Abstract
Serrated pathway polyps are a relatively new area of interest in the field of colorectal cancer screening and prevention. Akin to conventional adenomas, some serrated polyps (SPs) have the potential to develop into malignant serrated neoplasms, yet little is known regarding risk factors for these lesions. Early epidemiological studies of hyperplastic polyps (HPs) were performed without knowledge of the serrated pathway, and likely included a mixture of SPs. More recently, studies have specifically evaluated premalignant SPs, such as the sessile serrated adenoma (SSA) or surrogates for these polyps such as large or proximally-located SPs. SPs share some risk factors with conventional adenomas, and have been associated with tobacco use, obesity, and age. Nonsteroidal anti-inflammatory drug (NSAID) use, fiber, folic acid, and calcium have been associated with reduced risk of SPs. Studies focused on SSAs specifically have reported associations with age, female sex, smoking, obesity, diabetes, and possibly diets high in fat, carbohydrates, and calories. Higher education has also been associated with risk of SSAs, while an inverse association between NSAID use and SSAs has been reported. Risk factors for traditional serrated adenomas (TSAs) are largely unknown. Studies are largely limited by varying inclusion criteria, as well as differences in pathological classification schemes. Further epidemiological studies of SPs are needed to aid in risk stratification and screening, and etiological research.
Keywords: Colonic Neoplasms (MeSH), Colonic Polyps (MeSH), Adenoma (MeSH), Epidemiology (MeSH), Sessile serrated adenoma, Sessile serrated polyp, Hyperplastic polyp, Traditional serrated adenoma
Introduction
Serrated neoplasia is a relatively recent concept in the field of colorectal cancer pathogenesis. While knowledge of “hyperplastic” or “metaplastic” polyps of the colorectum has existed for over 50 years, these lesions were traditionally thought to be common yet harmless mucosal lesions without potential for progression [1]. It is now recognized that some polyps previously classified as benign HPs included more dangerous lesions, such as sessile serrated adenomas (SSAs) and traditional serrated adenomas (TSAs), which are important precursor lesions in the development of serrated pathway carcinomas. Serrated polyps (SPs) are thus a heterogeneous family of colorectal polyps that do pose a risk of malignant transformation.
SSAs are typically located in the proximal colon and are flat and subtle lesions that can be difficult to detect during colonoscopy [2, 3]. As such, SSAs may be missed and resected incompletely by endoscopists [4-6]. Additionally, multiple lines of evidence suggest that serrated pathway precursors contribute to the problem of interval cancers, which is an important concern for gastroenterologists and patients alike [7-12]. Furthermore, there is evidence that proximal SPs pose a greater risk of metachronous neoplasia than tubular adenomas, indicating their importance in screening and surveillance [13].
However, SPs have only recently been addressed by surveillance guidelines [14]. Furthermore, despite their importance, the epidemiology of serrated pathway polyps is relatively understudied compared to conventional adenomas. The aim of this review is to highlight several of the previous epidemiological studies that have been performed regarding SPs, and to identify established risk factors, and those that merit further research. Ultimately, a better understanding of the epidemiology of these important CRC precursors could help tailor screening and surveillance recommendations and inform etiological basic science research.
Overview of the Serrated Pathway and Serrated Polyp Types
Serrated pathway
Until relatively recently, it was thought that conventional adenomas were the sole precursors to sporadic CRC, as they contained dysplastic tissue and had the potential for malignant transformation via the well-characterized adenoma-carcinoma sequence involving genetic mutations in KRAS, p53, and APC genes [15]. However, within the last two decades it has been shown that certain SPs have the potential to develop into CRC as well. SSAs and TSAs are considered to be the primary precursors to serrated pathway cancers [10, 16-21]. The progression from SSA or TSA to CRC involves different molecular features than the adenoma-carcinoma sequence, such as BRAF mutation, CpG island methylation (CIMP), and microsatellite instability (MSI) [22]. As with the adenoma-carcinoma sequence, it is likely that risk factors act at different steps along the serrated pathway (Figure 1).
Figure 1.
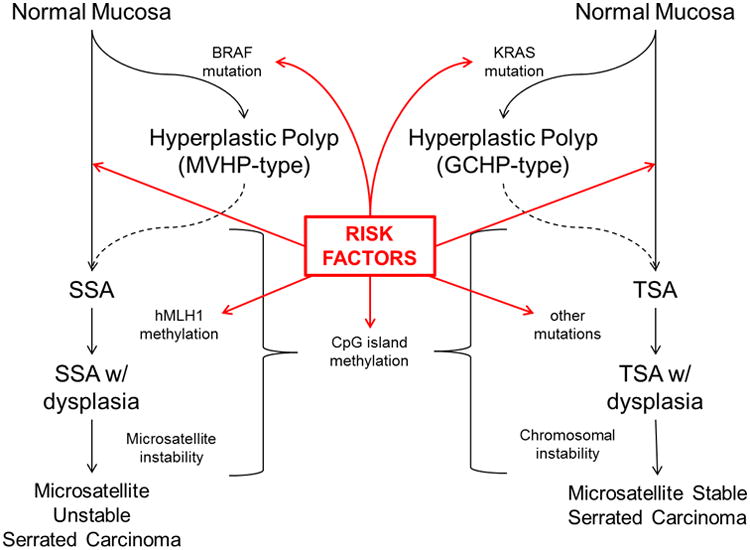
The Serrated Pathways to colorectal cancer, with proposed sequence of mutations and alterations leading to serrated carcinomas, and potential steps that could be influenced by risk factors (red arrows). Dotted lines represent proposed but unproven steps. See text for definition of abbreviations. Adapted from Snover [22] and Huang [3]
Description of serrated polyps
SPs are identified histologically as having a “saw-toothed” appearance of crypt epithelium. The World Health Organization subclassifies SPs into three categories: HPs, SSAs (with or without cytological dysplasia), and TSAs (with or without conventional dysplasia) depending primarily on the crypt architecture [23]. HPs are the most prevalent of the group and make up 75%-95% of SPs, while SSAs make up less than ¼ of SPs, and TSAs are much rarer than SSAs, comprising only about 1% of SPs [24-29].
HPs tend to be small (1 to 5mm) and distally-located, and have little or no risk of malignant potential [30-33]. HPs can be further classified into three subtypes: Microvesicular Hyperplastic Polyps (MVHP), Goblet Cell Hyperplastic Polyps (GCHP), and Mucin-Poor Hyperplastic Polyps (MPHP). While HPs as a group are not thought to have direct malignant potential, it is important to note that MVHPs exhibit molecular features (e.g. BRAF mutation) that are common to the premalignant SSA, and thus some have postulated that MVHPs may develop into SSAs and serrated pathway cancers, but this has not been definitively proven [10, 22, 34, 35].
SSAs represent 10-25% of all SPs [24, 25], are commonly located in the right colon, and are typically larger than HPs [26, 27]. SSAs are characterized histologically by distorted and dilated crypt bases, which resemble a boot-shaped pattern on cross-sectioning [22]. In contrast to adenomas, SSAs do not typically harbor dysplastic cells, but do have the potential to develop dysplasia and progress to malignancy [36]. The main route to malignant progression for SSAs involves CIMP-related inactivation of the hMLH1 gene, which leads to microsatellite instability and (probably) rapid growth, the development of cytological dysplasia, and malignant transformation into an MSI-high serrated carcinoma [3, 10, 35, 37] (Figure 1). It is worth noting that there remains controversy as to the best name for this lesion, and some authors prefer alternative terms such as “sessile serrated polyp.” Furthermore, due to issues of inconsistent pathological interpretation and changing nomenclature and taxonomy, some studies use surrogate definitions of important SPs such as proximal or large (typically ≥ 1 cm) SPs.
TSAs are the rarest form of SP, and are typically located in the distal colorectum [38]. TSAs resemble conventional adenomas, with a characteristic polypoid or pedunculated shape, though they are histologically distinct [2, 39, 40]. They contain conventional dysplasia more often than SSAs; however, the risk of progression to malignancy, as well as the time course of its progression is less certain given their rarity [28, 40].
