect a hazard ratio (HR) of 0.67 at a two-tailed significance level of .01. For OS, 773 events provided 85% power to detect an HR of 0.80 at a two-tailed significance level of .04. Three interim analyses were planned at approximately 15%, 40%, and 55% of the total events for OS using the O'Brien-Fleming boundary as implemented by the Lan-DeMets alpha spending method. The highly consistent rPFS end point assessed by both independent radiologist and investigator was also graphically evaluated. The Kaplan-Meier method was used to estimate the distribution of rPFS, and the stratified Cox model was used to estimate the HR and its associated 95% CI. The strength of the association between rPFS and OS was evaluated using Spearman's correlation and Kendall's tau coefficients, which were estimated through the Clayton copula.18 Alternative copula functions (Hougaard and Plackett) were also considered; however, the decision to use the Clayton copula was based on the fact that it provided the best fit according to the maximum likelihood values.
Table 1.
Consistency of Radiographic Progression-Free Survival Analyses
| Review | Interim Analysis Data Cutoff | Abiraterone Plus Prednisone |
Prednisone Alone |
HR | 95% CI | P | ||
|---|---|---|---|---|---|---|---|---|
| No. of Events | Median (months) | No. of Events | Median (months) | |||||
| rPFS | ||||||||
| Independent | December 2010 | 150 | NE | 251 | 8.3 | 0.43 | 0.35 to 0.52 | < .001 |
| Independent* | December 2010 | 174 | 12 | 294 | 7.9 | 0.42 | 0.35 to 0.51 | < .001 |
| Investigator | December 2010 | 174 | 13.7 | 261 | 8.3 | 0.49 | 0.41 to 0.60 | < .001 |
| Investigator | December 2011 | 271 | 16.5 | 336 | 8.3 | 0.53 | 0.45 to 0.62 | < .001 |
| OS | December 2011 | 147 | NE | 186 | 27.2 | 0.75 | 0.61 to 0.93 | .0097 |
NOTE. Data are reported for both independent and investigator review at first interim analysis to demonstrate degree of concordance between two readers per US Food and Drug Administration briefing document on radiologic reviews for PFS.19 Data at second interim analysis are also reported, because this clinical cutoff date represents that used to calculate Spearman's correlation between rPFS and OS.
Abbreviations: HR, hazard ratio; NE, not estimable; OS, overall survival; PFS, progression-free survival; rPFS, radiographic progression-free survival.
Including unequivocal clinical progression by investigator (ie, cancer pain requiring opiates, deterioration to grade 3 Eastern Cooperative Oncology Group performance status, initiation of cytotoxic chemotherapy, irradiation/surgical intervention for prostate cancer).
RESULTS
rPFS As Measured by Independent and Investigator Review
At the first interim analysis for OS, 401 rPFS events were observed based on the independent review (data cutoff, December 2010). Treatment with abiraterone plus prednisone led to a 57% reduction in the risk of radiographic progression or death compared with prednisone (abiraterone plus prednisone: median rPFS, not estimable; prednisone: median rPFS, 8.3 months; Table 1; Fig 2). When this independent assessment included unequivocal clinical progression17 as an event, a 58% reduction in risk was observed with abiraterone plus prednisone versus prednisone (abiraterone plus prednisone: median rPFS, 12 months; prednisone: median rPFS, 7.9 months; Table 1).
Fig 2.
Radiographic progression-free survival as assessed by blinded independent review (December 2010), blinded independent and investigator review (December 2010), and blinded independent and investigator review (December 2011).
When rPFS was based on investigator-reviewed assessments of scans at the first interim analysis, a 51% decrease in the hazard of radiographic progression or death was observed with abiraterone-plus-prednisone treatment (abiraterone plus prednisone: median rPFS, 13.7 months; prednisone: median rPFS, 8.3 months); this closely matched the independent review (Table 1; Fig 2). At the second interim analysis for OS, 607 rPFS events by investigator review were observed (43% of OS events as of December 20, 2011). Treatment with abiraterone plus prednisone led to a 47% reduction in the risk of radiographic progression or death compared with prednisone, which also closely matched the independent review at the first interim analysis (abiraterone plus prednisone: median rPFS, 16.5 months; prednisone: median rPFS, 8.3 months; Table 1; Fig 2).
