Case Report
A 61-year-old woman was diagnosed in 1990 with malignant melanoma arising on the left shoulder and underwent wide local excision. No other details are available regarding the initial histology or work-up. In late 2011, the patient developed a left axillary mass. An attempted resection took place in early 2012. This procedure was aborted after the mass was deemed unresectable as a result of close proximity to essential neurovascular structures. However, a biopsy revealed metastatic melanoma with immunohistochemistry positive for Melan-A and S100. Histologically, this biopsy revealed sparse, individually disposed CD4+ and CD8+ T cells that were scattered throughout the tumor nodule (Figs 1A through 1C; H&E, CD4 and CD8, respectively). Mutational analysis of the tumor revealed a point mutation in codon 61 of NRAS resulting in replacement of glutamine for arginine in the product protein. Only wild-type sequences were found in BRAF. A positron-emission tomography scan revealed extensive hypermetabolic left axillary, infraclavicular, supraclavicular, and cervical lymphadenopathy as well as a hypermetabolic intraparotid lymph node.
Fig 1.
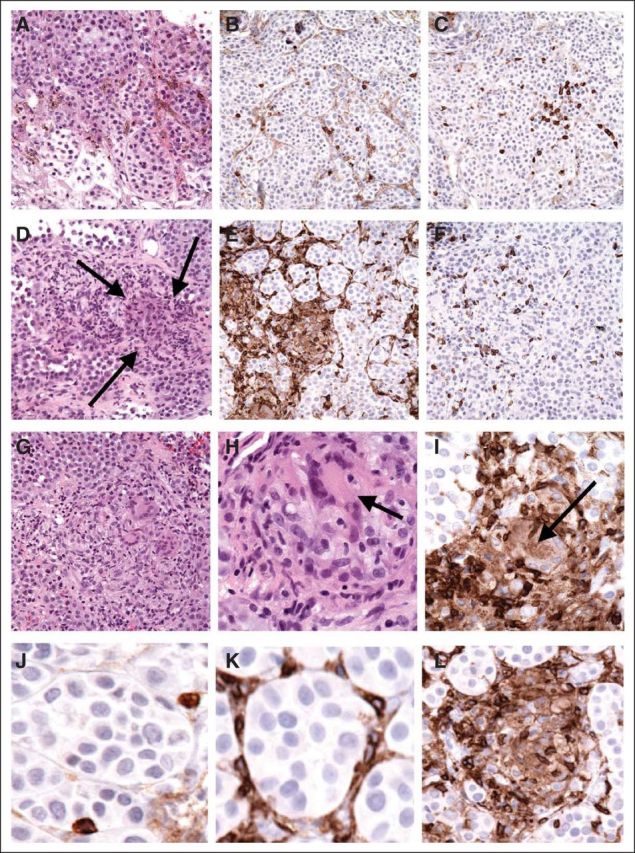
The patient was then referred to our institution, where she began receiving treatment with ipilimumab at a dose of 3 mg/kg every 3 weeks. In the 3 weeks between doses one and two, the patient was noted to have a massive increase in axillary lymphadenopathy. Before treatment, she had palpable disease within the axilla, although it did not affect her left arm. By the second dose, the mass had grown such that adduction of the patient's arm was limited in the final 45 degrees of motion. Two days after the second dose of ipilimumab, the patient began experiencing twice-daily relapsing fevers. These were consistently in the range of 38.9°C to 40°C and were associated with drenching sweats. The patient was evaluated on two occasions in emergency departments over the next 7 days, where blood cultures were negative, CBC was unremarkable, chest x-ray was clear, computed tomography (CT) of the abdomen was unremarkable, and no localizing signs of infection were observed. By day 10 of these fevers, the patient was admitted for palliative support. On physical examination, the left axillary lymphadenopathy had decreased to the size noted at her initial consultation. In the context of what seemed clinically to be an ipilimumab-induced cytokine storm, a biopsy of the left axillary tumor was obtained. This biopsy revealed focally brisk infiltration of tumor by T cells, with a marked CD4 predominance over CD8 (Figs 1D through 1F; H&E, CD4 and CD8, respectively) and multinucleated histiocytes (Fig 1D and 1H, arrows) that also stained for CD4 (Fig 1I, arrow) and formed small microgranulomas. The T cells varied from being singly disposed to aggregated at the perimeter of small tumor micronodules (Figs 1D through 1I). As opposed to the pretreatment specimen (Fig 1J), post-treatment infiltrating CD4+ T cells and histiocytes surrounded and obliterated small tumor nodules (Figs 1K and 1L, respectively). Correlation with adjacent hematoxylin and