Abstract
Among primary thyroid neoplasms, papillary thyroid carcinoma (PTC) and primary thyroid lymphoma (PTL) are known to coexist and are pathogenetically linked with Hashimoto's thyroiditis (HT). However, HT occurring in association with medullary thyroid carcinoma (MTC) is rarely documented. We report here an interesting case. A 34-year-old female with a solitary thyroid nodule underwent fine needle aspiration cytology (FNAC) that was interpreted as “MTC with admixed reactive lymphoid cells, derived possibly from a pretracheal lymph node.” Total thyroidectomy specimen showed “MTC with coexisting HT.” At a later stage, a follow-up FNAC from the recurrent thyroid swelling showed features consistent with HT. As an academic exercise, the initial smears on which a diagnosis of MTC was offered were reviewed to look for evidence of coexisting HT that showed scanty and patchy aggregates of reactive lymphoid cells without Hürthle cells. Our case highlights an unusual instance of MTC in concurrence with HT that can create a tricky situation for cytopathologists.
Keywords: Hashimoto's thyroiditis (HT), medullary thyroid carcinoma (MTC), concurrent lesions
Introduction
Hashimoto's thyroiditis (HT) often coexists with differentiated thyroid cancers such as papillary and follicular thyroid carcinomas and some studies have claimed a pathogenetic association between HT and papillary thyroid carcinoma (PTC).[1] Interestingly, rare studies have reported HT in combination with C-cell hyperplasia[2] and medullary thyroid carcinoma (MTC)[3,4] suggesting its possible pathogenetic link with C-cell lesions as well. The cases of concurrent MTC and HT documented in the literature are surgical specimens[1,3] and to our knowledge, fine needle aspiration cytologic (FNAC) description of such instances has not been on record. Although histologic diagnosis of concurrent MTC and HT is not difficult, its cytologic interpretation can be tricky. We report here an interesting case.
Case Report
A 34-year-old female presented with a history of thyromegaly for 1 year. Examination revealed a 4 cm × 3 cm, firm, solitary thyroid nodule freely moving with deglutition, along with a palpable left cervical lymph node. A contrast-enhanced computed tomography revealed multiple enlarged cervical lymph nodes. FNAC of thyroid and the palpable cervical node was performed by standard procedure. Cytologic smears were appropriately processed and stained by routine Papanicolaou and May-Grünwald-Giemsa (MGG) techniques. Thyroid aspirate showed a predominant population of discrete and few loosely clustered polygonal to plasmacytoid cells of variable size, at places exhibiting a pseudoglandular pattern. The cells had a medium-large, eccentrically placed nuclei displaying salt-and-pepper chromatin and a moderate to abundant basophilic cytoplasm. There were a few binucleate, trinucleate, and multinucleate cells exhibiting similar nuclear and cytoplasmic features. Some cells exhibited bizarre nuclei, conspicuous nucleoli, cytoplasmic vacuolation, and faint cytoplasmic red granules. The neoplastic cells were admixed with scattered, as well as, patchily distributed mature and transformed lymphocytes [Figure 1a and b] along with lymphoglandular bodies and a scanty amyloid-like material. Plasma cells were easily differentiated from plasmacytoid neoplastic cells. A diagnosis of MTC was rendered and the reactive lymphoid cells and plasma cells in the thyroid aspirate were reported as those of a pretracheal node. Cervical lymph node aspirate showed features consistent with metastatic MTC. A total thyroidectomy with lymph node dissection was carried out. Histologic sections from the tumor showed features consistent with “MTC coexisting with HT.” The tumor showed a significant amyloid deposit and the neoplastic cells were positive for calcitonin and synaptophysin [Figure 2 with insets a–c]. Lymph nodes revealed metastatic MTC. After 4 months, the patient presented with a right-sided thyroid nodule measuring 1.5 cm × 1 cm. Fine needle aspiration (FNA) of the nodule was performed to exclude recurrent MTC. A careful examination of representative and cellular FNA smears showed unambiguous features of HT without any evidence of MTC. Preoperative FNA smears were reviewed at this stage to know if features of HT were overlooked earlier; however, no Hürthle cells were appreciated even on review.
Figure 1.
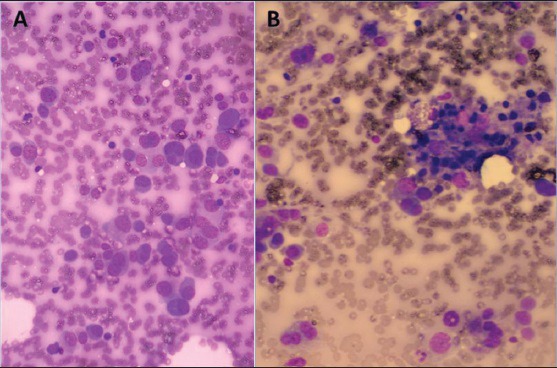
(a) Shows dissociated uni-, bi-, and multinucleated plasmacytoid neoplastic cells with a few scattered lymphocytes (MGG, ×400), while (b) Shows a patchy reactive lymphoid aggregate (MGG, ×400)
Figure 2.