Methods
To perform this review, we searched the MEDLINE-indexed medical literature using the PubMed search engine (http://www.pubmed.gov). The initial search string used was: (Epidemiology OR Risk Factor OR lifestyle) AND (Hyperplastic OR Serrated) AND polyps AND (colon OR colorectal). The results were limited to English language studies performed after 1990, and reviewed individually to identify relevant articles. Bibliographies of related articles were also reviewed to identify other pertinent studies.
Specific Risk Factors
Not surprisingly, studies analyzing risk factors for SSAs and TSAs are limited, given that the serrated pathway is still a burgeoning area of research, and many studies predate its inception. In fact, most epidemiologic studies regarding SPs performed prior to 2010 grouped all SPs under the heading “hyperplastic polyps” and did not recognize the different subclasses of SPs. Furthermore, studies on HPs or SPs in general largely fail to distinguish between those that are located proximally or distally in the colorectum, or large vs. small SPs. Nevertheless, several important studies of the epidemiology and risk factors of SPs will be reviewed here.
As a large number of early studies classified polyps as either adenomatous or hyperplastic, risk factors previously reported for hyperplastic polyps may better be generalized to SPs given its broader scope [33, 41-47]. It is likely that a small proportion of the “HPs” included in older studies (particularly large and/or proximally located HP) would be classified as SSAs based on the current pathologic criteria and nomenclature. However, it is also worth noting that prior to 5 years ago, the significance and endoscopic features of SSAs was not widely recognized, and therefore these lesions may have been less commonly identified or fully removed by endoscopists, so earlier studies likely included mostly distal HPs. For this reason, more contemporary studies are most informative with respect to the epidemiology of premalignant SPs. To date, there have been only two studies that specifically identified multiple risk factors for subclasses of SPs, particularly SSAs [48, 49]. Very little has been published on risk factors for TSAs.
Age
Older age is a major risk factor for development of conventional adenomas [50], and results have generally been similar in population-based studies of SPs. A German study reported an increased risk of HPs in those older than 55 years of age (age >55 vs. ≤55 years: OR 1.72, 95% CI 1.00, 2.96) [42] while a Japanese study reported similar results (age 55-64 vs. ≤44 years: OR 2.06, 95% CI 1.13, 3.78; age ≥65 vs. ≤44 years: OR 2.90, 95% CI 1.60, 5.30) [46] (Table 1). Two studies reported increased risk of SPs among individuals older than 50 years of age: Bouwens et al. (Age >50 vs. ≤50 years: OR 2.2, 95% CI 1.3, 3.8) and Burnett-Hartman et al. (age 50-59 vs. <50 years: OR 1.87, 95% CI 1.20, 2.91) [48, 51].
Table 1. Studies of serrated polyps and age.
| Author, year | Study description | n with SPs | Age OR/RR* (95% CI) |
|---|---|---|---|
| All SPs | |||
| Erhardt, 2002 [42] | German population Screening/diagnostic colonoscopy | 71 | ≤55 y: 1.00 [ref] >55 y: 1.72 (1.00-2.96) |
| Morimoto, 2002 [45] | US population (MN) Screening/diagnostic colonoscopy | 219 | <40 y: 1.0 [ref] 40-49 y: 0.9 (0.4-2.3) 50-59 y: 1.6 (0.7-3.7) 60-69 y: 1.4 (0.6-3.2) ≥70 y: 1.6 (0.5-5.0) |
| Lieberman, 2003 [33] | US population (veterans) Screening colonoscopy | 391 | 50-54 y: 1.00 [ref] 55-59 y: 1.10 (0.76-1.60) 60-64 y: 1.05 (0.76-1.46) 65-69 y: 1.01 (0.72-1.41) ≥70 y: 0.70 (0.48-1.02) |
| Omata, 2009 [46] | Japanese population, Diagnostic colonoscopy | 132 |

|
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Screening colonoscopy | 594 |

|
| Proximal/large SPs | |||
| Wallace, 2009 [52] | US population (multicenter/PPSG) Proximal SPs | 261 | <61 y: 1.00 [ref] ≥61 y: 0.86 (0.68-1.10) |
| Min, 2012 [56] | Korean population Proximal SPs | 60 | ≥70: 1.00 [ref] 60-69: 2.13 (0.62-7.29) 50-59: 1.53 (0.45-5.20) |
| Bouwens, 2013 [51] | Dutch population Proximal, large (>6mm) or dysplastic SPs | 141 |
|
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Proximal SPs | 199 | <50 y: 1.00 [ref] 50-59 y: 1.49 (0.70-3.14) 60-69 y: 1.72 (0.77-3.85) ≥70 y: 1.51 (0.60-3.81) |
| SSAs | |||
| Anderson, 2011 [49] | US population (CT) Pancolonic SSAs | 90 | Per year increase: 1.05 (1.02-1.08) |
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Pancolonic SSAs | 149 | <50 y: 1.00 [ref] 50-59 y: 1.63 (0.72-3.67) 60-69 y: 2.09 (0.87-5.00) ≥70 y: 2.19 (0.83-5.76) |
| Hetzel, 2010 [5] | US population (Boston) Pancolonic SSAs | 46 | Per year increase†: 1.00 (0.96-1.03) |
multivariate adjusted OR reported when provided
Incidence rate ratio
SP: serrated polyp; SSA: sessile serrated adenoma; OR: odds ratio; RR: risk ratio; CI: confidence interval; PPSG: Polyp Prevention study Group
Red text: statistically significant result
With respect to SSAs and age, published data are limited. A large pathology series of over 2000 patients with SSAs reported that the median age of those with SSAs was 62 years, which was the same as the median age of those with tubular adenomas [26]. Anderson et al. reported increasing risk of SSAs with age (per year increase in age: OR 1.05, 95% CI 1.02, 1.08) [49]. Similarly, Burnett-Hartmann et al. reported an elevated risk of SSAs with advanced age, but this did not reach statistical significance, perhaps due to small stratum size. (age ≥70 vs. <50 years: OR 2.19, 95% CI 0.83, 5.76) [48]. However, other studies have reported null associations between age and SSAs or advanced serrated lesions [5, 52]. Additional studies are therefore needed to clarify whether ageing clearly increases the risk of SSAs, but it appears that age is not as strong of a risk factor for SPs as it is for conventional adenomas.
Sex
Sex is one of the strongest known risk factors for adenomatous polyps, which are more common in men [53-55]. Regarding all SPs, there have been mixed results regarding predisposition based on sex. Men were found to have an increased risk of SPs by Erhardt et al. (OR 1.83, 95% CI 1.05, 3.18), just as women were found to have decreased risk of SPs by Morimoto et al. (OR 0.6, 95% CI 0.5, 0.9) [42, 45] (Table 2). However, other studies have not shown any significant association between sex and SPs, including Omata et al. (male OR 1.54, 95% CI 0.97, 2.44), Min et al. (male OR 1.82, 95% CI 0.99, 3.32), Bouwens (male OR 0.9, 95% CI 0.6, 1.2), Burnett-Hartman (female OR 1.06, 95% CI 0.8, 1.4), and Wallace (female proximal OR 1.07 95% CI 0.82, 1.39) [48, 51, 52, 56].
Table 2. Studies of serrated polyps and sex.