Agreement of rPFS by Independent and Investigator Review
There was general agreement of occurrence and timing of radiographic progression. As shown in Figure 2, a high degree of agreement was observed between the independent and investigator reviews at the first interim analysis. Agreement between independent and investigator assessment on rPFS event status in December 2010 was observed in 430 (79%) of 546 patients in the abiraterone-plus-prednisone group and 414 (76%) of 542 patients in the placebo group. With a longer follow-up in the investigator review (December 2011; Fig 2), agreement with the independent review in December 2010 persisted.
Application of 2 + 2 Rule for Confirmation or Nonconfirmation of Bone Scan Progression
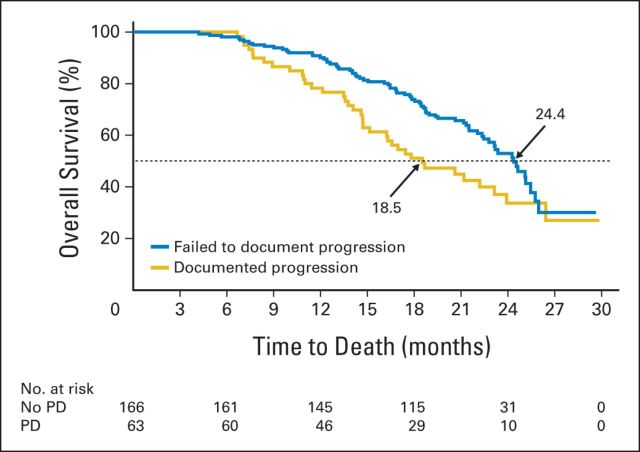
At the 8-week bone scan, 20% of patients (229; 108 of 546 in abiraterone-plus-prednisone group and 121 of 542 prednisone-treated patients) had ≥ two new lesions compared with baseline as measured by independent review. However, at the subsequent scan, 72% (166 of 229 patients [92 (86%) of 108 in abiraterone-plus-prednisone group and 74 (61%) of 121 in prednisone group]) did not show ≥ two additional lesions on their bone scan. Thus, 15% of the total study population (166 of 1,088 patients) would have been misclassified as having disease progression without implementation of the additional scan requirement. Conversely, 63 (28%) of 229 patients (abiraterone plus prednisone, n = 16 [25%]; prednisone, n = 47 [75%]) who had progressive disease on bone scan at week 8 had ≥ two new additional lesions, and thus confirmation of progression, on their next scan. OS was longer in those patients who did not meet criteria for progressive disease on their subsequent bone scan (n = 166) compared with those who did have confirmed progression on the subsequent scan (n = 63; median OS [December 2010 cutoff], 24.4 v 18.5 months; Fig 3). Forty-seven (28%; abiraterone plus prednisone, n = 33; prednisone, n = 14) of the 166 patients who did demonstrate progressive disease at week 8 ultimately demonstrated improvement on bone scan at week 16.
Fig 3.
Survival of patients with early bone scan progression (by 2 + 2 criteria) who failed to document progression (n = 166) versus those with documented progression on subsequent bone scan (n = 63). PD, progressive disease.
Progression by Bone Scan and RECIST
The specific radiologic events that defined progression events for the rPFS end point are listed in Table 2. The proportion of patients with disease progression by bone scan was similar to that observed by soft-tissue progression, with more events observed in patients receiving prednisone alone compared with those treated with abiraterone plus prednisone. At the independent review (December 2010), disease progression by bone scan only was 10%, compared with 12% for soft-tissue only in the abiraterone-plus-prednisone group; these values were 15% and 21%, respectively, in the prednisone-alone group. Trends were similar when comparing investigator-reviewed parameters at the first (December 2010) and second (December 2011) interim analyses. Unequivocal clinical progression without radiographic progression occurred in fewer abiraterone-treated patients compared with the prednisone group (abiraterone plus prednisone, 4%; prednisone, 8%).
Table 2.