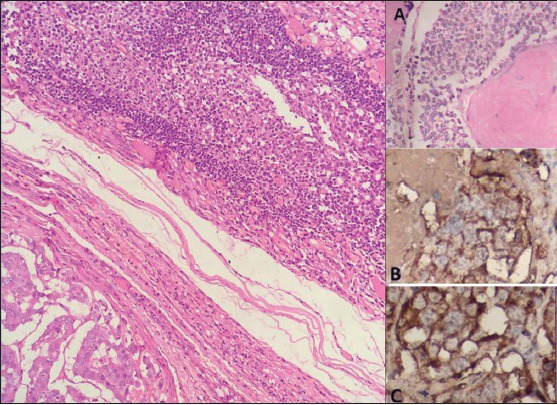
Shows the histology of coexisting medullary thyroid carcinoma and Hashimoto's thyroiditis (H and E, ×100). Inset A: Magnified view of neoplastic cells with amyloid (H and E, ×400); Inset B: Calcitonin positivity and Inset C: Synaptophysin expression (IHC, ×400)
Discussion
Although, cases are documented on surgically resected specimens of thyroid, the occurrence of MTC coexisting with HT is an extremely uncommon event. Although, our case was accurately diagnosed as MTC on preoperative FNA, there were some conflicting cytomorphologic features such as rare pseudoglandular structures and cells mimicking metastatic adenocarcinoma with conspicuous nucleoli and cytoplasmic vacuoles. Nonetheless, in view of a representative thyroid aspirate, with majority of the neoplastic cells displaying classic cytomorphology of MTC including the presence of amyloid-like material, metastatic adenocarcinoma was easily excluded. The most interesting aspect of our case was the striking presence of reactive lymphoid cells in combination with MTC, that we preoperatively interpreted as those derived from the pretracheal node, though actually they represented HT. Notably, no significant stromal lymphocytic response was observed within the tumor tissue to explain their presence in the preoperative FNA material. The postoperative evidence of HT in the recurrent nodule can be attributed to the diffuse nature of the autoinflammatory process of HT in the residual thyroid tissue. As there was no clinical suspicion of HT, serologic tests were not performed preoperatively; however, cytomorphologic features of HT were unambiguous on postoperative FNA material.
FNA interpretation of concurrent MTC and HT can be difficult due to two reasons:
A pretracheal node that moves with deglutition is likely to be incidentally sampled while aspirating thyroid, owing to which the presence of lymphoid cells in association with MTC can be interpreted as those derived from a pretracheal node (even when they are HT-derived) and
Extremely rare cases of Hürthle cell variant of MTC[5] in the presence of lymphocytes are likely to be misdiagnosed as HT.
The present case exemplifies the first situation. There has been a single case report in which calcitonin assay of FNAC fluid assisted the diagnosis of MTC in a previously known case of HT. FNAC performed thrice in that patient had yielded nondiagnostic material,[6] while in the present case there was an indication of concurrent MTC and HT in the FNA material itself. The prognosis of patients with concurrent thyroid carcinoma and HT is said to be better than that of patients with thyroid carcinoma alone. It is speculated that thyroid carcinoma stimulates the development of HT in some patients and that the autoimmune inflammatory reaction and the circulating antibodies retard the growth and dissemination of malignant cells.[1]
The purpose of documenting this case is to address the sampling and interpretative issues associated with an uncommon and perhaps an incidental occurrence of MTC in association with HT that cytopathologists should be aware of.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Segal K, Ben-Bassat M, Avraham A, Har-El G, Sidi J. Hashimoto's thyroiditis and carcinoma of the thyroid gland. Int Surg. 1985;70:205–9. [PubMed] [Google Scholar]
- 2.Libbey NP, Nowakowski KJ, Tucci JR. C-cell hyperplasia of the thyroid in a patient with goitrous hypothyroidism and Hashimoto's thyroiditis. Am J Surg Pathol. 1989;13:71–7. doi: 10.1097/00000478-198901000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Weiss LM, Weinberg DS, Warhol MJ. Medullary carcinoma arising in a thyroid with Hashimoto's disease. Am J Clin Pathol. 1983;80:534–8. doi: 10.1093/ajcp/80.4.534. [DOI] [PubMed] [Google Scholar]
- 4.Gul K, Sen DO, Ugras NS, Inancli SS, Ersoy R, Cakir B. Concurrent thyroid medullary, papillary carcinoma and Hashimoto thyroiditis: Case report. Endocrine Abstracts. 2009;20:271. [Google Scholar]
- 5.Dedivitis RA, Di Giovanni JH, Silva GF, Marinho LC, Guimarães AV. Oncocytic variant of medullary thyroid carcinoma: Case report. Arq Bras Endocrinol Metabol. 2004;48:315–7. doi: 10.1590/s0004-27302004000200017. [DOI] [PubMed] [Google Scholar]
- 6.Mousa U, Gursoy A, Ozdemir H, Moray G. Medullary thyroid carcinoma in a patient with Hashimoto's thyroiditis diagnosed by calcitonin washout from a thyroid nodule. Diagn Cytopathol. 2013;41:644–6. doi: 10.1002/dc.21850. [DOI] [PubMed] [Google Scholar]


