Abstract
Purpose
CS-1008 (tigatuzumab) is a humanized, monoclonal immunoglobulin G1 (IgG1) agonistic antibody to human death receptor 5. The purpose of this study was to investigate the impact of CS-1008 dose on the biodistribution, quantitative tumor uptake, and antitumor response in patients with metastatic colorectal cancer (mCRC).
Patients and Methods
Patients with mCRC who had received at least one course of chemotherapy were assigned to one of five dosage cohorts and infused with a weekly dose of CS-1008. Day 1 and day 36 doses were trace-labeled with indium-111 (111In), followed by whole-body planar and regional single-photon emission computed tomography (SPECT) imaging at several time points over the course of 10 days.
Results
Nineteen patients were enrolled. 111In-CS-1008 uptake in tumor was observed in only 12 patients (63%). 111In-CS-1008 uptake and pharmacokinetics were not affected by dose or repeated drug administration. 111In-CS-1008 biodistribution showed gradual blood-pool clearance and no abnormal uptake in normal tissue. No anti–CS-1008 antibody development was detected. One patient achieved partial response (3.7 months duration), eight patients had stable disease, and 10 patients had progressive disease. Clinical benefit rate (stable disease + partial response) in patients with 111In-CS-1008 uptake in tumor was 58% versus 28% in patients with no uptake. An analysis of individual lesions showed that lesions with antibody uptake were one third as likely to progress as those without antibody uptake (P = .07). Death-receptor–5 expression in archived tumor samples did not correlate with 111In-CS-1008 uptake (P = .5) or tumor response (P = .6).
Conclusion
Death-receptor–5 imaging with 111In-CS-1008 reveals interpatient and intrapatient heterogeneity of uptake in tumor, is not dose dependent, and is predictive of clinical benefit in the treatment of patients who have mCRC.
INTRODUCTION
Death receptor 5 (DR5), also known as tumor necrosis factor-related apoptosis-inducing ligand receptor 2 (TRAIL-R2), is a cell surface receptor with a cytoplasmic death domain that, when activated by its ligand (apoptosis ligand 2 [Apo2L/TRAIL]), triggers the extrinsic apoptotic pathway by activating caspases.1 DR5 is overexpressed in a variety of tumor types, including colon, gastric, pancreatic, lung, and cervical cancer, but with limited expression in normal tissues.2
CS-1008 (tigatuzumab) is a humanized, monoclonal immunoglobulin G1 (IgG1) antibody to human DR5 created by complementarity determining region–grafting the murine antibody TRA-8 (mTRA-8).3,4 Both mTRA-8 and CS-1008 showed potent in vitro cytotoxicity4 and significant in vivo antitumor activity against solid tumor xenografts.3,5 Preclinical studies demonstrated a direct correlation of CS-1008 uptake in tumor, receptor occupancy, and tumor growth inhibition, and receptor saturation in vivo was also associated with a threshold level of therapeutic effect.6
Clinically, CS-1008, similar to other DR5 agonists,7–14 showed a favorable toxicity profile with no dose-limiting toxicity at doses as high as 8 mg/kg/wk, and long-term disease stabilization was observed.9 However, none of the combination studies with DR5 agonists15–22 (Appendix Table A1, online only) achieved their end points of improving disease outcomes, thereby highlighting the potential importance of patient selection and/or rational therapeutic combinations.
Tumors are genetically unstable, as this is the most efficient way for them to evolve,23–25 and this may lead to significant heterogeneity between tumors as well as within a single tumor for receptor expression. In addition, antibody penetration into tumors may be nonuniform26 as a result of a variety of biophysical factors27 such as intervessel distance, interstitial pressure, receptor density, and internalization rate. Thus, heterogeneity of receptor expression or of antibody biodistribution may affect results of phase II studies by diluting the therapeutic benefit seen in a subset of patients or tumor masses.
In view of the linkage of DR5 activation and therapeutic efficacy,6 and the lack of clinical data on the relationship of dose to receptor occupancy and saturation, this study aimed to determine the biodistribution and tumor uptake of CS-1008, and to correlate these results with antitumor response in patients with metastatic colorectal cancer (mCRC).
PATIENTS AND METHODS
Eligibility Criteria
Patients with histologically proven mCRC who had received at least one course of chemotherapy for metastatic disease, with one target lesion ≥ 2 cm evaluable by gamma camera imaging and with an Eastern Cooperative Oncology Group performance status ≤ 2, were eligible. Other inclusion criteria were an age of ≥ 18 years; a life expectancy of at least 3 months; and adequate bone marrow, liver, and renal function. Patients on regular corticosteroid, nonsteroidal anti-inflammatory drug, or other immunosuppressive treatment within 3 weeks before first drug administration were excluded. Written informed consent from all patients and approval from the appropriate independent ethics committee were obtained.
Overall Study Design and Drug Administration
The trial was an open-label, single-site, phase I study. The primary objectives were to determine the impact of different loading doses on initial biodistribution, pharmacokinetics, and tumor uptake of indium-111 labeled CS-1008 (111In-CS-1008) and changes in biodistribution, pharmacokinetics, and tumor uptake following continuous sequential doses of CS-1008. Secondary objectives were to determine changes in tumor metabolism, antitumor response, changes in serum apoptosis biomarkers, and serum tumor response markers. Two to five patients were assigned to five nonsequential cohorts (Table 1) to facilitate optimal data acquisition for analysis of biodistribution, pharmacokinetics, and imaging characteristics across dosage levels.
Table 1.
Study Dose Cohorts
| Cohort No. | Day 1 111In-CS-1008 (mg/kg) | Day 8 CS-1008 (mg/kg) | Days 15, 22, 29 CS-1008 (mg/kg) | Day 36 111In-CS-1008 (mg/kg) | Day 43 CS-1008 (mg/kg) | Additional Cycles CS-1008 (mg/kg) |
|---|---|---|---|---|---|---|
| 1 | 0.2 | 6 | 2 | 2 | 2 | 2 |
| 2 | 1 | 6 | 2 | 2 | 2 | 2 |
| 3 | 2 | 6 | 2 | 2 | 2 | 2 |
| 4 | 4 | 4 | 2 | 2 | 2 | 2 |
| 5 | 6 | 2 | 2 | 2 | 2 | 2 |
Abbreviation: 111In-CS-1008, indium-111 labeled to CS-1008.