Progression Events in rPFS End Point
| Review | Interim Analysis Data Cutoff | Abiraterone Plus Prednisone (n = 546) |
Prednisone Alone (n = 542) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Events |
Bone Scan Only |
Soft Tissue Only |
Bone and Soft Tissue |
Death |
Total Events |
Bone Scan Only |
Soft Tissue Only |
Bone and Soft Tissue |
Death |
||||||||||||
| No. | % of Patients | No. | % of Patients With Events | No. | % of Patients With Events | No. | % of Patients With Events | No. | % of Patients With Events | No. | % of Patients | No. | % of Patients With Events | No. | % of Patients With Events | No. | % of Patients With Events | No. | % of Patients With Events | ||
| Independent | December 2010 | 150 | 28 | 57 | 38 | 66 | 44 | 18 | 12 | 9 | 6 | 251 | 46 | 79 | 32 | 115 | 46 | 46 | 18 | 11 | 4 |
| Investigator | December 2010 | 174 | 32 | 74 | 43 | 69 | 40 | 20 | 12 | 11 | 6 | 261 | 48 | 118 | 45 | 105 | 40 | 28 | 11 | 10 | 4 |
| Investigator | December 2011 | 271 | 50 | 107 | 40 | 102 | 38 | 42 | 16 | 20 | 7 | 336 | 62 | 146 | 44 | 135 | 40 | 44 | 13 | 11 | 3 |
Abbreviation: rPFS, radiographic progression-free survival.
Progression events that resulted in treatment termination are listed in Table 3 and specify the manifestations of disease progression. Both radiographic progression alone and unequivocal clinical progression alone led to greater discontinuation in the prednisone group than the abiraterone-plus-prednisone group. Discontinuation resulting from both radiographic and unequivocal clinical progression was comparable between groups.
Table 3.
Reasons for Discontinuation
| Reason | Abiraterone Plus Prednisone (n = 542; %) | Prednisone Alone (n = 540; %) |
|---|---|---|
| Radiographic progression only | 21 | 30 |
| Unequivocal clinical progression only | 21 | 25 |
| Radiographic and unequivocal clinical progression | 11 | 10 |
| Adverse event | 7 | 5 |
| Withdrew consent | 6 | 9 |
| Other | 4 | 5 |
NOTE. December 2011 cutoff.
The percentages of patients who progressed per target lesion assessment via modified RECIST were 4.9% in the abiraterone-plus-prednisone group and 10.0% in the prednisone-alone group, with independent review conducted at the December 2010 cutoff date. Progression by nontarget lesion as assessed by modified RECIST was similarly increased in the prednisone-alone group (6.4% v 12.2%); patients treated with prednisone alone showed increased progression by modified RECIST, presenting with either new lesions (5.0% v 2.6%) or no new lesions (7.2% v 3.8%). Overall progression with new lesions was lower in the abiraterone-plus-prednisone group (11.0% v 20.5%). At the December 2010 cutoff date, more patients had experienced PSA progression initially (n = 357) compared with the first appearance of either soft-tissue progression (n = 126) or bone progression (n = 125).
Correlation of rPFS With OS
As previously reported,17 at the second interim analysis of OS (43% of events), use of abiraterone plus prednisone led to an estimated 25% decrease in the hazard of death (HR, 0.75; 95% CI, 0.61 to 0.93; P = .0097). rPFS was positively associated with OS (Spearman's correlation coefficient, 0.72; Kendall's tau statistic, 0.53; maximum likelihood values for Clayton, Hougaard, and Plackett copulas were −3796.11, −3796.99, and −3797.79, respectively) in each treatment group (Appendix Table A2, online only). No single subpart of rPFS was so clearly dominant among the total number of events that a statistically valid determination of the association with OS could be conducted within a single subpart (Table 2).
Clinical Benefit of Abiraterone on Secondary End Points
All secondary end points showed superiority of abiraterone plus prednisone over prednisone alone and were consistent with the imaging results, including time to cytotoxic chemotherapy initiation (P < .001), time to opiate use for cancer-related pain (P < .001), time to PSA progression (P < .001), and time to performance status deterioration (P = .005).17
DISCUSSION
The COU-AA-302 study is the first randomized, double-blind, placebo-controlled phase III trial in mCRPC to our knowledge to prospectively demonstrate a highly positive association between OS and rPFS. The study, which included blinded central radiology review, provides the highest level of evidence to date that rPFS is highly associated with OS in chemotherapy-naive asymptomatic or mildly symptomatic mCRPC, with a Spearman correlation coefficient of 0.72. The Kendall's tau statistic was higher than that observed in previous reports, although comparisons across trials using other, less rigorous definitions of progression and data collection are limited.9,10 The rigor of the independently validated data showing significant benefit in rPFS and a strong trend in OS as coprimary end points in combination with clinically relevant secondary end points supported the regulatory approval of abiraterone acetate plus prednisone for the treatment of patients with mCRPC who have not received chemotherapy in many countries, including the United States, the European Union, and Canada.20,21 Our trial was the first to our knowledge to use rPFS as a registration end point in this clinical state per FDA approval of a supplemental new drug application.22 The results suggest that this objective, prospectively defined end point may serve as a response indicator biomarker that is evaluable in future studies. The end point definition described in this study arose from a need to improve the reliability and utility of traditionally defined composite PFS16 or time-to-progression23 end points that were not based on a validated assay as used in previous studies.
