Abstract
Background:
The role of Papanicolaou (Pap) test in cervical cancer screening need not be overemphasized. While most Western countries have adopted the liquid-based cytology (LBC), which is considered superior, many developing countries are still using the conventional Pap smear (CPS) technique.
Objective:
To compare the staining and cytomorphological features on conventional versus liquid-based cervicovaginal smears.
Materials and Methods:
One hundred and forty cervicovaginal smears prepared by the standard conventional and LBC techniques were interpreted as per the Bethesda system of reporting cervicovaginal smears. Twelve parameters were studied, compared, and statistically analyzed. A P value <0.05 was considered to be statistically significant.
Results:
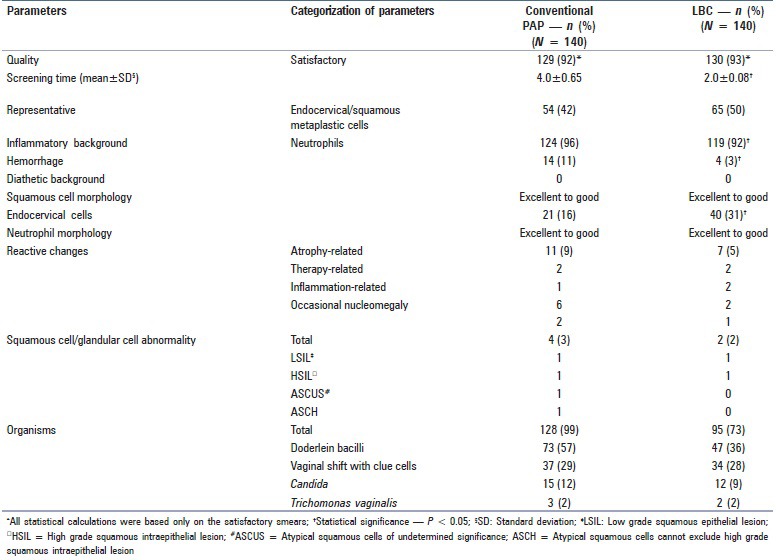
129/140 (92%) of CPSs and 130/140 (93%) LBC smears were satisfactory. LBC had a significantly shorter screening time (2.0 ± 0.08 vs 4.0 ± 0.65) and better representative material than that of CPS (50% vs 42%). Neutrophils were significantly more in CPS than LBC (96% vs 92%) with a P value <0.05 while hemorrhagic background and red blood cells (RBCs) were more prominent in CPS. LBC showed significant artifactual changes in squamous epithelial cells. Epithelial abnormalities ranging from atypical squamous cells of undetermined significance (ASCUS) to high grade squamous intraepithelial lesion (HSIL) were seen in 3% (4) and 2% (2) of CPSs and LBCs, respectively. Organisms were better picked up in CPS (99% in CPS vs 73% LBC) with a value of P = 0.0001.
Conclusion:
Although a shorter screening time and cleaner background are the major advantages of LBC, CPS is not inferior to LBC. Considering the high cost, rather than the advantages associated with LBC, we feel that CPS is a better option for developing countries.
Keywords: Conventional Pap smear (CPS), morphology, liquid-based cytology (LBC)
Introduction
Papanicolaou (Pap) smear is a useful cytological screening method for the prevention of cervical cancer, thereby reducing cervical cancer mortality rates,[1] which is done by conventional Pap smear (CPS) examination.[2] However, limitations of CPS such as suboptimal smears with insufficient squamous cells, presence of obscuring blood, dense inflammation, mucin, and thick smears with overlapping epithelial cells reduce its sensitivity to as low as 50% with a rise in false negativity rate ranging between 14% and 33%.[1,2,3] Liquid-based cytology (LBC), which is widely practiced in the Western setup,[1,2] was developed to improve the diagnostic reliability of Pap smears by reducing the number of inadequate smears and false negativity rate, and also allow important ancillary tests such as human papillomavirus (HPV) testing.[1,4] Although there is sufficient Western literature on LBC, the studies from India comparing CPS and LBC techniques are sparse.[1,2] Moreover, there have been conflicting results with regard to the quality of LBC results.[5,6]
The aim of the present work was to study the morphological differences between the LBC and CPS techniques and also to assess their relative advantages and limitations, particularly, in the Indian context.
Materials and Methods
After obtaining the permission and clearance of the Scientific Advisory and the Institute Ethics Committees of our hospital, 140 patients who attended the gynecology outpatient department (OPD) during a period of 2 months from June 2013 to July 2013 were randomly selected. The chief complaints of these patients were bleeding per vaginum, vaginal itching, ulcer, white discharge, irregular menstrual cycles, pain in the lower abdomen, and postcoital bleeding.
After obtaining informed consent from the patients, two samples were collected in one sitting. Initially, a direct Pap smear was made (CPS) using an Ayre spatula, which was immediately fixed in 95% ethanol for conventional Pap staining. The second sample was collected in a vial containing 10 mL of SurePath preservative for LBC, using the detachable endocervical brush provided by the company. The sample thus collected was processed using the Tripath machine (BD SurePath™ liquid-based Pap test Rotina 380 (Hettich Zentrifugen D-78532 Tullinger type 1701-30); Prepmate automated accessory (MDC1 Ltd., 1 liver pool garden Worthing BN (Burlington) 11 1SL UK; multivial vortexer (Henry Troemner, LLC. USA); Prepstain easy aspirator, ejector, and vacuum pump (KNF Neuberger, D79112, Freiburg made in Germany, type PM15454-026) and the slides were stained by BD stains (Prep stains). Both conventional and LBC smears were studied in detail (initially by a trained undergraduate student under the supervision of a trained cytotechnologist) by two experienced cytopathologists and were interpreted as per the Bethesda system of reporting Pap smears.[7]
Twelve parameters were studied and compared between the two techniques. The parameters included were:
Quality,
Representation,
Inflammatory background,
Red blood cells (RBCs)/hemorrhagic background,
Diathetic background,
Squamous cell morphology,
Endocervical cell morphology,
Inflammatory cell morphology,
Reactive changes,
Squamous/glandular cells abnormality,
Organisms, and
The screening time.