As outlined in Table 1, different CS-1008 loading doses were administered on day 1 and day 8 in each cohort, followed by an intravenous weekly dose of 2 mg/kg. These loading doses were selected on the basis of previous phase I data.9 Day 1 and day 36 doses were trace-labeled with 111In (2 mg of CS-1008 radiolabeled with 185 to 259 MBq [5-7 mCi]). The duration of the first cycle was 7 weeks, and patients with partial response (PR) or stable disease (SD) could receive additional CS-1008 until the occurrence of progressive disease (PD), unacceptable toxicity, or study withdrawal at the request of the patient or the treating physician. Additional cycles were scheduled as 4-week cycles and were administered weekly at a dose of 2 mg/kg.
Radiolabeling of CS-1008
The antibody CS-1008 was labeled with 111In (Nordion; Ottawa, Ontario, Canada) via the bifunctional metal ion chelate, CHX-A″-DTPA, according to methods previously described.28,29
Biodistribution and Tumor Uptake of 111In-CS-1008
Gamma camera imaging.
Gamma camera imaging with anterior and posterior whole-body sweep scans and single-photon emission computed tomography (SPECT) imaging of relevant regions with known tumor(s) were performed at five time points (day 1, day 2, day 4 or 5, day 7 or 8, and day 11 or 12), after the completion of the initial infusion of 111In-CS-1008. The whole-body gamma camera imaging and SPECT imaging after the day-36 infusion of 111In-CS-1008 (256.80 ± 13.29 MBq) were acquired on four time points (day 36, day 37, day 39 or 40, and day 42 or 43). All gamma camera imaging was performed on a dual-head gamma camera (SKYLight, Philips Medical Systems, North Milpitas, CA).
Quantitative Tumor Uptake
SPECT images acquired at different time points were coregistered with computed tomography (CT) images. Nonuniform CT attenuation correction of the coregistered SPECT images was performed with use of a simplified Chang algorithm.30–32 Volumes of interest (VOIs) were drawn around the whole tumor mass on the transverse slices of SPECT image at the time points at which the tumors were most clearly identified. The tumor VOI was then transposed onto all aligned images for a particular patient. Resultant counts in the tumor VOIs were then background corrected and converted to activity by calibrating counts to a standard of known activity that was in the field of view of the patient image.
Efficacy
Tumor response was assessed according to the response evaluation criteria in solid tumors (RECIST) version 1.1 guidelines.33 Disease assessment was based on CT and other appropriate imaging obtained at the time of screening, at the end of cycle 1 (EOC1) (day 44 to day 50), and, for patients receiving additional cycles, at the end of odd-numbered cycles and at the end of the study. The duration of overall response was measured according to RECIST guidelines.33
Tumor metabolic response to CS-1008 was assessed by [18F]fluorodeoxyglucose ([18F] FDG) positron emission tomography (PET)/CT scan performed at screening, at day 15, and at EOC1 according to the European Organisation for Research and Treatment of Cancer guidelines.34
Pharmacokinetics
Serum obtained from patients following 111In-CS-1008 infusion was aliquoted and counted in a gamma scintillation counter (Packard Instruments, Canberra, Australia). The results were expressed as the percent of injected dose per liter and μg/mL. A two-compartment intravenous bolus model (WNL model 8) was fitted to individual labeled infusions for each patient with use of unweighted, nonlinear least squares with WinNonlin (Scientific Consultant, Apex, NC) version 5.2 (Pharsight, Mountain View, CA). A validated sandwich enzyme-linked-immunosorbent–assay (ELISA) method was also used to measure CS-1008 concentrations in sera.
Human Anti–CS-1008 Antibody
Human anti–CS-1008 antibodies (HAHAs) were measured by Medpace Reference Laboratories (Cincinnati, OH) with use of a validated ELISA protocol.
DR5 Expression in Archived Tumor-Tissue Sample
DR5 (goat polyclonal) immunohistochemical testing was performed in formalin-fixed paraffin-embedded (FFPE) human cancer tissues in accordance with Mosaic Laboratories' standard operating procedures. DR5 immunohistochemistry staining was evaluated by a pathologist who assigned an H-score as follows: percentage of cells staining 0 (unstained), 1+ (weak staining), 2+ (moderate staining) and 3+ (strong staining) were recorded; the H-score was then calculated on the basis of the summation of the product of percent of cells stained at each intensity with use of the following equation: (3 × percentage of cells staining at 3+) + (2 × percentage of cells staining at 2+) + (1 × percentage of cells staining at 1+).
Serum Apoptotic Markers and Serum Tumor Response Biomarkers
Serum samples for biomarkers of apoptosis (caspase 3/7, 8, and M30) and tumor biomarkers (carcinoembryonic antigen [CEA]) were drawn at screening, before 111In-CS-1008 infusions on day 1 and day 36; and at 4 hours, 24 hours, and day 3 or 4 after these infusions. Blood samples for the measurement of CEA were also drawn on day 1 of additional cycles and at EOC1.
Statistical Considerations
All comparisons across cohorts were performed using a one-way analysis of variance. Comparison of paired data was performed by means of paired t test. The statistical significance of any correlation between tumor uptake, tumor response and DR5 expression was examined using Fisher's exact test. Simple least square linear regression was used to calculate the correlation coefficient between dose and uptake. A repeated measures analysis of variance was used to assess changes in serum biomarkers across all time points. All statistical tests were conducted using a two-sided alpha level of .05.
RESULTS
Patient Characteristics and Treatment
Nineteen patients (11 male, eight female) with a mean age of 64 years (range, 50 to 83 years) were entered into the trial between October 2010 and March 2012. Patient characteristics are summarized in Table 2.
Table 2.
Patient Characteristics
| Characteristic | All Patients (N = 19) |
|---|---|
| Age, years | |
| Mean | 64 |
| Range | 50-83 |
| Sex, no. of patients (%) | |
| Male | 11 (58%) |
| Female | 8 (42%) |
| ECOG performance status | |
| 0 | 6 |
| 1 | 12 |
| 2 | 1 |
| No. of prior chemotherapy regimens | |
| Median | 4 |
| Range | 2-6 |
| Primary site of disease | |
| Colon | 11 |
| Rectum | 8 |
| Histologic type of primary tumor | |
| Adenocarcinoma | 18 |
| Mucinous carcinoma (> 50% mucinous carcinoma) | 1 |
| Histologic grade (G)/differentiation | |
| G2: Moderately differentiated | 15 |
| G3: Poorly differentiated | 3 |
| Unknown | 1 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Eighteen patients completed cycle 1, and one patient was prematurely withdrawn on day 36 as the result of symptomatic deterioration secondary to PD. Nine patients received from one to five additional cycles of CS-1008. The number of CS-1008 infusions ranged from five to 27 (median, seven) with a cumulative dose per patient ranging from 899 to 5563 mg (mean, 2101 mg).