| Author, year | Study description | n with SPs | Sex OR/RR* (95% CI) |
|---|---|---|---|
| All SPs | |||
| Erhardt, 2002 [42] | German population Screening/diagnostic colonoscopy | 71 |
|
| Morimoto, 2002 [45] | US population (MN) Screening/diagnostic colonoscopy | 219 |
|
| Omata, 2009 [46] | Japanese population Diagnostic colonoscopy | 132 | Female: 1.00 [ref] Male: 1.54 (0.97-2.44) |
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Screening colonoscopy | 594 | Male: 1.00 [ref] Female: 1.06 (0.80-1.40) |
| Proximal/large SPs | |||
| Wallace, 2009 [52] | US population (multicenter/PPSG) Proximal SPs | 261 | Male: 1.00 [ref] Female: 1.07 (0.82-1.39) |
| Min, 2012 [56] | Korean population Proximal SPs | 60 | Female: 1.00 [ref] Male: 1.82 (0.99-3.32) |
| Bouwens, 2013 [51] | Dutch population Proximal, large (>6mm) or dysplastic SPs | 141 | Female: 1.0 [ref] Male: 0.9 (0.6-1.2) |
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Proximal SPs | 199 | Male: 1.00 [ref] Female: 1.05 (0.66-1.67) |
| SSAs | |||
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Pancolonic SSAs | 149 | Male: 1.00 [ref] Female: 1.37 (0.82-2.28) |
| Lash, 2010 [26] | US population (multicenter) Pancolonic SSAs | 2416 |
|
| Hetzel, 2010 [5] | US population (Boston) Pancolonic SSAs | 46 | Female: 1.00 [ref]† Male: 1.55 (0.93-2.61) |
multivariate adjusted OR reported when provided
Incidence rate ratio
SP: serrated polyp; SSA: sessile serrated adenoma; OR: odds ratio; RR: risk ratio; CI: confidence interval; PPSG: Polyp Prevention study Group
Red text: statistically significant result
With regards to SSAs specifically, studies have also been mixed regarding sex-related risk. Burnett Hartman et al. (n = 149 SSAs) found that women were not at a statistically significant increased risk of SSAs (OR 1.37, 95% CI 0.82, 2.28), similar to Wallace et al. (proximal advanced serrated lesion RR 1.16, 95% CI 0.72, 1.87) [48, 52]. Similarly, in an average risk screening cohort, Hetzel et al. found that an equal proportion of men and women (50% each) had SSAs, and sex was not independently associated with SSA detection (male OR: 1.55, 95% CI 0.93, 2.61) [5]. However, a much larger study performed by Lash et al. examined 179,111 patients who had polyps removed on colonoscopy, and identified a sample of 2416 SSAs. Fifty-four percent of SSAs were found in women, and the authors did report an association between SSAs and female sex (OR 1.21, 95% CI 1.11, 1.32) [26]. Notably, this study also found that the female:male ratio increases as SSAs progress, as women made up 53% of those with nondysplastic SSAs, but 57%, 69%, and 76% of those with SSAs with low grade dysplasia, high grade dysplasia, and cancer respectively. A number of other studies have reported that a higher proportion of SSAs are removed from women, including Carr et al. (65% female) and Spring et al. (65% female) [24, 25]. It appears that there is a slight female predominance for SPs and SSAs in particular, and the sex distribution of SPs certainly differs from that of adenomas, where male sex is a clear risk factor.
Race and Ethnicity
African Americans have higher incidence of both colorectal adenomas and CRC [57, 58], but this does not appear to be true for SPs. In one US study, African Americans and Hispanics had lower risks of SPs compared to non-Hispanic whites (RR 0.65, 95% CI 0.50, 0.85 and RR 0.33, 95% CI 0.20, 0.55, respectively) [52]. Similarly, another study reported a decreased risk of SPs in African Americans versus Caucasians, although not statistically significant (OR 0.49 95% CI: 0.23, 1.02) [48]. There have been a number of studies of serrated polyps in Japanese and other East Asian populations, but direct comparisons to other racial groups are limited [2, 56, 59]. With respect to SSAs specifically, the data are limited as well. Similar to the data for SPs in general, two studies have reported lower, but not statistically-significant associations between black race and SSAs or advanced SPs (Supplementary Table 3) [48, 52]. It is worth noting that the association between race and SPs appears quite strong based on point estimates, yet small numbers of non-whites in these studies likely limited the statistical power for these comparisons.
Socioeconomic status
Two early studies did not find a significant correlation between education level and presence of SPs [41, 45] (Supplementary Table 1). More recently, however, Burnett-Hartman et al. reported a positive association with SPs and higher education (college graduates vs. high school or less: OR 1.66, 95% CI 1.14, 2.43; graduate degree vs. high school or less: OR 1.46, 95% CI 1.00, 2.12) [48]. The effect of higher education appeared to be slightly stronger for proximal vs. distal SPs, but this difference was not statistically significant (Supplementary Table 2). Interestingly, a stronger association was reported between higher education and SSAs specifically (college graduates vs. high school or less: OR 3.35, 95% CI 1.41, 7.99; graduate degree vs. high school or less: OR 3.63, 95% CI 1.55, 8.54) (Supplementary Table 3). Given the lack of a plausible biologic link between higher education and serrated neoplasia, it is possible that this effect is confounded by other factors, such as quality of bowel preparation, race, diet, or access to care. Due to the lack of other corroborating studies on this and other socioeconomic risk factors, this area certainly deserves more consideration in the future.
Smoking
Tobacco use is a commonly-cited risk factor for SPs. One of the earliest studies to report this association was performed by Kearney et al., using a combined cohort of the Nurses' Health Study and Health Professionals Follow-up Study. Smoking was found to have a strong association with distal SPs in both men and women, (current vs. never smokers: males RR 2.45, 95% CI 1.59, 3.75; females RR 1.96 95% CI 1.16, 2.86) [44]. A smaller study performed by Martinez et al. also reported that tobacco was associated with increased risk of SPs (current vs. never smokers: OR 2.10, 95% CI 1.12, 3.94) [41]. Other studies in larger populations, including US, German and Japanese patients have also provided evidence that smoking is a risk factor for distal SPs and SPs in general [33, 42, 43, 45, 46] (Table 3). Two contemporary studies also reported associations between smoking and SPs, including Fu et al. (current vs. never smokers: OR 4.44, 95% CI 3.47, 5.67) and Burnett-Hartman et al. (current vs. never smokers OR 3.00, 95% CI 1.93, 4.66) [48, 60] (Table 3).
Table 3. Studies of serrated polyps and smoking.
| Author, year | Study description | n with SPs | Smoking* OR/RR† (95% CI) |
|---|---|---|---|
| All SPs | |||
| Kearney, 1995 [44] | US population (NHS/HPFS) Screening sigmoidoscopy or colonoscopy, distal HP/SP only | 394 |
|
|
| |||
| Martinez, 1997 [41] | US population (TX) Screening sigmoidoscopy or colonoscopy | 81 |
|
| Erhardt, 2002 [42] | German population Screening/diagnostic colonoscopy | 71 | Ever smoker: 1.79 (1.04-3.06) |
| Morimoto, 2002 [45] | US population (MN) Screening/diagnostic colonoscopy | 219 | Former smoker: 2.5 (1.4-4.4) Current smoker: 4.1 (2.2-7.6) |
| Lieberman, 2003 [33] | US population (veterans) Screening colonoscopy | 391 | Former smoker: 1.65 (1.23-2.23) Current smoker: 2.71 (1.93-3.81) |
| Ji, 2006 [43] | US population (PLCO) Screening sigmoidoscopy, distal HP/SP only | 1545 | Former smoker: 1.8 (1.6-2.1) Current smoker: 4.4 (3.7-5.2) Ever smoker: 2.1 (1.9-2.4) |
| Omata, 2009 [46] | Japanese population Diagnostic colonoscopy | 132 |
|
| Fu, 2012 [60] | US population (TN) Screening/diagnostic colonoscopy | 662 | Former smoker: 1.93 (1.56-2.38) Current smoker: 4.44 (3.47-5.67) <30 pack-years: 4.74 (3.51-6.42) ≥30 pack-years: 4.04 (3.02-5.42) |
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Screening colonoscopy | 594 | Former smoker: 1.70 (1.34-2.16) Current smoker: 3.00 (1.93-4.66) |
| Proximal/large SPs | |||
| Wallace, 2009 [52] | US population (multicenter/PPSG) Proximal SPs | 261 | Former smoker: 0.98 (0.75-1.28) Current smoker: 1.11 (0.80-1.54) |
| Bouwens, 2013 [51] | Dutch population Proximal, large (>6mm) or dysplastic SPs | 141 |
|
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Proximal SPs | 199 | Former smoker: 1.16 (0.79-1.71) Current smoker: 0.88 (0.32-2.39) |
| SSAs | |||
| Anderson, 2011 [49] | US population (CT) Pancolonic SSAs | 90 |
|
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Pancolonic SSAs | 149 |
|
Referent category (OR/RR=1.0) is nonsmokers or never smokers for all studies unless otherwise stated
multivariate adjusted OR reported when provided
SP: serrated polyp; SSA: sessile serrated adenoma; OR: odds ratio; RR: risk ratio; CI: confidence interval; NHS/HPFS: Nurses Health Study/Health Professionals Followup Study; PLCO: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; PPSG: Polyp Prevention study Group
Red text: statistically significant result
While the evidence largely supports a positive association between smoking and risk of SPs in general, data on anatomical location are mixed. Using pooled data from three different polyp chemoprevention studies, Wallace et al. reported that smoking was associated with development of SPs in the distal colon, but not the proximal colon (current vs. never smokers: proximal colon RR 1.11 95% CI 0.80, 1.54; distal colon RR 2.18 95% CI 1.80, 2.65) [52]. Burnett-Hartman found no correlation with tobacco use of 199 proximal SPs (current vs. never smokers OR 0.88 95% CI 0.32, 2.39) [48]. In contrast, Bouwens et al. did find an increased risk of large (>6 mm), proximal or dysplastic SPs associated with smoking (current vs. never smokers OR 2.2, 95% CI 1.4, 3.6) [51].