Biomarker development is contingent on building an evidentiary database that demonstrates that a biomarker is associated with a clinical outcome within a specific clinical context of use.24 The rPFS end point adapted from modified PCWG2 and RECIST used a standardized objective assessment of imaging studies that reported in simple binary terms either progression or nonprogression. The PCWG2 criteria focus on the number of bone lesions for defining progression of bone scans, not the intensity of uptake, allowing for a less subjective interpretation of progression in osseous metastases.
The Kaplan-Meier rPFS curves were virtually identical, whether assessed by an investigator or independent reviewer, regardless of treatment arm. The temptation is to conclude that independent radiographic assessment is not necessary in clinical trials in mCRPC. However, this study was not designed to test that issue, and most likely, these results reflect the high degree of training, the education of participating investigators, and the intensive real-time monitoring by the sponsor, so the independent radiologist reviewers and investigators understood PCWG2 and modified RECIST 1.0 guidelines to appropriately evaluate bone and soft-tissue disease using the protocol-defined guidelines.
In addition to establishing a reproducible definition of progression in bone, PCWG2 criteria enabled patients to remain on study despite an apparent worsening of the first post-treatment scan.25 In our study, patients were required to follow the 2 + 2 criteria for radiographic progression by bone scan during the possible flare period at the first post-treatment scan (8 weeks). As a result, 72% of patients (166 of 229) who had ≥ two new lesions on their 8-week scan remained on study because they did not develop two additional new lesions on the subsequent confirmatory scan, following the 2 + 2 rule. Using this definition of progression, we identified a population of patients who avoided premature treatment discontinuation.
Of note, not all patients experienced progression by bone scintigraphy. The investigator review from the first interim analysis indicated that 13% of patients in the abiraterone-plus-prednisone group and 19% in the prednisone-alone group experienced progression by soft-tissue only (19% and 25%, respectively, at second interim analysis review). This suggests that abiraterone impedes progression of disease not only in bone but also in the soft tissues, and the combination of using both the adapted PCWG2 and RECIST criteria is important for capturing the complexity of an rPFS end point.
rPFS data are highly dependent on the schedule of study assessments.26 On the basis of the outcome of the atrasentan trial, where rapid progression was attributable to flare,15,16 our trial, in which PSA was not used to terminate treatment, used 8-week assessments for the first 24 weeks and then reverted to 12-week assessments. Not all trials will require this specific scanning schedule, but studies that use alternative scanning schedules risk a reduction in the interpretability and significance of rPFS relative to OS, as determined in this trial.
In summary, our trial demonstrated that rPFS is highly positively associated with OS in patients with mCRPC. These data make no surrogate claims of rPFS and do not provide support for the use of rPFS to serve as a substitute for OS at this juncture.
Supplementary Material
Acknowledgment
We thank Ira Mills, PhD, of PAREXEL, for writing assistance.
Presented in part at the Annual Meeting of the European Society for Medical Oncology, Vienna, Austria, September 28-October 2, 2012.
Glossary Terms
- biomarker:
a functional biochemical or molecular indicator of a biologic or disease process that has predictive, diagnostic, and/or prognostic utility.
Appendix
Table A1.
Statistical Plan and Outcomes of COU-AA-302
| Overall Assumption | rPFS | OS |
|---|---|---|
| Alpha | .01 | .04 |
| Power, % | 91 | 85 |
| HR | 0.67 | 0.80 |
| No. of expected events | 378 | 773 |
NOTE. Additional information on study by Ryan et al17 (http://www.nejm.org/doi/suppl/10.1056/NEJMoa1209096/suppl_file/nejmoa1209096_protocol.pdf).
Abbreviations: COU-AA-302, Cougar–Abiraterone Acetate–Study 302; HR, hazard ratio; NA, not applicable; OS, overall survival; rPFS, radiographic progression-free survival.
13% OS events.
43% OS events.
56% OS events.
Estimated through Clayton copula.