Biodistribution and Dosimetry Analyses
A similar biodistribution pattern was observed in all patients following all 111In-CS-1008 infusions. Evaluation of gamma camera imaging showed initial blood pooling, followed by some hepatic uptake by day 4 and gradual blood-pool clearance. Hepatic uptake was consistent with excretion of catabolized 111In-chelate, rather than specific CS-1008 uptake (Appendix Table A2, online only). There was also no discernible uptake of 111In-CS-1008 in any other normal tissue (Fig 1A). High, specific uptake of 111In-CS-1008 in tumor was visualized in the target lesions of 12 patients (Table 3) and was observed to peak on day 7 or 8 after each labeled infusion (Fig 1B).
Fig 1.
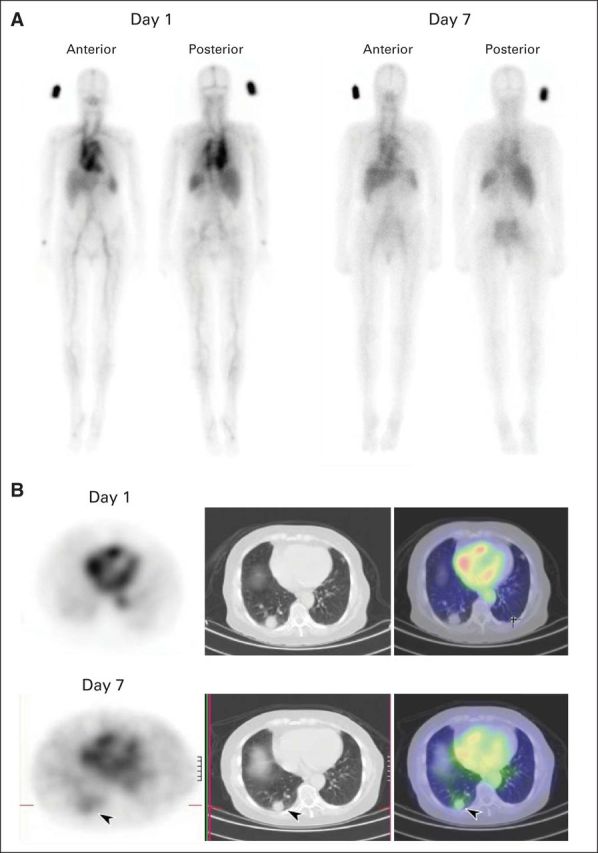
(A) Whole-body biodistribution of indium-111 labeled to CS-1008 (111In-CS-1008) in patient 014, showing gradual blood-pool clearance and no specific normal tissue uptake. (B) 111In-CS-1008 single-photon emission computed tomography and computed tomography (SPECT/CT) in patient 014 (left, SPECT; middle, CT; right, merged SPECT/CT), showing excellent uptake of 111In-CS-1008 in tumor (arrow) in right lung by day 7.
Table 3.
Patient Outcome and Disease Response
| Patient ID | Cohort | Age (years) | Sex | Best Response by RECIST (months on study) | Metabolic Response | 111In-CS-1008 Tumor Uptake |
|---|---|---|---|---|---|---|
| 001 | 1 | 68 | Male | PD (1.6) | PMD | No |
| 002 | 1 | 64 | Male | SD (6.9) | SMD | No |
| 004 | 3 | 50 | Male | SD (2.9) | SMD | No |
| 006 | 2 | 52 | Male | PD (1.7) | PMD | No |
| 009 | 5 | 80 | Male | PD (1.7) | PMD | No |
| 015 | 5 | 63 | Female | PD (1.5) | PMD | No |
| 019 | 3 | 63 | Male | PD (1.6) | SMD | No |
| 003 | 3 | 68 | Female | SD (3.7) | SMD | Yes |
| 005 | 2 | 59 | Female | SD (3.7) | SMD | Yes |
| 007 | 4 | 83 | Male | SD (3.7) | SMD | Yes |
| 008 | 4 | 57 | Female | SD (5.5) | SMD | Yes |
| 010 | 5 | 66 | Male | SD (3.8) | PMD | Yes |
| 011 | 2 | 65 | Female | PD (1.8) | PMD | Yes |
| 012 | 3 | 65 | Male | PD (1.8) | PMD | Yes |
| 013 | 5 | 51 | Female | PD (1.6) | PMD | Yes |
| 014 | 4 | 65 | Female | PR (6.3) | PMR | Yes |
| 016 | 3 | 74 | Female | PD (1.4) | PMD | Yes |
| 017 | 5 | 54 | Male | PD (1.7) | PMD | Yes |
| 018 | 2 | 62 | Male | SD (3.2) | SMD | Yes |
Abbreviations: ID, identification; 111In-CS-1008, indium-111 labeled to CS-1008; PD, progressive disease; PMD, progressive metabolic disease; PMR, partial metabolic response; SD, stable disease; SMD, stable metabolic disease.
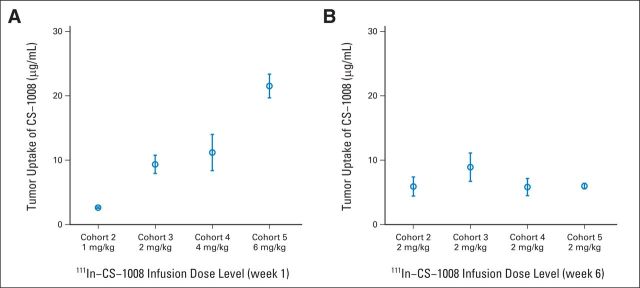
Five cohorts received different doses of CS-1008 as shown in Table 1. There was a strong, positive correlation between protein dose and quantitative tumor uptake (r = .95, P < .001). As shown in Figure 2A, increasing total CS-1008 protein dose on day 1 resulted in a corresponding proportional increase in tumor concentration of 111In-CS-1008. Similarly, in the comparison between day 1 and day 36 (Fig 2A and 2B), tumor concentration varied proportionally with the dose, without alteration in biodistribution, which was maintained and consistent with repeat infusions. Intervening cold doses did not change the amount or degree of biodistribution within the same patients. No receptor saturation was seen at the doses examined.
Fig 2.
Quantitative tumor uptake of indium-111 labeled to CS-1008 (111In-CS-1008; μg/mL) uptake after (A) week-1 and (B) week-6 infusions. Week-1 infusion showed linear increase in μg/mL 111In-CS-1008 uptake in tumors across all dose levels. Week-6 infusion, at 2 mg/kg in all patients, showed consistent CS-1008 uptake compared with week-1 infusion, indicating no death receptor 5 saturation with repeat infusions.