Several studies have examined the relationship of tobacco and SSAs or advanced SPs (Table 3). Anderson et al. found a positive association with tobacco and SSAs (≥20 vs. <20 pack-years OR 7.31, 95% CI 3.92, 13.63) [49]. In a slightly larger study, Burnett-Hartman et al. also reported an association between smoking and SSAs (current vs. never smokers OR 2.91, 95% CI 1.36, 6.21) [48]. Wallace et al. found that tobacco use was associated with a significantly increased risk of advanced SPs in the distal colorectum, but not in the proximal colon (current vs. never smokers: proximal RR 1.51, 95% CI 0.80, 2.86; distal RR 3.42, 95% CI 1.91, 6.11) [52] (Supplementary Table 3). Given the somewhat discordant results regarding smoking in studies of proximal SPs (a high proportion of which are SSAs) and those of SSAs specifically, the role of smoking in serrated neoplasia remains somewhat uncertain.
Alcohol
Alcohol use has been shown to increase risk of development of HP/SPs in a few early studies, as shown by Martinez et al. (>9.4 g/day vs. nonuse: OR 2.01, 95% CI 1.11, 3.63) and Kearney et al. (>30 g/day vs. nonuse: male RR 1.69, 95% CI 1.01, 2.80; female RR 1.79, 95% CI 1.02, 3.15) [41, 44]. However, alcohol has generally not been found to be a risk factor for SPs in more contemporary studies of SPs [33, 42, 45, 46, 48, 60] (Supplementary Table 1).
With respect to more advanced SPs, 3 studies reported no association between alcohol and risk of proximal or large SPs [48, 51, 52] (Supplementary Table 2). Burnett-Hartman et al. reported that alcohol use was not associated with SSAs specifically (≥14 vs. <1 drinks/week: OR 1.09 95% CI 0.54, 2.20). Wallace et al. also reported lack of association between alcohol and advanced SPs (≥1 drink/day vs. nonuse: proximal RR 1.20 95% CI 0.73, 1.98; distal RR 1.19 95% CI 0.73, 1.92) [48, 52] (Supplementary Table 3). Therefore, the relationship between alcohol and SPs in general is questionable, and there does not appear to be a strong association between alcohol use and advanced SPs or SSAs specifically.
Diet
There are several well-established relationships between certain dietary components and both conventional adenomas and CRC, such as higher intake of processed and red meat, refined carbohydrates, and lower intake of fruits and vegetables [16, 61]. The relationship between diet and SPs is less clear, but the few published studies examining the nutritional epidemiology of SPs will be reviewed below.
Macronutrients and specific foods
A positive association between red meat intake and SPs was reported in a recent colonoscopy-based case-control study by Fu and colleagues (≥44.2 vs. <10 g/day: OR 1.36, 95% CI 1.04, 1.78) while Erhardt et al. also found that high intake of meat (ham and sausage in particular), was associated with a higher prevalence of SPs in a German population (≥15 vs. <15 g/day: OR 3.70, 95% CI 1.49, 9.19) [42, 60] (Supplementary Table 1). Fu et al. failed to find a significant association of HPs with fiber intake or overall fat intake [60]. Kearney et al. similarly found that animal fat intake was not associated with SPs [44]. However, Wallace et al. reported that high fat diets were associated with SPs, including advanced lesions, at all anatomical sites (quartile 4 vs. quartile 1 fat intake, proximal SPs: RR 1.45 95% CI 1.01, 2.10; distal SPs RR 1.27 95% CI 1.03, 1.56; quartile 3 versus quartile 1 proximal advanced SPs: RR 2.38, 95% CI 1.24, 4.57; quartile 3 versus quartile 1 distal advanced SPs: RR 1.86, 95% CI 1.02, 3.41) [52]. However, the authors did not find an association between red meat intake and SPs. High carbohydrate diets were not clearly associated with SPs in general or advanced SPs. High calorie diets were only associated with distal advanced SPs (quartile 4 vs. quartile 1 total energy intake: RR 2.28, 95% CI 1.23, 4.24). There were no specific foods or macronutrient categories that were associated with significant protective effects in these studies.
Micronutrients
An array of vitamin and mineral supplements have been analyzed in epidemiological studies of HPs, including vitamins D, E and C, beta-carotene, and multivitamin supplements, however, no conclusive evidence of a significant association has been reported [33, 41, 44, 45]. Calcium has been shown to have an inverse association with SPs by Fu (≥1169.3 vs. ≤229.3 mg/day: OR 0.73, 95% CI 0.56, 0.96) and Martinez (≥1094 vs. ≤558 mg/day: OR 0.41, 95% CI 0.19, 0.90) [41, 60]. Regarding folate intake, there were three studies that looked at its relationship to SPs. Kearney et al. found that female subjects taking higher doses of folate had decreased incidence of distal SPs (>672 vs. <280 vs. mcg/day: RR 0.45, 95% CI 0.28, 0.74) [44]. Additionally, Fu et al. reported an inverse association with folate intake and SPs (≥584.4 vs. ≤421.4 mcg/day: OR 0.73, 95% CI 0.56, 0.96) [60] (Supplementary Table 1). Conversely, in the study performed by Wallace et al., folate was shown to actually increase the risk of proximal advanced SPs (1 mg folate daily vs. placebo: RR 2.07, 95% CI: 1.14, 3.77) [52] (Supplementary Table 3). This difference may have to do with the fact that supplemental folate was administered as an investigational chemo-preventive agent in the Wallace study vs. dietary folate content in the Fu et al. and Nurses' Health studies. However, given these seemingly discordant results, the association between folate intake and SPs is not entirely clear, and merits further research.
Obesity
Obesity, assessed by elevated body mass index (BMI) is associated with increased incidence of both colorectal adenomas and CRC [62-65]. Regarding serrated pathway lesions, several studies show a direct relationship with obesity. Early studies showed an increased risk of HPs and SPs in general associated with higher BMI, as reported by Martinez et al. (BMI >29.8 vs. <23.4: OR 3.79, 95% CI 1.61, 8.92) and Morimoto (BMI 26.5-29.6 vs. <24.2: male OR 2.2, 95% CI 1.2, 4.3) [41, 45] (Table 4). Notably, in the Morimoto study, the relationship between obesity and SPs was limited to men, with an essentially null finding reported for women [45] Fu et al. also found a positive correlation between BMI and SPs (BMI ≥ 30 vs. 18.5-24.9: OR 1.36, 95% 1.08, 1.70). Later studies using contemporary criteria for SPs confirmed an association between higher BMI and distal, but not proximal SPs, as shown by Wallace (BMI ≥30 vs. <25: distal RR 1.27, 95% CI: 1.06, 1.53) and Burnett-Hartman (BMI ≥30 vs. <25: distal OR 1.48, 95% CI: 1.04, 2.11) (Table 4).