Table A2.
rPFS Positively Associated With OS, Overall and Within Both Treatment Groups
| Group | Spearman's Correlation Coefficient | 95% CI | Kendall's Tau Statistic | 95% CI |
|---|---|---|---|---|
| Overall | 0.71 | 0.65 to 0.77 | 0.52 | 0.47 to 0.58 |
| Abiraterone plus prednisone | 0.82 | 0.75 to 0.88 | 0.63 | 0.56 to 0.70 |
| Prednisone alone | 0.60 | 0.50 to 0.69 | 0.43 | 0.35 to 0.51 |
NOTE. Total of 1,064 observations from 10 countries analyzed.
Abbreviations: OS, overall survival; rPFS, radiographic progression-free survival.
Support information appears at the end of this article.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Clinical trial information: NCT00887198.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Support
Supported by Ortho Biotech Oncology Research and Development (unit of Cougar Biotechnology, now Janssen Research & Development), by the Prostate Cancer Clinical Trials Consortium, sponsored by the Department of Defense, and by Janssen Global Services, which provided writing assistance.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Arturo Molina, Janssen Pharmaceuticals (C); Jinhui Li, Janssen Pharmaceuticals (C); Thian Kheoh, Janssen Pharmaceuticals (C); Shannon L. Matheny, Janssen Pharmaceuticals (C); Vahid Naini, Janssen Pharmaceuticals (C); Tomasz Burzykowski, International Drug Development Institute (C); Thomas W. Griffin, Janssen Pharmaceuticals (C) Consultant or Advisory Role: Johann S. de Bono, Johnson & Johnson (C); Karim Fizazi, Janssen Pharmaceuticals (C); Paul de Souza, GlaxoSmithKline (C), Janssen Pharmaceuticals (C), Pfizer (C); Philip W. Kantoff, Janssen Pharmaceuticals (C); Celestia S. Higano, Bayer (C), Dendreon (C), AbbVie (C), Algeta (C), Astellas Pharma (C), Ferring Pharmaceuticals (C), Medivation (C), Pfizer (C); Howard I. Scher, AstraZeneca (U), Astellas Pharma (U), BIND Pharmaceuticals (U), Bristol-Myers Squibb (U), Celgene (U), Chugai Academy for Advanced Oncology (C), Endocyte (U), Endo/Orion Pharmaceuticals (C), Exelixis (U), Foundation Medicine (U), Genentech (U), Janssen Pharmaceuticals (U), Veridex (U), Medivation (U), Novartis (U), OncologySTAT (C), Palmetto (C), Pfizer (U), sanofi-aventis (U), Takeda Millennium (U), TEVA Pharmaceutical (U), Ventana (U) Stock Ownership: Arturo Molina, Johnson & Johnson; Jinhui Li, Johnson & Johnson; Thian Kheoh, Johnson & Johnson; Vahid Naini, Johnson & Johnson; Tomasz Burzykowski, International Drug Development Institute; Thomas W. Griffin, Johnson & Johnson Honoraria: Johann S. de Bono, Johnson & Johnson, Astellas Pharma, sanofi-aventis; Paul de Souza, GlaxoSmithKline, Janssen Pharmaceuticals Research Funding: Celestia S. Higano, Cougar Biotechnology, Algeta, Aragon, AstraZeneca, Bayer, Dendreon, Exelixis, Genentech, Medivation, Millennium Pharmaceuticals, sanofi-aventis, TEVA Pharmaceuticals, Novartis, OncoGenex Technologies, ImClone Systems; Howard I. Scher, BIND Therapeutics, Exelixis, Genentech, Janssen Pharmaceuticals, Medivation, Janssen Diagnostics; Charles J. Ryan, Janssen Pharmaceuticals Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Michael J. Morris, Arturo Molina, Eric J. Small, Johann S. de Bono, Christopher J. Logothetis, Karim Fizazi, Celestia S. Higano, Thian Kheoh, Steven M. Larson, Thomas W. Griffin, Howard I. Scher, Charles J. Ryan
Provision of study materials or patients: Michael J. Morris, Eric J. Small, Johann S. de Bono, Christopher J. Logothetis, Karim Fizazi, Celestia S. Higano, Howard I. Scher, Charles J. Ryan
Collection and assembly of data: Michael J. Morris, Arturo Molina, Eric J. Small, Johann S. de Bono, Christopher J. Logothetis, Paul de Souza, Thian Kheoh, Steven M. Larson, Shannon L. Matheny, Vahid Naini, Thomas W. Griffin, Charles J. Ryan
Data analysis and interpretation: Michael J. Morris, Arturo Molina, Johann S. de Bono, Christopher J. Logothetis, Karim Fizazi, Philip W. Kantoff, Celestia S. Higano, Jinhui Li, Thian Kheoh, Steven M. Larson, Shannon L. Matheny, Vahid Naini, Tomasz Burzykowski, Thomas W. Griffin, Howard I. Scher, Charles J. Ryan