Pharmacokinetics
Pharmacokinetic results (Appendix Table A3, online only) demonstrated a terminal (β) half-life of 6 to 12 days (6 to 8.8 days by ELISA), with total serum clearance ranging from 12 to 18.5 mL/h. Maximum serum concentration (Cmax) and area under the serum concentration-time curve (AUC) values were dose proportional, showing a dose-dependent increase. The pharmacokinetic parameters were not significantly affected by repeated drug administration. The ELISA measurements were comparable with the results obtained with 111In-CS-1008 (Appendix Table A4, online only) analysis.
Antitumor Activity
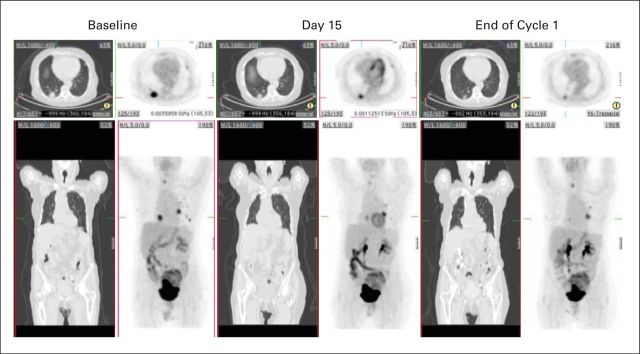
All patients were evaluable for antitumor response by RECIST criteria (Table 3). One patient achieved PR lasting 3.7 months (Fig 3). Eight patients had SD lasting a median of 4 months (range, 2.6 to 6.7 months). Ten patients had PD.
Fig 3.
Whole-body [18F]fluorodeoxyglucose ([18F]FDG) positron emission tomography (PET) and computed tomography (CT) in patient 014 with a partial response on CT and partial metabolic response on PET. Axial (top row) and coronal (bottom row) images of maximum-intensity projection CT and [18F]FDG PET images are displayed. Metastatic lesions in the right and left lungs show substantial shrinkage after treatment (reduction in maximum standardized update value, 43% and 38%, respectively), with the shrinkage identified as early as 2 weeks after commencement of treatment with CS-1008.
Metabolic response assessed by [18F]FDG PET and RECIST response at EOC1 were concordant in 17 patients. Patient 010 had SD on CT scan; however, this patient had a partial metabolic response on FDG PET.
111In-CS-1008 Uptake in Tumor
Of the 19 patients entered into the study, seven patients showed no uptake in any tumor site, while 12 patients showed some degree of tumor uptake: three at each of the 1, 2, 4 and 6 mg/kg dose levels. Interestingly, liver metastatic lesions showed generally poor 111In-CS-1008 uptake, with only one patient showing definite uptake equivalent or above liver background activity in any hepatic metastatic lesion. No significant differences were observed in visual tumor uptake between day 1 and day 36, and comparison between day-1 infusions also showed no effect of different loading doses on tumor uptake. Importantly, outcomes were notably different in patients whose tumors showed no 111In-CS-1008 uptake. Of the 12 patients with 111In-CS-1008 tumor uptake, seven patients had overall SD (n = 6) or PR (n = 1), for a clinical benefit rate of 58% (SD + PR). In contrast, five of the seven patients without CS-1008 tumor uptake experienced PD, for a clinical benefit rate of 28% (Fisher's exact test, P = .37).
111In-CS-1008 Tumor Uptake in Individual Target Lesions
We also examined the 111In-CS-1008 uptake in all reference lesions of each patient and correlated the uptake with the tumor response in those specific lesions. In the 12 patients for whom some uptake was seen, there were 24 evaluable lesions located in lung (n = 13), lymph nodes (n = 5), liver (n = 2), abdominal soft tissue (n = 2), thyroid (n = 1), and rectum (n = 1). Twenty-two of these lesions showed mild, moderate, or markedly increased 111In-CS-1008 scans uptake. The seven patients whose tumors showed no uptake had 20 evaluable lesions distributed between liver (n = 13), lung (n = 4), lymph nodes (n = 2), and soft tissue (n = 1).
For the 12 patients whose tumors showed 111In-CS-1008 uptake, we found that the lesions with uptake had an 88% probability of being stable or responding to treatment, even in patients with overall PD on restaging. The risk of PD in the lesions with no uptake was approximately three times higher than those lesions with uptake (40% v 12.5%, respectively). Lesions with uptake were one third as likely to progress compared with lesions without uptake, although the correlation between tumor response and CS-1008 uptake did not reach statistical significance (Fisher's exact test, P = .07).
HAHAs
There was no serologic evidence of HAHAs in any patient.
Pharmacodynamic Biomarkers
There was a trend toward an increase in caspase 8 on day 4 and an increase in M30 after sequential infusions. Caspase 3/7 tended to decrease in the postinfusion time points (Appendix Table A5, online only). Serum apoptotic markers were not significantly different between the two groups with and without 111In-CS-1008 tumor uptake and between the two groups with and without clinical benefit. CEA levels increased significantly (P = .027) at EOC1 in patients showing PD as compared with patients who had SD or PR.
DR5 Expression
Overall positive expression of DR5 was observed in all tumor specimens (range, 5% to 100%; average, 60%). Five patients had both primary and metastatic tumor available for the analyses of DR5. Metastatic tumor sites had a significantly higher mean H-score than that assigned to primary tumor sites for both membrane (68.6 ± 51.5 v 17.8 ± 17.2, respectively; P = .048) and cytoplasmic (81.8 ± 55.2 v 39.2 ± 38.6, respectively; P = .047) compartments. DR5 expression in archived tumor samples (with use of both H-score and percentage of positive cells) did not correlate with 111In-CS-1008 uptake nor with clinical outcome (Appendix Table A6, online only).
Toxicity
CS-1008 was well tolerated. Only one adverse event (grade 1 nausea) was considered possibly related to 111In-CS-1008. No serious adverse events were related to study treatment.
DISCUSSION
DR agonists represent a new class of therapeutics that selectively target apoptosis. Several monotherapeutic studies using DR4-DR5 agonist antibodies7–14 have demonstrated a favorable safety profile at the doses tested, with occasional but sustained responses and prolonged stable disease seen in isolated patients. However, when used in combination with established cancer therapeutics,15–22 none of the trials involving DR5 agonist antibodies reported to date have achieved their primary end points of improving response rate or progression-free survival. This indicates that agonistic antibodies against DR5 are only active in a subset of patients and that, therefore, it is crucial to identify accurate ways to preselect patients. Moreover, intrapatient molecular heterogeneity within tumors and evolutionary dynamics are critical obstacles for all targeted therapies, and the ability to monitor these factors noninvasively in real time is essential to address this issue optimally.35
In the current study, a novel molecular imaging signature predictive of DR5 agonist efficacy has been identified. We demonstrated that the reference lesions with 111In-CS-1008 uptake were less likely to progress even in patients with overall PD at restaging. Furthermore, we observed intra- and interpatient variability in DR5 uptake, with only 12 patients showing 111In-CS-1008 uptake in tumor. This variability is likely related to heterogeneous DR5 expression in tumor, although variable antibody penetration due to biophysical properties of individual tumor masses may also influence the concentration of antibody in tumor. A preclinical study6 of DR5 occupancy in vivo has shown that 111In-CS-1008 uptake correlated both with DR5 expression on tumor cells and the degree of antitumor activity. We also used SPECT imaging in this study, and a PET imaging approach may have allowed greater sensitivity in lesion detection.