Table 4. Studies of serrated polyps and obesity.
| Author, year | Study description | n with SPs | BMI OR/RR* (95% CI) |
|---|---|---|---|
| All SPs | |||
| Martinez, 1997 [41] | US population (TX) Screening sigmoidoscopy or colonoscopy | 81 |

|
| Erhardt, 2002 [42] | German population Screening/diagnostic colonoscopy | 71 | BMI ≤24: 1.00 [ref] BMI >24: 1.39 (0.79-2.46) |
| Morimoto, 2002 [45] | US population (MN) Screening/diagnostic colonoscopy | 219 | Females BMI <22.7: 1.0 [ref] BMI 22.7-25.8: 0.8 (0.4-1.7) BMI 25.9-29.8: 0.9 (0.5-1.7) BMI ≥29.9: 1.1 (0.6-2.0) |

| |||
| Omata, 2009 [46] | Japanese population Diagnostic colonoscopy | 132 | BMI <22: 1.00 [ref] BMI ≥22: 1.51 (0.97-2.33) BMI ≥25: 1.41 (0.81-2.44) |
| Fu, 2012 [60] | US population (TN) Screening/diagnostic colonoscopy | 662 |
|
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Screening colonoscopy | 594 | BMI <25: 1.00 [ref] BMI 25-29: 0.97 (0.75-1.26) BMI ≥30: 1.26 (0.93-1.71) |
| Proximal/large SPs | |||
| Wallace, 2009 [52] | US population (multicenter/PPSG) Proximal SPs | 261 | BMI <25: 1.00 [ref] BMI 25-29.9: 0.96 (0.73-1.27) BMI ≥30: 1.13 (0.83-1.56) |
| Bouwens, 2013 [51] | Dutch population Proximal, large (>6mm) or dysplastic SPs | 141 | BMI <25: 1.0 [ref] BMI 25-29.9: 1.0 (0.7-1.5) BMI ≥30: 1.4 (0.9-2.3) |
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Proximal SPs | 199 | BMI <25: 1.00 [ref] BMI 25-29: 0.79 (0.53-1.20) BMI ≥30: 0.71 (0.41-1.22) |
| SSAs | |||
| Anderson, 2011 [49] | US population (CT) Pancolonic SSAs | 90 |
|
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Pancolonic SSAs | 149 | BMI <25: 1.00 [ref] BMI 25-29.9: 0.77 (0.48-1.23) BMI ≥30: 1.13 (0.66-1.94) |
multivariate adjusted OR reported when provided
SP: serrated polyp; SSA: sessile serrated adenoma; BMI: body mass index; OR: odds ratio; RR: risk ratio; CI: confidence interval; PPSG: Polyp Prevention study Group
Red text: statistically significant result
The association between obesity and SSAs specifically has been examined in two studies, with conflicting results. Anderson et al. found that obesity was associated with all SSAs (BMI ≥30 vs. <30: OR 2.57, 95% CI 1.44, 4.62) and large SSAs (≥ 1cm) in particular (OR 3.96, 95% CI 1.27, 12.36) [49]. In contrast, Burnett-Hartman did not find an association between obesity and SSAs in a larger cohort, (BMI ≥30 vs. <25, OR 1.13, 95% CI: 0.66, 1.94) [48].
Aspirin/NSAID use
A number of studies have examined the role of NSAIDs and aspirin in the epidemiology of SPs. Early studies did find a risk reduction of SPs in subjects who used NSAIDs, including Lieberman et al. (daily vs. never use: OR 0.75, 95% CI 0.56, 0.99) and Martinez et al. (use ≥7 times/week vs. never use: OR 0.32, 95% CI 0.14, 0.72) [33, 41] (Table 5). Later studies performed by Bouwens et al. (nondaily or no use vs. daily aspirin use: OR 1.8, 95% CI: 1.1, 3.0) and Wallace et al. (daily aspirin use vs. placebo: proximal RR 0.56, 95% CI 0.34, 0.91; distal RR 0.77, 95% CI 0.58, 1.04) found that aspirin use decreased risk of SPs, particularly in proximal lesions [51, 52]. Several studies have reported trends toward inverse associations between NSAIDs and SSAs or advanced SPs specifically, but these estimates did not reach statistical significance, perhaps due to sample size limitations [48, 52] (Table 5 and Supplementary Table 3). Overall, evidence suggests that aspirin and NSAIDs may have a chemopreventive effect for proximal SPs (particularly SSAs), but further conclusive evidence is needed.
Table 5. Studies of serrated polyps and nonsteroidal anti-inflammatory drug (NSAID) or aspirin usage.
| Author, year | Study description | n with SPs | NSAID/ASA OR/RR* (95% CI) |
|---|---|---|---|
| All SPs | |||
| Martinez, 1997 [41] | US population (TX) Screening sigmoidoscopy or colonoscopy | 81 |
|
| Morimoto, 2002 [45] | US population (MN) Screening/diagnostic colonoscopy | 219 | Never use: 1.0 [ref] ≥once/week aspirin: 1.0 (0.6-1.6) ≥once/week NSAID: 0.6 (0.3-1.1) |
| Lieberman, 2003 [33] | US population (veterans) Screening colonoscopy | 391 |
|
| Fu, 2012 [60] | US population (TN) Screening/diagnostic colonoscopy | 662 | Never use: 1.00 [ref] Former use: 0.96 (0.68-1.35) Current Use: 0.84 (0.69-1.01) |
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Screening colonoscopy | 594 | Nonuse: 1.00 [ref] Former use: 0.89 (0.59-1.33) Current use: 0.81 (0.64-1.03) |
| Proximal/large SPs | |||
| Wallace, 2009 [52] | US population (multicenter/PPSG) Proximal SPs | 261 |
|
| Bouwens, 2013 [51] | Dutch population Proximal, large (>6mm) or dysplastic SPs | 141 |
|
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Proximal SPs | 199 | Nonuse: 1.00 [ref] Former use: 1.21 (0.67-2.19) Current use: 0.68 (0.46-1.03) |
| SSAs | |||
| Burnett-Hartman, 2013 [48] | US population (Group Health, WA) Pancolonic SSAs | 149 | Nonuse: 1.00 [ref] Former use: 1.39 (0.75-2.56) Current use: 0.64 (0.41-1.01) |
multivariate adjusted OR reported when provided
SP: serrated polyp; SSA: sessile serrated adenoma; BMI: body mass index; OR: odds ratio; RR: risk ratio; CI: confidence interval; PPSG: Polyp Prevention study Group
Red text: statistically significant result
Family history
Family history of CRC is a major factor in cancer screening guidelines yet there have been mixed results regarding its implications on SPs [66]. Of the six studies that examined family history of CRC as a risk factor for SPs [25, 33, 44, 48, 49, 52], only two reported an association. Kearney et al. reported that family history of CRC was positively associated with SPs among both males (RR 1.95, 95% CI 1.39, 2.74) and females (RR 2.03, 95% CI 1.39, 2.95) (Supplementary Table 1). Similarly, Spring et al. reported that patients with SSAs specifically may be more likely to have a family history of CRC, as 42% of patients with SSAs had a 1st degree relative with CRC, compared to 25% of controls, although this did not reach statistical significance [25]. Thus, while family history of CRC is an important risk factor in screening in general, its importance in development of SPs is at this point is unclear, and needs to be further elucidated. It is possible, for example, that persons with a “specific” family history of SPs or MSI-high CRC have an increased risk of developing SSAs. Along these lines, several studies have shown that relatives of persons with CRC with serrated pathway genetic alterations (e.g. BRAF mutation) do have an elevated risk of CRC [67, 68]. One would presume that those relatives are also at risk for serrated pathway precursor lesions (i.e. SSAs), but that has not been definitively shown. Also, it is clearly established that in patients with serrated polyposis syndrome (SPS), there is a heritable and genetic predisposition for developing SPs and serrated pathway cancers [69, 70].
Personal history of serrated lesions
It is well established that persons with a personal history of conventional adenomas have an increased risk of adenoma recurrence, and for this reason, post-polypectomy surveillance is recommended when adenomas are found [14, 71, 72]. With the exception of SPS populations, the risk of metachronous serrated neoplasia in those with SPs (and the importance of a prior history of SPs) has been less studied. With respect to SPs in general, Benson et al. reported that a history of SPs was associated with an increased risk of future SPs, but not adenomas (OR for SPs: 3.67, 95% CI 2.54, 5.31) [73]. Interestingly, having both HP and adenomas on the index exam was a stronger risk factor for having SPs and adenomas at follow up, compared to those with only one type of polyp on their index exam. Two other studies performed by Spring et al. and Burnett-Hartman et al. also found that a history of polyps was associated with SPs, however, these studies did not distinguish if the index polyps were conventional adenomas or SPs [25, 74]. Two contemporary studies addressed this question directly. Bouwens et al. reported that a personal history of SPs was an independent risk factor for finding a large, proximal, or dysplastic SP on follow-up colonoscopy (OR 2.6, 95% CI 1.3, 4.9) [51]. Additionally, Teriaky et al. reported that among 22 patients with SSAs on index colonoscopy, 50% had a metachronous SSA during a 5 year follow-up period [75]. Therefore it appears that a history of SPs and SSAs specifically is a risk factor for future SPs.