eosin–stained sections revealed that the obliterated micronodules were composed of both lymphocytes and multinucleated histiocytes that formed discrete granulomatous foci. Forkhead box P3–positive T cells were rare. The patient continued to experience high fevers and drenching sweats that were refractory to antipyretic agents (including acetaminophen, nonsteroidal anti-inflammatory drugs, and meperidine) for 2 additional days. Fifteen days after the second dose of ipilimumab, the patient experienced defervescence, although she was noted to have lost 11 kg in weight during the fever episode. After discharge, the patient developed facial asymmetry, low energy, and vision changes, which she described as like looking through a piece of paint-specked glass. Biochemical interrogation of the pituitary axis revealed no abnormalities, and magnetic resonance imaging of the brain with pituitary cuts indicated no stroke or hypophysitis. Neurologic and ophthalmologic consultations suggested diagnoses of Bell's palsy and uveitis, with supportive care and prednisolone eye drops as therapy, respectively.
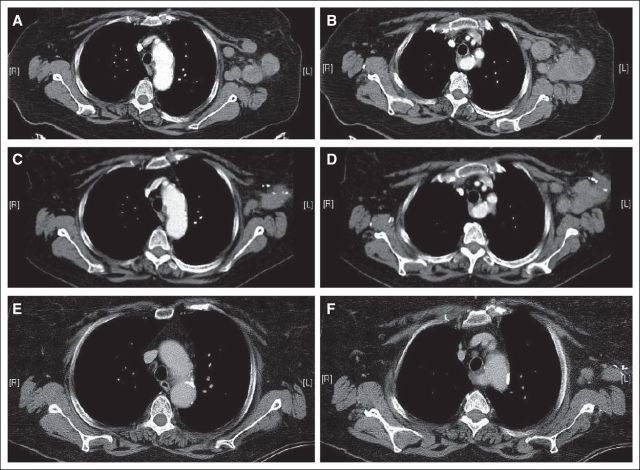
The patient returned to the clinic approximately 3 weeks later, after treatment with corticosteroid eye drops, and demonstrated resolution of facial asymmetry and no observable immune-related toxicity. A restaging CT scan confirmed that the melanoma had decreased in size to approximately the size seen on the patient's initial CT scan at diagnosis. Given that lymphadenopathy persisted and given the lack of future treatment options with significant expected efficacy, the patient requested a third dose of ipilimumab, and this was administered after a discussion of risks and benefits. Two weeks later, the patient described a return of the visual symptoms as well as the development of peripheral neuropathy involving the fingers, toes, and soles of her feet. Uveitis was again treated with corticosteroid eye drops. The neuropathy pain was described as a constant burning and tingling sensation. This increased over time, eventually involving her feet up to her knees bilaterally as well as her fingertips and the T10 right-sided cutaneous distribution. Functionally, the patient became unable to knit and began to have unsteadiness when walking down stairs. Interventions, including high-doses of gabapentin and prednisone, failed to provide benefit. Electromyography demonstrated a distal symmetric polyneuropathy with degeneration of sensory and motor axons in the feet as well as sensory axons in the hands. Given these symptoms, the fourth dose of ipilimumab was held, and over time, these neuropathy symptoms improved somewhat without other intervention. A subsequent restaging CT scan 6 weeks later showed near resolution of the adenopathy in the left axilla. This is shown in Figure 2, where two CT cuts demonstrate tumor shrinkage over time. Pretreatment scans are shown in Figures 2A and 2B, scans after two doses of ipilimumab and immune induction with fevers are shown in Figures 2C and 2D, and scans after three doses of ipilimumab and the development of neuropathy are shown in Figures 2E and 2F.