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 3.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 6.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 7.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 8.Scher HI, Morris MJ, Kelly WK, et al. Prostate cancer clinical trial end points: “RECIST”ing a step backwards. Clin Cancer Res. 2005;11:5223–5232. doi: 10.1158/1078-0432.CCR-05-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halabi S, Vogelzang NJ, Ou SS, et al. Progression-free survival as a predictor of overall survival in men with castrate-resistant prostate cancer. J Clin Oncol. 2009;27:2766–2771. doi: 10.1200/JCO.2008.18.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scher HI, Warren M, Heller G. The association between measures of progression and survival in castrate-metastatic prostate cancer. Clin Cancer Res. 2007;13:1488–1492. doi: 10.1158/1078-0432.CCR-06-1885. [DOI] [PubMed] [Google Scholar]
- 11.FDA Oncologic Drugs Advisory Committee Meeting on Prostate Cancer Endpoints; March 3, 2005; Bethesda, MD. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/CancerDrugs/ucm094586.htm. [Google Scholar]
- 12.Scher HI, Halabi S, Tannock I, et al. The Prostate Cancer Clinical Trials Working Group (PCCTWG) consensus criteria for phase II clinical trials for castration-resistant prostate cancer. J Clin Oncol. 2013;31(suppl 15s):321s. abstr 5057. [Google Scholar]
- 13.Fox JJ, Morris MJ, Larson SM, et al. Developing imaging strategies for castration resistant prostate cancer. Acta Oncol. 2011;50:39–48. doi: 10.3109/0284186X.2011.572914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris MJ, Farrelly JS, Fox JJ, et al. The Prostate Cancer Clinical Trials Consortium (PCCTC) bone scan data capture tool for clinical trials using Prostate Cancer Working Group 2 (PCWG2) criteria: Effect on data accuracy and workload. J Clin Oncol. 2011;(suppl):29. abstr 121. [Google Scholar]
- 15.Carducci MA, Saad F, Abrahamsson PA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–1966. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 16.Sternberg CN, Petrylak DP, Sartor O, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: The SPARC trial. J Clin Oncol. 2009;27:5431–5438. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 17.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burzykowski T, Molenberghs G, Buyse M, et al. Validation of surrogate end points in multiple randomized clinical trials with failure time end points. J Roy Stat Soc: Series C (Appl Stat) 2001;50:405–422. [Google Scholar]
- 19.US Food and Drug Administration. FDA Briefing Document: Oncologic Drugs Advisory Committee Meeting July 24, 2012—Examination of Radiologic Review of Progression-Free Survival in Non-Hematologic Malignancies. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/oncologicdrugsadvisorycommittee/ucm311141.pdf.
- 20.US Food and Drug Administration. Abiraterone acetate. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm331628.htm.
- 21.European Medicines Agency. Zytiga. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002321/human_med_001499.jsp&mid=WC0b01ac058001d124.
- 22.Kluetz PG, Ning YM, Maher VE, et al. Abiraterone acetate in combination with prednisone for the treatment of patients with metastatic castration-resistant prostate cancer: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res. 2013;19:6650–6656. doi: 10.1158/1078-0432.CCR-13-2134. [DOI] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration. FDA Briefing Document: Oncology Drugs Advisory Committee Meeting July 24, 2007—NDA 21801 Orplatna (satraplatin capsules) http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4309b1-04-fda.pdf.
- 24.Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu Rev Med. 2008;59:1–12. doi: 10.1146/annurev.med.59.090506.155819. [DOI] [PubMed] [Google Scholar]
- 25.Ryan CJ, Shah S, Efstathiou E, et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17:4854–4861. doi: 10.1158/1078-0432.CCR-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gignac GA, Morris MJ, Heller G, et al. Assessing outcomes in prostate cancer clinical trials: A twenty-first century tower of Babel. Cancer. 2008;113:966–974. doi: 10.1002/cncr.23719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.