Other risk factors
Physical activity
There is evidence that physical activity may have protective effects against conventional adenomas as well as CRC in both proximal and distal locations [76, 77]. With respect to SPs, a similar protective effect has not been established, though this has been studied by several investigators [33, 41, 45, 48, 60] (Supplementary Tables 1-3).
Diabetes
Similarly, diabetes has been reported to be a possible risk factor for CRC and conventional adenomas [78, 79]. There are sparse data regarding the association between diabetes and SPs. Bouwens et al found no relation between diabetes and SPs, while Anderson et al. did report an association with SSAs in diabetics versus nondiabetics (OR 4.57, 95% CI 2.36, 8.82) [49, 51] (Supplementary Table 3).
Hormone replacement therapy
Two studies have examined the role of hormone replacement therapy (HRT) in development of SPs, in analyses limited to women. Burnett-Hartman et al. found that there was an inverse relationship in the use of estrogen-only HRT and development of SPs (OR 0.63, 95% CI: 0.44, 0.90). Morimoto reported a borderline association between ever use of HRT and SPs (OR 0.7, 95% CI 0.4, 1.1) [45]. Interestingly, these findings do seem to correlate with the protective effects of HRT against colorectal cancer as reported in 2004 by the Women's Health Initiative study [80]. As SSAs appear to be more common in women, the role of estrogen in the pathogenesis of serrated pathway lesions merits further research.
Risk Factors for Traditional Serrated Adenomas
Since TSAs are rare, there are scant epidemiologic data on these polyps. In the largest series of TSAs (n=709 patients), Lash et al. reported that the median age of those harboring TSAs was 63 years, and roughly half were women (50.9%) [26]. There was a trend towards higher risk of dysplasia associated with female sex, but this did not reach statistical significance (OR 1.83 (95% CI 0.48, 6.95). Some have suggested a connection between TSAs and inflammatory bowel disease, but this is inconclusive [81, 82].
Risk factors for specific genetic and epigenetic alterations
A variety of genetic mutations contribute to the pathway by which SPs develop and progress towards serrated carcinoma. While the studies reviewed previously have examined risk factors for histologic outcome of SPs, a few studies have identified risk factors for serrated pathway mutations as well. One study analyzed risk factors for BRAF mutations and CIMP-high phenotypes, it was shown that BRAF mutations were associated with male sex (OR 1.47 95% CI: 1.04, 2.09), BMI (≥30 vs. <25: OR 1.57 95% CI: 1.03, 2.40), tobacco use (current vs. never smoker OR 2.75 95% CI: 1.48, 5.11) and history of prior colorectal polyps (OR 1.60 95% CI: 1.11, 2.30), while CIMP-high was associated with Caucasian race (OR 2.27 95% CI: 1.08, 4.76), tobacco use (current vs. never smoker OR 2.71 95% CI: 1.19, 6.18) and history of prior colorectal polyps (OR 2.35 95% CI: 1.46, 3.79) [74]. These results are consistent with several of the risk factors for SPs identified above.
Discussion
There have been few established risk factors identified for SPs. Tobacco, alcohol, and obesity have been shown fairly consistently to be associated with increased risk of SPs in general [33, 41-46, 48, 49, 52]. There is also limited evidence that Caucasians and those with diets high in fat and calories have a greater risk of SPs [52]. Some data suggest that folate intake and NSAID use may serve a protective role against SPs, however results are somewhat mixed [48, 49, 52]. Estrogen-only HRT has also been associated with a protective effect against SPs [48]. With regards to SSAs specifically, the published risk factor research is limited. Female sex has been associated with SSAs in multiple studies [5, 24-26]. Smoking has also consistently been identified as a risk factor for SSAs [48, 49]. There have been mixed results involving obesity, as one study did find an association with SSAs [49], however, two others did not [48, 52]. Interestingly, one study found a relation between higher levels of education and SSAs [48]. Aspirin and NSAID use may have a protective effect, at least for proximal SPs, and probably for SSAs as well [48, 51, 52]. Even less data exists regarding the epidemiology of TSAs, and risk factors for these polyps are largely unknown. Given that SSAs and TSAs are understood to be precursors to a substantial proportion of sporadic CRC, more research is warranted to establish which populations are most prone to developing these polyps. While risk factors for SPs appear to overlap with those of conventional adenomas, there are some clear differences (Figure 2).
Figure 2.
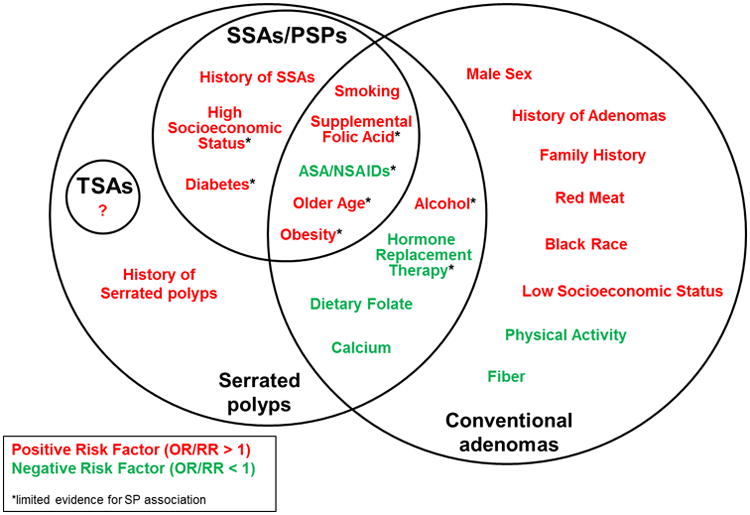
Established and probable risk factors for serrated and adenomatous colorectal polyps, and sessile serrated adenomas/proximal serrated polyps specifically, based on current literature. See text for definition of abbreviations.
A major limitation in the epidemiology of SPs is related to the shift in pathological classification that has occurred over the past decade, as our understanding of the serrated pathway has developed. In early studies, SPs were not routinely differentiated between more benign HPs versus premalignant SSAs or TSAs, and as such, epidemiologic studies prior to the mid to late-2000s are largely limited by outdated pathological interpretations and terminology [33, 41, 43-46]. Furthermore, many of these studies focused solely on distal polyps (primarily small HPs), which are not thought to have malignant potential, limiting their clinical relevance [43, 44]. As such, it is difficult to interpret the findings from many prior studies of SPs, and we recognize that risk factors for polyps previously categorized under the (now outdated) umbrella term “hyperplastic polyps” may not be the same as risk factors for premalignant SPs. For these reasons, more contemporary studies are likely to be most informative with respect to the epidemiology of SPs. This review underscores the need for a reassessment of risk factors for all categories of SPs based on the current pathologic criteria and nomenclature, especially for SSAs, which have arguably the most relevance clinically in CRC screening given their premalignant potential and relative abundance with respect to TSAs.
As with the adenoma-carcinoma sequence, different risk factors may influence different steps of the serrated pathway (Figure 1). Also, it is clear that some persons have a genetic predisposition to serrated pathway polyps and cancers. In the prototypical case, this is manifest by SPS, a condition of multiple SPs and an increased risk of CRC [83]. While the specific genetic defects underlying this condition have yet to be elucidated, there is likely a continuum of risk associated with a number of genes (or epigenetic modifications) with some persons experiencing more attenuated phenotypes. Environmental risk factors likely interact with a given person's genetic susceptibility [84]. Therefore, future epidemiologic research on SPs should take into account molecular aspects of these lesions as well [85].