In this clinical study, 111In-CS-1008 uptake in tumor demonstrated trends toward predicting clinical benefit (SD or PR) on both a per-patient and per-lesion basis, suggesting that lesions that progressed were likely DR5 negative or low DR5 expressers. It is noted that patients were not required to have PD at study entry, and therefore SD may be due to the biologic nature of disease in individual patients.
The 111In-CS-1008 biodistribution and dosimetry analyses showed that doses up to 6 mg/kg did not result in DR5 receptor saturation. Repeat 111In-CS-1008 infusions also demonstrated no saturation of DR5 receptors, with results that were similar to those observed after the first 111In-CS-1008 infusion, indicating a dynamic turnover of receptors on the tumor cell surface. Pharmacokinetic analysis showed proportional increases in Cmax and AUC with higher doses, and minor differences in T1/2α and elimination clearance between dose levels is likely a reflection of patient numbers in each cohort.
In agreement with previous studies,9,15,16 CS-1008 was well tolerated at doses as high as 6 mg/kg in heavily pretreated patients who had mCRC. Antitumor activity of CS-1008 was observed, with one patient achieving a PR and 8 patients having SD for a median duration of of 4 months.
Although several factors that predict sensitivity and resistance to DR5 agonists have been described in vitro,36–43 biomarkers that aid in patient selection or predict response to these agents in the clinic remain to be determined. In agreement with a prior study,14 a relationship between DR5 expression and antitumor activity could not be established in our study, and no relationship was found between DR5 expression and degree of 111In-CS-1008 tumor uptake. These findings suggest that it would be premature to use DR5 staining for screening, especially with archival tissue.
Taken together, our data suggest that tumor DR5 expression, assessed with use of molecular imaging of DR5 receptor occupancy (111In-CS-1008 imaging) reveals real-time heterogeneous DR5 expression and appears to be a promising predictive imaging biomarker of clinical benefit in patients with mCRC treated with DR5 agonist antibody.
Acknowledgment
Presented in part at the 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013. We thank the staff of the Cancer Clinical Trials Centre and the Department of Nuclear Medicine and Centre for PET, Austin Health, for their assistance with this study, and the patients and their families who participated in this trial. The study sponsor was the Ludwig Institute for Cancer Research, New York, NY.
Appendix
Table A1.
Published Clinical Trials of DR5 Agonistss
| Agonist | Site | Drug | Outcome | Safety |
|---|---|---|---|---|
| Conatumumab (C) AMG 655 (fully human monoclonal antibody DR5 agonist) | Advanced solid tumors7 | Phase I: single agent | 1 PR | No DLTs |
| Advanced solid tumors12 | Phase I: single agent | No antitumor responses | No DLTs | |
| NSCLC17 | Randomized phase II: paclitaxel and carboplatin + C (3 mg/kg, 15 mg/kg or placebo) | Did not improve PFS | Well tolerated | |
| Sarcoma18 | Phase I/randomized phase II: doxorubicin + C (15 mg/kg or placebo) | Did not improve PFS | Well tolerated | |
| CRC19 | Phase Ib/randomized phase II: mFOLFOX/bevacizumab + C (2 mg/kg, 10 mg/kg or placebo) | Did not improve PFS | Well tolerated | |
| CRC20 | Randomized phase II: FOLFIRI + C (10 mg/kg), ganitumab 12 mg/kg or placebo | Trend toward improved PFS | Well tolerated | |
| Pancreatic cancer21 | Randomized phase II: gemcitabine + C (10 mg/kg), ganitumab 12 mg/kg or placebo | Trend toward improved 6-mo survival rate | Well tolerated | |
| Tigatuzumab (T) CS-1008 (humanized monoclonal antibody DR5 agonist) | Advanced solid tumors9 | Phase I: single agent | No antitumor responses | No DLTs |
| NSCLC16 | Randomized phase II: paclitaxel/carboplatin + T versus placebo | Did not improve PFS | Well tolerated | |
| Pancreatic cancer15 | Single arm phase II: gemcitabine + T (single arm) | PFS similar to historical gemcitabine data | Well tolerated | |
| Lexatumumab (fully human monoclonal antibody DR5 agonist) | Advanced solid tumors14 | Phase I: single agent | No antitumor responses | 4 DLTs |
| Advanced solid tumors11 | Phase I: single agent | No antitumor responses | 1 DLT | |
| Drozitumab (D) PRO95780 (fully human monoclonal antibody DR5 agonist) | Advanced solid tumors13 | Phase I: single agent | No antitumor responses | 2 DLTs |
| CRC22 | Phase Ib: FOLFOX/bevacizumab + D (two dose cohorts) | 2 PRs | No DLTs |
Abbreviations: CRC, colorectal cancer; DLT, dose-limiting toxicity; DR5, death receptor 5; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; NSCLC, non–small-cell lung cancer; PFS, progression-free survival; PR, partial response.
Table A2.
Quantitative Uptake of 111In-CS-1008 in Liver
| 111In-CS-1008 Infusion | Dose Cohort Group (mg/kg) | Liver Uptake |
|||
|---|---|---|---|---|---|
| %ID/g |
μg/mL |
||||
| Mean | SD | Mean | SD | ||
| Week-1 infusion day 7 or 8 scan | 1 | .0077 | .0024 | 5.22 | .99 |
| 2 | .0059 | .0000 | 9.50 | .00 | |
| 4 | .0084 | .0034 | 22.40 | 7.39 | |
| 6 | .0068 | .0001 | 34.63 | 4.29 | |
| Week-6 infusion day 42 or 43 scan | 1 | .0075 | .0026 | 9.85 | 1.55 |
| 2 | .0067 | .0000 | 10.81 | .00 | |
| 4 | .0102 | .0024 | 13.87 | .97 | |
| 6 | .0062 | .0006 | 11.00 | 2.34 | |
Abbreviations: 111In-CS-1008, indium-111 labeled to CS-1008; %ID/g, percent of injected dose per gram.
Table A3.