Fig 2.
Discussion
Although T-cell infiltrates in primary melanomas are not uncommon and are linked to prognosis,1,2 similar findings are rare in untreated metastases. Ipilimumab is a recombinant human monoclonal antibody that binds the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4). The rationale for its use in the treatment of melanoma is to block immune system downregulation by CTLA-4, which is normally achieved by competing with the costimulatory molecule CD28 for their common ligands CD80 and CD86.3,4 Current reports of ipilimumab-induced antimelanoma immune responses implicate antigen-specific CD8+ T cells as dominant effectors, although CD4+ T cells also are activated.5,6 In our patient, the dominant immune response was mediated by CD4+ rather than CD8+ T cells and discrete microgranulomas formed by activated CD4+ multinucleated histiocytes that seemed to collaborate in the destruction of tumor micronodules.
Antitumor granulomatous reactions have been described previously in primary tumors as well as in draining lymph nodes with or without metastases in a wide variety of solid tumors including breast, larynx, lung, stomach, colon, and kidney.7–14 In terms of their pathophysiology, it has been speculated that such granulomatous responses could represent hypersensitivity reactions to certain tumor antigens or constitute innate immune reactions to tumor cell necrosis.12,15 Ipilimumab, by unleashing T-cell–mediated immune responses, facilitates local and systemic production of cytokines.4 Granulomatous reactions are known to be triggered and sustained by cytokines such as interferon gamma, tumor necrosis factor α, and interleukin-12 that are produced by activated T cells and macrophages.16 In our patient, the antitumor granulomatous response was associated with tumor regression clinically and features of cytokine storm, both potentially relevant to the extent and nature of the immunohistochemical findings. Unfortunately, it has not been determined yet whether this type of immune reaction has any prognostic relevance.10,17
Although, to our knowledge, this is the first report documenting an active and effective granulomatous antimelanoma response as a result of ipilimumab treatment, previous granulomatous phenomena have been associated with this agent. Interstitial nephritis and pulmonary sarcoid-like infiltrates have been described in patient-derived samples, with dendritic cells, macrophages, as well as variable populations of CD4+, CD8+, and forkhead box P3–positive T cells being documented.18–20 However, these granulomas were described in the context of toxicity to the drug and were not directly implicated in the antitumor activity of anti–CTLA-4 therapy.
Preclinical studies have suggested that melanomas harboring activation of the mitogen-activated protein kinase pathway are associated with an immunosuppressive phenotype.21 However, paradoxically, patients with NRAS-mutated melanomas, as in our patient, have been preliminarily associated with increased response rates to high-dose interleukin-2 immunotherapy.22 Although it is unknown whether this association holds for immune checkpoint blockade approaches, it is of interest to speculate that the robust and effective antimelanoma response noted herein may relate to upstream factors that are integral to this patient's melanoma.
Clinically, this case highlights the diversity of immune-related adverse effects that are possible after anti–CTLA-4 treatment. Several reviews have described the incidence and management of these so-called events of special interest,23,24 as they are termed by the US Food and Drug Administration, although, to our knowledge, this is the first report of a syndrome of relapsing, spiking fevers associated with significant morbidity. Our patient case also highlights potential challenges related to achieving a balance between patient expectations for therapeutic benefit and the risk of immunotherapy-related toxicities. Early clinical trial data suggested a correlation between the development of immune-mediated toxicity and survival. However, this has not been validated in larger studies.25 Therefore, it is understandable that some patients are willing to risk additional toxicity with subsequent immunotherapy. This case emphasizes the ongoing need for improved therapeutic options for patients with unresectable or metastatic BRAF wild-type malignant melanoma.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: F. Stephen Hodi, Bristol-Myers Squibb (U) Stock Ownership: None Honoraria: None Research Funding: F. Stephen Hodi, Bristol-Myers Squibb Expert Testimony: None Patents: None Other Remuneration: None