In conclusion, the number of contemporary epidemiological studies on SPs is limited, and results have been mixed, with few established risk factors identified thus far. Given the increasingly recognized importance of SPs (and particularly SSAs) as CRC precursors, further research is needed on this topic [12]. Specifically, tobacco, folate, NSAIDs, sex, diet, HRT, and socioeconomic status are risk factors that merit further investigation. Rigorously conducted cross-sectional or case-control studies of patients undergoing high-quality colonoscopic screening examinations with expert gastrointestinal pathologist readings will provide the best evidence in this regard. Future studies should compare patients with SSAs to those conventional adenomas and negative colonoscopies, and ideally would further characterize SSAs by their specific genetic and epigenetic alterations. Compared to the adenoma-carcinoma sequence, the serrated pathway is relatively understudied, and therefore identification of risk factors is important not only for risk stratification, but also in order to better understand the cascade of events that lead to a serrated pathway cancer. A better understanding of the epidemiology of serrated neoplasia and its associated molecular defects is warranted in order to optimally impact screening and prevention of all sporadic CRC.
Supplementary Material
Supplementary Table 1: Risk factors for serrated polyps (primarily hyperplastic polyps)
Supplementary Table 2: Contemporary studies of risk factors for proximal and/or large serrated polyps
Supplementary Table 3: Contemporary studies of risk factors for sessile serrated adenomas and other advanced serrated polyps
Acknowledgments
Financial Support: This work was supported, in part, by a grant from the American College of Gastroenterology, ACG-JR-000-2012 (SDC)
Footnotes
Potential competing interests: None
References
- 1.Morson B. Some peculiarities in the histology of intestinal polyps. Dis Colon Rectum. 1962;5:337–44. [Google Scholar]
- 2.Oka S, Tanaka S, Hiyama T, et al. Clinicopathologic and endoscopic features of colorectal serrated adenoma: differences between polypoid and superficial types. Gastrointest Endosc. 2004;59(2):213–9. doi: 10.1016/s0016-5107(03)02693-2. [DOI] [PubMed] [Google Scholar]
- 3.Huang CS, Farraye FA, Yang S, et al. The clinical significance of serrated polyps. Am J Gastroenterol. 2011;106(2):229–40. doi: 10.1038/ajg.2010.429. quiz 241. [DOI] [PubMed] [Google Scholar]
- 4.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144(1):74–80 e1. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105(12):2656–64. doi: 10.1038/ajg.2010.315. [DOI] [PubMed] [Google Scholar]
- 6.Kahi CJ, Hewett DG, Norton DL, et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9(1):42–6. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131(6):1700–5. doi: 10.1053/j.gastro.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8(10):858–64. doi: 10.1016/j.cgh.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010;105(5):1189–95. doi: 10.1038/ajg.2009.699. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30(12):1491–501. doi: 10.1097/01.pas.0000213313.36306.85. [DOI] [PubMed] [Google Scholar]
- 11.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095–105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crockett SD, Snover DC, Ahnen DJ, et al. Sessile Serrated Adenomas: An evidence-based guide to management. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology. 2010;139(5):1497–502. doi: 10.1053/j.gastro.2010.06.074. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319(9):525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 16.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14(6):524–37. doi: 10.1097/00000478-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 17.East JE, Saunders BP, Jass JR. Sporadic and syndromic hyperplastic polyps and serrated adenomas of the colon: classification, molecular genetics, natural history, and clinical management. Gastroenterol Clin North Am. 2008;37(1):25–46, v. doi: 10.1016/j.gtc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Lee EJ, Choi C, Park CK, et al. Tracing origin of serrated adenomas with BRAF and KRAS mutations. Virchows Arch. 2005;447(3):597–602. doi: 10.1007/s00428-005-1226-2. [DOI] [PubMed] [Google Scholar]
- 19.Torlakovic E, Skovlund E, Snover DC, et al. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27(1):65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Miwa S, Mitomi H, Igarashi M, et al. Clinicopathologic differences among subtypes of serrated adenomas of the colorectum. Hepatogastroenterology. 2005;52(62):437–40. [PubMed] [Google Scholar]
- 21.Sawyer EJ, Cerar A, Hanby AM, et al. Molecular characteristics of serrated adenomas of the colorectum. Gut. 2002;51(2):200–6. doi: 10.1136/gut.51.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42(1):1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Bosman FT, C F, Hruban RH, et al. Tumours of the Digestive System. 4th. Berlin: Springer-Verlag; 2010. WHO Classification of Tumours Pathology and Genetics. [Google Scholar]
- 24.Carr NJ, Mahajan H, Tan KL, et al. Serrated and non-serrated polyps of the colorectum: their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J Clin Pathol. 2009;62(6):516–8. doi: 10.1136/jcp.2008.061960. [DOI] [PubMed] [Google Scholar]
- 25.Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131(5):1400–7. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 26.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63(8):681–6. doi: 10.1136/jcp.2010.075507. [DOI] [PubMed] [Google Scholar]
- 27.Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology. 2005;47(1):32–40. doi: 10.1111/j.1365-2559.2005.02180.x. [DOI] [PubMed] [Google Scholar]
- 28.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107(9):1315–29. doi: 10.1038/ajg.2012.161. quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein NS, Bhanot P, Odish E, et al. Hyperplastic-like colon polyps that preceded microsatellite-unstable adenocarcinomas. Am J Clin Pathol. 2003;119(6):778–96. doi: 10.1309/DRFQ-0WFU-F1G1-3CTK. [DOI] [PubMed] [Google Scholar]
- 30.Weston AP, Campbell DR. Diminutive colonic polyps: histopathology, spatial distribution, concomitant significant lesions, and treatment complications. Am J Gastroenterol. 1995;90(1):24–8. [PubMed] [Google Scholar]
- 31.Tedesco FJ, Hendrix JC, Pickens CA, et al. Diminutive polyps: histopathology, spatial distribution, and clinical significance. Gastrointest Endosc. 1982;28(1):1–5. doi: 10.1016/s0016-5107(82)72954-2. [DOI] [PubMed] [Google Scholar]
- 32.Lambert R, Kudo SE, Vieth M, et al. Pragmatic classification of superficial neoplastic colorectal lesions. Gastrointest Endosc. 2009;70(6):1182–99. doi: 10.1016/j.gie.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman DA, Prindiville S, Weiss DG, et al. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959–67. doi: 10.1001/jama.290.22.2959. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien MJ. Hyperplastic and serrated polyps of the colorectum. Gastroenterol Clin North Am. 2007;36(4):947–68, viii. doi: 10.1016/j.gtc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien MJ, Yang S, Clebanoff JL, et al. Hyperplastic (serrated) polyps of the colorectum: relationship of CpG island methylator phenotype and K-ras mutation to location and histologic subtype. Am J Surg Pathol. 2004;28(4):423–34. doi: 10.1097/00000478-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein NS. Small colonic microsatellite unstable adenocarcinomas and high-grade epithelial dysplasias in sessile serrated adenoma polypectomy specimens: a study of eight cases. Am J Clin Pathol. 2006;125(1):132–45. [PubMed] [Google Scholar]
- 37.Oh K, Redston M, Odze RD. Support for hMLH1 and MGMT silencing as a mechanism of tumorigenesis in the hyperplastic-adenoma-carcinoma (serrated) carcinogenic pathway in the colon. Hum Pathol. 2005;36(1):101–11. doi: 10.1016/j.humpath.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Fu B, Yachida S, Morgan R, et al. Clinicopathologic and genetic characterization of traditional serrated adenomas of the colon. Am J Clin Pathol. 2012;138(3):356–66. doi: 10.1309/AJCPVT7LC4CRPZSK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Limketkai BN, Lam-Himlin D, Arnold CA, et al. The cutting edge of serrated polyps: a practical guide to approaching and managing serrated colon polyps. Gastrointest Endosc. 2013;77(3):360–75. doi: 10.1016/j.gie.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA) Am J Surg Pathol. 2008;32(1):21–9. doi: 10.1097/PAS.0b013e318157f002. [DOI] [PubMed] [Google Scholar]
- 41.Martinez ME, McPherson RS, Levin B, et al. A case-control study of dietary intake and other lifestyle risk factors for hyperplastic polyps. Gastroenterology. 1997;113(2):423–9. doi: 10.1053/gast.1997.v113.pm9247459. [DOI] [PubMed] [Google Scholar]