Mean Pharmacokinetic Parameters After Initial Dose of 111In-CS-1008
| No. of Patients* | Cohort No. (day-1 dose, mg/kg) | t½α Mean ± SD (hr) | t½β Mean ± SD (hr) | V1 Mean ± SD (mL) | AUC Mean ± SD, (hr × μg/mL) | CL Mean ± SD (mL/h) | Cmax Mean ± SD (μg/mL) |
|---|---|---|---|---|---|---|---|
| 2 | 1 (0.2) | 14.51 ± 5.95 | 284.76 ± 0.38 | 3,209.83 ± 321.96 | 986.58 ± 125.11 | 14.92 ± 2.50 | 4.59 ± 0.89 |
| 4 | 2 (1) | 21.52 ± 5.76 | 264.89 ± 122.12 | 2,592.36 ± 303.21 | 5,706.38 ± 2,632.72 | 12.31 ± 3.38 | 24.60 ± 3.75 |
| 5 | 3 (2) | 5.29 ± 4.76 | 163.08 ± 39.86 | 3,329.11 ± 624.49 | 8,386.68 ± 855.70 | 18.23 ± 0.51 | 47.29 ± 10.19 |
| 2 | 4 (4) | 10.45 ± 6.85 | 243.39 ± 52.21 | 2,658.70 ± 112.60 | 18,714.05 ± 1,511.25 | 12.00 ± 2.02 | 84.14 ± 11.00 |
| 4 | 5 (6) | 14.73 ± 4.72 | 247.50 ± 52.90 | 4,036.84 ± 424.72 | 28,492.39 ± 1,598.03 | 18.52 ± 2.61 | 131.32 ± 20.05 |
| One-way ANOVA, P | .01 | .219 | .007 | < .001 | .006 | < .001 |
Abbreviations: ANOVA, analysis of variance; AUC, area under the serum concentration time-curve; CL, total serum clearance; Cmax, maximum serum concentration; 111In-CS-1008, indium-111 labeled to CS-1008; t1/2α, half-life of distribution phase of drug; t1/2β, half-life of elimination phase of drug; V1, volume of central compartment.
Patients 007 and 009 were not included in the determination of mean parameter values because of curve fit solution instability.
Table A4.
111In-CS-1008 AUC, Clearance, and Cmax Compared With CS-1008 Protein (ELISA)
| Parameter | Cohort |
111In-CS-1008 |
Serum CS-1008 (ELISA) |
P (paired t test) | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| AUC, hr × μg/mL | 1 | 986.58 | 125.11 | 872.03 | 39.27 | .43 |
| 2 | 5,706.38 | 2,632.72 | 7,434.69 | 3,083.27 | .49 | |
| 3 | 8,386.68 | 855.70 | 14,445.42 | 5,160.21 | .34 | |
| 4 | 18,714.05 | 1,511.25 | —* | —* | —* | |
| 5 | 28,492.39 | 1,598.03 | 33,683.99 | 11,876.96 | .53 | |
| CL, mL/h | 1 | 14.92 | 2.50 | 17.16 | 5.71 | .70 |
| 2 | 12.31 | 3.38 | 9.35 | 2.02 | .20 | |
| 3 | 18.23 | 20.51 | 10.62 | 2.46 | .14 | |
| 4 | 12.00 | 2.03 | —* | —* | —* | |
| 5 | 18.52 | 2.61 | 16.39 | 5.05 | .57 | |
| Cmax, μg/mL | 1 | 4.59 | 0.89 | 10.54 | 7.11 | .45 |
| 2 | 24.60 | 10.19 | 31.34 | 4.74 | .07 | |
| 3 | 47.29 | 10.19 | 83.58 | 0.16 | .001 | |
| 4 | 84.14 | 11.00 | —* | —* | —* | |
| 5 | 131.3 | 20.05 | 170.20 | 52.08 | .25 | |
Abbreviations: AUC, area under the serum concentration-time curve; CL, total serum clearance; Cmax, maximum serum concentration; ELISA, enzyme-linked immunosorbent assay; 111In-CS-1008, indium-111 labeled to CS-1008; SD, standard deviation.
Outliers with a disproportionate effect on estimated parameters were excluded from data analysis; therefore, because of the lack of data, no statistics could be performed in cohort 4.
Table A5.
Serum Apoptotic Biomarker Levels
| Time Points | Caspase 8 (μ/mL) | Caspase 3/7 (μ/mL) | M30 (μ/L) |
|---|---|---|---|
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | |
| Baseline | .21 ± .20 | .15 ± .26 | 490.86 ± 616.89 |
| Day 1, 4 hrs postinfusion | .17 ± .19 | .05 ± .04 | 442.03 ± 625.39 |
| Day 2 | .14 ± .13 | .06 ± .07 | 429.63 ± 494.15 |
| Day 4 or 5 | .34 ± .66 | .07 ± .06 | 401.22 ± 466.13 |
| Day 36, preinfusion | .23 ± .31 | .05 ± .06 | 714.65 ± 1264.56 |
| Day 36, 4 hrs postinfusion | .21 ± .29 | .04 ± .04 | 553.53 ± 734.82 |
| Day 37 | .28 ± .46 | .06 ± .08 | 620.12 ± 979.83 |
| Day 39 or 40 | .23 ± .32 | .10 ± .15 | 532.95 ± 616.73 |
| Repeated measures ANOVA, P | .633 | .042 | .1 |
Abbreviations: ANOVA, analysis of variance; SD, standard deviation.
Table A6.
DR5 Expression, 111In-CS-1008 Uptake, and Antitumor Response
| Patient ID | 111In-CS-1008 Uptake in Tumor* | DR5 IHC (H-score membrane staining) | DR5 IHC (H-score cytoplasmic staining) | Best Response (RECIST) |
|---|---|---|---|---|
| Patients with uptake in tumor and overall SD or PR | ||||
| 003 | 4 | 20 | 56 | SD |
| 005 | 4 | 14 | 21 | SD |
| 007 | 3 | 90 | 127 | SD |
| 008 | 3 | 63 | 93 | SD |
| 014 | 3 | 92 | 102 | PR |
| 018 | 4 | 15 | 110 | SD |
| 010 | 3 | 93 | 102 | SD |
| Patients with uptake in tumor and overall PD | ||||
| 011 | 3 | 19 | 25 | PD |
| 012 | 4 | 70 | 80 | PD |
| 013 | 4 | 76 | 87 | PD |
| 016 | 2 | 34 | 68 | PD |
| 017 | 3 | 21 | 34 | PD |
| Patients with no uptake in tumor | ||||
| 001 | 1 | 30 | 70 | PD |
| 002 | 1 | 110 | 135 | SD |
| 004 | 1 | 35 | 60 | SD |
| 006 | 1 | 90 | 127 | PD |
| 009 | 1 | 30 | 70 | PD |
| 015 | 1 | 106 | 124 | PD |
| 019 | 1 | 0 | 80 | PD |
NOTE. No significant associations were found between membrane staining (P > .9) or cytoplasmic staining (P = .5) and 111In-CS-1008 tumor uptake. Tumor response was not associated with DR5 expression (P = .6 for membrane staining; P > .9 for cytoplasmic staining).