REFERENCES
- 1.Spatz A, Gimotty PA, Cook MG, et al. Protective effect of a brisk tumor infiltrating lymphocyte infiltrate in melanoma: An EORTC melanoma group study. J Clin Oncol. 2007;25(suppl):476s. abstr 8519. [Google Scholar]
- 2.Clemente CG, Mihm MC, Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peggs KS, Quezada SA, Korman AJ, et al. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Yuan J, Ginsberg B, Page D, et al. CTLA-4 blockade increases antigen-specific CD8(+) T cells in prevaccinated patients with melanoma: Three cases. Cancer Immunol Immunother. 2011;60:1137–1146. doi: 10.1007/s00262-011-1011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: Clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58:1297–1306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyne JD. Necrobiotic palisading granulomas associated with breast carcinoma. J Clin Pathol. 2005;58:1290–1293. doi: 10.1136/jcp.2004.023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bässler R, Birke F. Histopathology of tumour associated sarcoid-like stromal reaction in breast cancer: An analysis of 5 cases with immunohistochemical investigations. Virchows Arch A Pathol Anat Histopathol. 1988;412:231–239. doi: 10.1007/BF00737147. [DOI] [PubMed] [Google Scholar]
- 9.Ophir D, Nissim F, Marshak G. Granulomatous reaction in lymph nodes draining laryngeal carcinoma. Head Neck Surg. 1986;8:214–217. doi: 10.1002/hed.2890080315. [DOI] [PubMed] [Google Scholar]
- 10.Kamiyoshihara M, Hirai T, Kawashima O, et al. Sarcoid reactions in primary pulmonary carcinoma: Report of seven cases. Oncol Rep. 1998;5:177–180. [PubMed] [Google Scholar]
- 11.Shigematsu H, Kurita A, Omura Y, et al. Gastric cancer with sarcoid reactions in the regional lymph nodes, the stomach wall, and the splenic parenchyma: Report of a case. Surg Today. 1999;29:549–552. doi: 10.1007/BF02482351. [DOI] [PubMed] [Google Scholar]
- 12.Coyne JD. Colonic carcinoma with granulomatous (sarcoid) reaction. J Clin Pathol. 2002;55:708–709. doi: 10.1136/jcp.55.9.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii T, Tabe Y, Yajima R, et al. Adenocarcinoma of ascending colon associated with sarcoid reaction in regional lymph nodes. Case Rep Gastroenterol. 2010;4:118–123. doi: 10.1159/000275064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregorie HB, Jr, Othersen HB, Jr, Moore MP., Jr The significance of sarcoid-like lesions in association with malignant neoplasms. Am J Surg. 1962;104:577–586. doi: 10.1016/0002-9610(62)90399-9. [DOI] [PubMed] [Google Scholar]
- 15.Brincker H. Sarcoid reactions in malignant tumours. Cancer Treat Rev. 1986;13:147–156. doi: 10.1016/0305-7372(86)90002-2. [DOI] [PubMed] [Google Scholar]
- 16.Sneller MC. Granuloma formation, implications for the pathogenesis of vasculitis. Cleve Clin J Med. 2002;69(suppl 2):SII40–SII43. doi: 10.3949/ccjm.69.suppl_2.sii40. [DOI] [PubMed] [Google Scholar]
- 17.Tomimaru Y, Higashiyama M, Okami J, et al. Surgical results of lung cancer with sarcoid reaction in regional lymph nodes. Jpn J Clin Oncol. 2007;37:90–95. doi: 10.1093/jjco/hyl141. [DOI] [PubMed] [Google Scholar]
- 18.Berthod G, Lazor R, Letovanec I, et al. Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J Clin Oncol. 2012;30:e156–e159. doi: 10.1200/JCO.2011.39.3298. [DOI] [PubMed] [Google Scholar]
- 19.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thajudeen B, Koppula S, Madhrira M. Ipilimumab induced granulomatous interstitial nephritis. National Kidney Foundation Spring Clinical Meetings; May 9-13, 2012; Washington, DC. (abstr 31) [Google Scholar]
- 21.Sumimoto H, Imabayashi F, Iwata T, et al. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph RW, Sullivan RJ, Harrell R, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immunother. 2012;35:66–72. doi: 10.1097/CJI.0b013e3182372636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: Detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119:1675–1682. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 24.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 25.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]