- 42.Erhardt JG, Kreichgauer HP, Meisner C, et al. Alcohol, cigarette smoking, dietary factors and the risk of colorectal adenomas and hyperplastic polyps--a case control study. Eur J Nutr. 2002;41(1):35–43. doi: 10.1007/s003940200004. [DOI] [PubMed] [Google Scholar]
- 43.Ji BT, Weissfeld JL, Chow WH, et al. Tobacco smoking and colorectal hyperplastic and adenomatous polyps. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(5):897–901. doi: 10.1158/1055-9965.EPI-05-0883. [DOI] [PubMed] [Google Scholar]
- 44.Kearney J, Giovannucci E, Rimm EB, et al. Diet, alcohol, and smoking and the occurrence of hyperplastic polyps of the colon and rectum (United States) Cancer causes & control: CCC. 1995;6(1):45–56. doi: 10.1007/BF00051680. [DOI] [PubMed] [Google Scholar]
- 45.Morimoto LM, Newcomb PA, Ulrich CM, et al. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11(10 Pt 1):1012–8. [PubMed] [Google Scholar]
- 46.Omata F, Brown WR, Tokuda Y, et al. Modifiable Risk Factors for Colorectal Neoplasms and Hyperplastic Polyps. Internal Medicine. 2009;48(3):123–128. doi: 10.2169/internalmedicine.48.1562. [DOI] [PubMed] [Google Scholar]
- 47.Shrubsole MJ, Wu H, Ness RM, et al. Alcohol drinking, cigarette smoking, and risk of colorectal adenomatous and hyperplastic polyps. American journal of epidemiology. 2008;167(9):1050–8. doi: 10.1093/aje/kwm400. [DOI] [PubMed] [Google Scholar]
- 48.Burnett-Hartman AN, Passarelli MN, Adams SV, et al. Differences in epidemiologic risk factors for colorectal adenomas and serrated polyps by lesion severity and anatomical site. Am J Epidemiol. 2013;177(7):625–37. doi: 10.1093/aje/kws282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson JC, Rangasamy P, Rustagi T, et al. Risk factors for sessile serrated adenomas. J Clin Gastroenterol. 2011;45(8):694–9. doi: 10.1097/MCG.0b013e318207f3cf. [DOI] [PubMed] [Google Scholar]
- 50.Heitman SJ, Ronksley PE, Hilsden RJ, et al. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7(12):1272–8. doi: 10.1016/j.cgh.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 51.Bouwens MW, Winkens B, Rondagh EJ, et al. Simple clinical risk score identifies patients with serrated polyps in routine practice. Cancer Prev Res (Phila) 2013;6(8):855–63. doi: 10.1158/1940-6207.CAPR-13-0022. [DOI] [PubMed] [Google Scholar]
- 52.Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2310–7. doi: 10.1158/1055-9965.EPI-09-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imperiale TF, Wagner DR, Lin CY, et al. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343(3):169–74. doi: 10.1056/NEJM200007203430302. [DOI] [PubMed] [Google Scholar]
- 54.Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352(20):2061–8. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 55.Rex DK. Colonoscopy: a review of its yield for cancers and adenomas by indication. Am J Gastroenterol. 1995;90(3):353–65. [PubMed] [Google Scholar]
- 56.Min YW, Lee JH, Lee SH, et al. Prevalence of proximal colon serrated polyps in a population at average risk undergoing screening colonoscopy: a multicenter study. Clin Res Hepatol Gastroenterol. 2012;36(6):604–8. doi: 10.1016/j.clinre.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 57.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 58.Lieberman DA, Holub JL, Moravec MD, et al. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008;300(12):1417–22. doi: 10.1001/jama.300.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hiraoka S, Kato J, Fujiki S, et al. The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology. 2010;139(5):1503–10. 1510 e1–3. doi: 10.1053/j.gastro.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 60.Fu Z, Shrubsole MJ, Smalley WE, et al. Lifestyle factors and their combined impact on the risk of colorectal polyps. Am J Epidemiol. 2012;176(9):766–76. doi: 10.1093/aje/kws157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller PE, Lesko SM, Muscat JE, et al. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 62(4):413–24. doi: 10.1080/01635580903407114. [DOI] [PubMed] [Google Scholar]
- 62.Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122(5):327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 63.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 64.Martinez ME, Giovannucci E, Spiegelman D, et al. Leisure-time physical activity, body size, and colon cancer in women. Nurses' Health Study Research Group. J Natl Cancer Inst. 1997;89(13):948–55. doi: 10.1093/jnci/89.13.948. [DOI] [PubMed] [Google Scholar]
- 65.Ben Q, An W, Jiang Y, et al. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology. 2012;142(4):762–72. doi: 10.1053/j.gastro.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 66.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 67.Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p. V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1831–9. doi: 10.1158/1055-9965.EPI-10-0055. [DOI] [PubMed] [Google Scholar]
- 68.Vandrovcova J, Lagerstedt-Robinsson K, Pahlman L, et al. Somatic BRAF-V600E mutations in familial colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2270–3. doi: 10.1158/1055-9965.EPI-06-0359. [DOI] [PubMed] [Google Scholar]
- 69.Edelstein DL, Axilbund JE, Hylind LM, et al. Serrated polyposis: rapid and relentless development of colorectal neoplasia. Gut. 2012;62(3):404–8. doi: 10.1136/gutjnl-2011-300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110(3):748–55. doi: 10.1053/gast.1996.v110.pm8608884. [DOI] [PubMed] [Google Scholar]
- 71.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136(3):832–41. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077–85. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 73.Bensen SP, Cole BF, Mott LA, et al. Colorectal hyperplastic polyps and risk of recurrence of adenomas and hyperplastic polyps. Lancet. 1999;354(9193):1873–1874. doi: 10.1016/s0140-6736(99)04469-4. [DOI] [PubMed] [Google Scholar]
- 74.Burnett-Hartman AN, Newcomb PA, Potter JD, et al. Genomic Aberrations Occurring in Subsets of Serrated Colorectal Lesions but not Conventional Adenomas. Cancer Res. 2013;73(9):2863–72. doi: 10.1158/0008-5472.CAN-12-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teriaky A, Driman DK, Chande N. Outcomes of a 5-year follow-up of patients with sessile serrated adenomas. Scand J Gastroenterol. 2012;47(2):178–83. doi: 10.3109/00365521.2011.645499. [DOI] [PubMed] [Google Scholar]
- 76.Boyle T, Keegel T, Bull F, et al. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104(20):1548–61. doi: 10.1093/jnci/djs354. [DOI] [PubMed] [Google Scholar]
- 77.Wolin KY, Yan Y, Colditz GA. Physical activity and risk of colon adenoma: a meta-analysis. Br J Cancer. 2011;104(5):882–5. doi: 10.1038/sj.bjc.6606045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuhara H, Steinmaus C, Cohen SE, et al. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. 2011;106(11):1911–21. doi: 10.1038/ajg.2011.301. quiz 1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eddi R, Karki A, Shah A, et al. Association of type 2 diabetes and colon adenomas. J Gastrointest Cancer. 2012;43(1):87–92. doi: 10.1007/s12029-011-9316-7. [DOI] [PubMed] [Google Scholar]
- 80.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350(10):991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 81.Bossard C, Denis MG, Bezieau S, et al. Involvement of the serrated neoplasia pathway in inflammatory bowel disease-related colorectal oncogenesis. Oncol Rep. 2007;18(5):1093–7. [PubMed] [Google Scholar]
- 82.Klarskov L, Mogensen AM, Jespersen N, et al. Filiform serrated adenomatous polyposis arising in a diverted rectum of an inflammatory bowel disease patient. APMIS. 2011;119(6):393–8. doi: 10.1111/j.1600-0463.2011.02717.x. [DOI] [PubMed] [Google Scholar]
- 83.Edelstein DL, Axilbund JE, Hylind LM, et al. Serrated polyposis: rapid and relentless development of colorectal neoplasia. Gut. 2013;62(3):404–8. doi: 10.1136/gutjnl-2011-300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosty C, Hewett DG, Brown IS, et al. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013;48(3):287–302. doi: 10.1007/s00535-012-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ogino S, Lochhead P, Chan AT, et al. Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Mod Pathol. 2013;26(4):465–84. doi: 10.1038/modpathol.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Risk factors for serrated polyps (primarily hyperplastic polyps)
Supplementary Table 2: Contemporary studies of risk factors for proximal and/or large serrated polyps
Supplementary Table 3: Contemporary studies of risk factors for sessile serrated adenomas and other advanced serrated polyps