Abbreviations: DR5, death receptor 5; IHC, immunohistochemistry; 111In-CS-1008, indium-111 labeled to CS-1008; PD, progressive disease; PR, partial response; SD, stable disease.
Visual grading of maximum intensity of 111In-CS-1008 uptake in tumor: 1, no uptake; 2, mild uptake; 3, moderate uptake; 4, marked uptake.
Footnotes
See accompanying editorial on page 2585
Supported by Daiichi Sankyo Co., Ltd.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01220999.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Archie N. Tse, Mendel Jansen, Ralph Venhaus, Robert A. Beckman, Andrew M. Scott
Administrative support: Ralph Venhaus
Collection and assembly of data: Marika Ciprotti, Niall C. Tebbutt, Fook-Thean Lee, Sze-Ting Lee, Hui K. Gan, David C. McKee, Geoffrey Chong, Wendie Hopkins, Bridget Chappell, Fiona E. Scott, Martin W. Brechbiel, Rira Watanabe, Ralph Venhaus, Jonathan Greenberg, Andrew M. Scott
Data analysis and interpretation: Marika Ciprotti, Niall C. Tebbutt, Fook-Thean Lee, Sze-Ting Lee, Hui K. Gan, Graeme J. O'Keefe, Sylvia J. Gong, Fiona E. Scott, Archie N. Tse, Mendel Jansen, Manabu Matsumura, Masakatsu Kotsuma, Ralph Venhaus, Robert A. Beckman, Andrew M. Scott
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase I Imaging and Pharmacodynamic Trial of CS-1008 in Patients With Metastatic Colorectal Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Marika Ciprotti
Employment: Daiichi Sankyo
Niall C. Tebbutt
No relationship to disclose
Fook-Thean Lee
No relationship to disclose
Sze-Ting Lee
No relationship to disclose
Hui K. Gan
Honoraria: Novartis
Consulting or Advisory Role: Merck Serono
Speakers' Bureau: Merck Serono, Pfizer
Travel, Accommodations, Expenses: Bayer
David C. McKee
No relationship to disclose
Graeme J. O'Keefe
No relationship to disclose
Sylvia J. Gong
No relationship to disclose
Geoffrey Chong
Research Funding: Amgen (Inst), Novartis (Inst), Astellas Pharma (Inst), Incyte (Inst)
Wendie Hopkins
No relationship to disclose
Bridget Chappell
No relationship to disclose
Fiona E. Scott
Research Funding: Daiichi Sankyo (Inst)
Martin W. Brechbiel
No relationship to disclose
Archie N. Tse
Employment: Daiichi Sankyo
Stock or Other Ownership: Daiichi Sankyo
Patents, Royalties, Other Intellectual Property: Daiichi Sankyo
Mendel Jansen
Employment: Daiichi Sankyo
Stock or Other Ownership: Daiichi Sankyo
Manabu Matsumura
Employment: Daiichi Sankyo
Stock or Other Ownership: Daiichi Sankyo
Masakatsu Kotsuma
Employment: Daiichi Sankyo
Rira Watanabe
Employment: Daiichi Sankyo
Ralph Venhaus
Stock or Other Ownership: Agenus, Dynavax, Soligenix
Robert A. Beckman
Employment: Daiichi Sankyo
Stock or Other Ownership: Johnson and Johnson
Patents, Royalties, Other Intellectual Property: Onco-Mind
Other Relationship: Consultant for the Cancer Institute of New Jersey
Jonathan Greenberg
Employment: Daiichi Sankyo
Stock or Other Ownership: Daiichi Sankyo
Andrew M. Scott
Research Funding: Daiichi Sankyo, Abbvie, Avipep
Patents, Royalties, Other Intellectual Property: Patents held
REFERENCES
- 1.de Vries EGE, Gietema JA, de Jong S. Tumor necrosis factor-related apoptosis-inducing ligand pathway and its therapeutic implications. Clin Cancer Res. 2006;12:2390–2393. doi: 10.1158/1078-0432.CCR-06-0352. [DOI] [PubMed] [Google Scholar]
- 2.Daniels RA, Turley H, Kimberley FC, et al. Expression of TRAIL and TRAIL receptors in normal and malignant tissues. Cell Res. 2005;15:430–438. doi: 10.1038/sj.cr.7290311. [DOI] [PubMed] [Google Scholar]
- 3.Ichikawa K, Liu W, Zhao L, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–960. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 4.Yada A, Yazawa M, Ishida S, et al. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann Oncol. 2008;19:1060–1067. doi: 10.1093/annonc/mdn015. [DOI] [PubMed] [Google Scholar]
- 5.Buchsbaum DJ, Zhou T, Grizzle WE, et al. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res. 2003;9:3731–3741. [PubMed] [Google Scholar]
- 6.Burvenich IJG, Lee FT, Cartwright GA, et al. Molecular imaging of death receptor 5 occupancy and saturation kinetics in vivo by humanized monoclonal antibody CS-1008. Clin Cancer Res. 2013;19:5984–5993. doi: 10.1158/1078-0432.CCR-12-3104. [DOI] [PubMed] [Google Scholar]
- 7.Herbst RS, Kurzrock R, Hong DS, et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res. 2010;16:5883–5891. doi: 10.1158/1078-0432.CCR-10-0631. [DOI] [PubMed] [Google Scholar]
- 8.Hotte SJ, Hirte HW, Chen EX, et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:3450–3455. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 9.Forero-Torres A, Shah J, Wood T, et al. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5) Cancer Biother Radiopharm. 2010;25:13–19. doi: 10.1089/cbr.2009.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolcher AW, Mita M, Meropol NJ, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25:1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 11.Wakelee HA, Patnaik A, Sikic BI, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol. 2010;21:376–381. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi T, Murakami H, Ohtsu A, et al. Phase I study of conatumumab, a pro-apoptotic death receptor 5 agonist antibody, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;68:733–741. doi: 10.1007/s00280-010-1544-1. [DOI] [PubMed] [Google Scholar]
- 13.Camidge DR, Herbst RS, Gordon MS, et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res. 2010;16:1256–1263. doi: 10.1158/1078-0432.CCR-09-1267. [DOI] [PubMed] [Google Scholar]
- 14.Plummer R, Attard G, Pacey S, et al. Phase I and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 15.Forero-Torres A, Infante JR, Waterhouse D, et al. Phase II, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer. Cancer Med. 2013;2:925–932. doi: 10.1002/cam4.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reck M, Krzakowski M, Chmielowska E, et al. A randomized, double-blind, placebo-controlled phase II study of tigatuzumab (CS-1008) in combination with carboplatin/paclitaxel in patients with chemotherapy-naive metastatic/unresectable non-small cell lung cancer. Lung Cancer. 2013;82:441–448. doi: 10.1016/j.lungcan.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Paz-Ares L, Balint B, de Boer RH, et al. A randomized phase II study of paclitaxel and carboplatin with or without conatumumab for first-line treatment of advanced non-small-cell lung cancer. J Thorac Oncol. 2013;8:329–337. doi: 10.1097/JTO.0b013e31827ce554. [DOI] [PubMed] [Google Scholar]
- 18.Demetri GD, Le Cesne A, Chawla SP, et al. First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: A phase I/II open-label and double-blind study. Eur J Cancer. 2012;48:547–563. doi: 10.1016/j.ejca.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs CS, Fakih M, Schwartzberg L, et al. TRAIL receptor agonist conatumumab with modified FOLFOX6 plus bevacizumab for first-line treatment of metastatic colorectal cancer: A randomized phase 1b/2 trial. Cancer. 2013;119:4290–4298. doi: 10.1002/cncr.28353. [DOI] [PubMed] [Google Scholar]
- 20.Cohn AL, Tabernero J, Maurel J, et al. A randomized, placebo-controlled phase II study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer. Ann Oncol. 2013;24:1777–1785. doi: 10.1093/annonc/mdt057. [DOI] [PubMed] [Google Scholar]
- 21.Kindler HL, Richards DA, Garbo LE, et al. A randomized, placebo-controlled phase II study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol. 2012;23:2834–2842. doi: 10.1093/annonc/mds142. [DOI] [PubMed] [Google Scholar]
- 22.Rocha Lima CM, Bayraktar S, Flores AM, et al. Phase Ib study of drozitumab combined with first-line mFOLFOX6 plus bevacizumab in patients with metastatic colorectal cancer. Cancer Invest. 2012;30:727–731. doi: 10.3109/07357907.2012.732163. [DOI] [PubMed] [Google Scholar]
- 23.Beckman RA, Loeb LA. Efficiency of carcinogenesis with and without a mutator mutation. Proc Natl Acad Sci U S A. 2006;103:14140–14145. doi: 10.1073/pnas.0606271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckman RA. Efficiency of carcinogenesis: Is the mutator phenotype inevitable? Semin Cancer Biol. 2010;20:340–352. doi: 10.1016/j.semcancer.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams GP, Schier R, McCall AM, et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001;61:4750–4755. [PubMed] [Google Scholar]
- 27.Beckman RA, Weiner LM, Davis HM. Antibody constructs in cancer therapy: Protein engineering strategies to improve exposure in solid tumors. Cancer. 2007;109:170–179. doi: 10.1002/cncr.22402. [DOI] [PubMed] [Google Scholar]
- 28.Wu C, Kobayashi H, Sun B, et al. Stereochemical influence on the stability of radio-metal complexes in vivo. Synthesis and evaluation of the four stereoisomers of 2-(p-nitrobenzyl)-trans-CyDTPA. Bioorg Med Chem. 1997;5:1925–1934. doi: 10.1016/s0968-0896(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 29.Lee FT, Rigopoulos A, Hall C, et al. Specific localization, gamma camera imaging, and intracellular trafficking of radiolabelled chimeric anti-G(D3) ganglioside monoclonal antibody KM871 in SK-MEL-28 melanoma xenografts. Cancer Res. 2001;61:4474–4482. [PubMed] [Google Scholar]
- 30.Chang L-T. A method for attenuation correction in radionuclide computed tomography. IEEE Transactions on Nuclear Science. 1978;25:638–643. [Google Scholar]
- 31.Tsui BM, Gullberg GT, Edgerton ER, et al. Correction of nonuniform attenuation in cardiac SPECT imaging. J Nucl Med. 1989;30:497–507. [PubMed] [Google Scholar]
- 32.Seo Y, Wong KH, Hasegawa BH. Calculation and validation of the use of effective attenuation coefficient for attenuation correction in In-111 SPECT. Med Phys. 2005;32:3628–3635. doi: 10.1118/1.2128084. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 35.Beckman RA, Schemmann GS, Yeang CH. Impact of genetic dynamics and single-cell heterogeneity on development of nonstandard personalized medicine strategies for cancer. Proc Natl Acad Sci U S A. 2012;109:14586–14591. doi: 10.1073/pnas.1203559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 37.Lane D, Cote M, Grondin R, et al. Acquired resistance to TRAIL-induced apoptosis in human ovarian cancer cells is conferred by increased turnover of mature caspase-3. Mol Cancer Ther. 2006;5:509–521. doi: 10.1158/1535-7163.MCT-05-0362. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Xu X, Bai L, et al. Epidermal growth factor receptor-mediated tissue transglutaminase overexpression couples acquired tumor necrosis factor-related apoptosis-inducing ligand resistance and migration through c-FLIP and MMP-9 proteins in lung cancer cells. J Biol Chem. 2011;286:21164–21172. doi: 10.1074/jbc.M110.207571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falschlehner C, Emmerich CH, Gerlach B, et al. TRAIL signalling: Decisions between life and death. Int J Biochem Cell Biol. 2007;39:1462–1475. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Song JJ, Szczepanski MJ, Kim SY, et al. C-Cbl-mediated degradation of TRAIL receptors is responsible for the development of the early phase of TRAIL resistance. Cell Signal. 2010;22:553–563. doi: 10.1016/j.cellsig.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang JH, Moritz W, Graf R, et al. Preconditioning with death ligands FasL and TNF-alpha protects the cirrhotic mouse liver against ischaemic injury. Gut. 2008;57:492–499. doi: 10.1136/gut.2007.137703. [DOI] [PubMed] [Google Scholar]
- 42.Song JJ, An JY, Kwon YT, et al. Evidence for two modes of development of acquired tumor necrosis factor-related apoptosis-inducing ligand resistance. Involvement of Bcl-xL. J Biol Chem. 2007;282:319–328. doi: 10.1074/jbc.M608065200. [DOI] [PubMed] [Google Scholar]
- 43.Malhi H, Gores GJ. TRAIL resistance results in cancer progression: A TRAIL to perdition? Oncogene. 2006;25:7333–7335. doi: 10.1038/sj.onc.1209765. [DOI] [PubMed] [Google Scholar]