Only satisfactory smears were included for statistical analysis. Simple percentage analysis, Fisher's exact test, chi-square test, and unpaired t-test were performed wherever applicable. A P value <0.05 was considered to be statistically significant.
Results
Out of 140 cases, 129 (92%) CPSs were satisfactory and 11 (8%) were unsatisfactory; LBC also had a similar rate of satisfactoriness with 130 (93%) satisfactory and 10 (7%) unsatisfactory smears with a statistically insignificant two-sided P value. The time taken for screening a CPS was 4.0 ± 0.65 min while it was 2.0 ± 0.08 min for LBC with a P value of 0.0001. As for the representativeness (based on the presence or absence of endocervical/metaplastic squamous cells), LBC smears were more representative [65 (50%)] than CPS [54 (42%)]. The endocervical cells were more frequently encountered in LBC [40 (31%)], as compared to CPS [21 (16%)] with a statistically significant P value of 0.008. Inflammation was graded as 1+, 2+, and 3+ based on the visual impression of the extent of neutrophils present. Neutrophils were seen in 124 (96%) conventional Pap smears in comparison with 119 (92%) LBC smears with P value of <0.05. The morphology of neutrophils ranged from “good” to “excellent” in both CPS and LBC and the neutrophils showed more clumping in LBC smears while they were scattered in CPS. Hemorrhagic background and RBCs were encountered more frequently in CPS [14 (11%)], as compared to LBC 4 (3%) with a statistically significant P value of <0.05. However, none of the conventional Pap/LBC smears showed diathetic background due to lack of any invasive carcinoma in our study.
The reactive changes such as atrophy, therapy, and inflammation-associated alterations and occasionally, nucleomegaly were appreciated in 11 (9%) CPSs and 7 (5%) LBC smears, which were not statistically significant (P = 0.34). Squamous cell/glandular cell abnormalities such as low grade squamous epithelial lesion (LSIL), high grade squamous intraepithelial lesion (HSIL), atypical squamous cells of undetermined significance (ASCUS), and atypical squamous cells cannot exclude high grade squamous intraepithelial lesion (ASCH) were seen in four (3%) CPSs and two (2%) LBC smears. Organisms were appreciable in 128 (99%) the conventional smears and 95 (73%) LBC smears; this was statistically significant (P = 0.0001). The organisms observed were Doderlein bacilli (normal commensals), Gardenerella vaginalis (shift in vaginal flora with clue cells), Candida species, and Trichomonas vaginalis. Details of all 12 parameters and their statistical significances are provided in Table 1.
Table 1.
Comparison between the conventional Pap smear and SurePath LBC
Discussion
Our study assessed the morphological differences between the conventional Pap smear and LBC smear and their impact on their interpretation. Twelve of the parameters were analyzed, out of which the parameters that showed statistical significance were:
Representativeness,
Inflammation,
Hemorrhagic background and RBCs, and
Organisms.
There was no significant difference in the remaining parameters between the two techniques. With regard to satisfactoriness of the smears, most studies claim a reduced number of unsatisfactory smears with LBC.[1,8,9,10,11,12,13] However, similar to our study, a study by Davey et al.[14] showed no significant difference in the rate of inadequate/unsatisfactory smears between LBC and CPS. An almost equal number of unsatisfactory smears were encountered in conventional Pap (8%) and LBC (7%) in our study. Hemorrhage, dense inflammation, drying artefact, and scanty cellular elements were the unsatisfactory features noted in our CPS while a scanty cellular component was the only unsatisfactory feature in LBC. Unlike most other studies involving split smears,[4,8] our study involved the direct vial transfer for LBC and collection by Ayre spatula for CPS.
There was a drastic reduction in the screening time, which was obviously attributable to a smaller screening area of 13 mm in the SurePath preparations. Our results were comparable to those of other studies.[11,15,16] With regard to the presence of endocervical cells/metaplastic squamous cells, LBC gave better results in our study, which was attributable to the cleaner background of LBC smears that allowed a better appreciation of the transformation zone component. The other reason was that CPS is usually associated with some loss of cells in the collecting device while LBC is likely to be more representative due to direct transfer of the entire collecting device for the preservation and homogenization of the sample during processing. Our study showed more number of endocervical cells in LBC, which was in accordance with a study by Bergerone et al.[10] who reported that only 7% of their CPSs contained endocervical cells while 13% of LBC smears had them. However, in the study of Strander et al. most LBC smears lacked endocervical cells when compared with CPS.[11]
In our study, inflammation was graded as 1+, 2+, and 3+. A 3+ inflammation was noted in 52 (42%) CPSs and 24 (20%) LBC smears while 1+ inflammation was noted in 62 (52%) LBC smears and in 38 (31%) CPSs, with a P value of <0.005. Neutrophils were seen in 124 (96%) conventional and 119 (92%) LBC smears. LBC showed a clumping of neutrophils [Figures 1a–d]; this was said to be due to tendency of LBC preservative fluid to stick to the inflammatory exudate forming clumps.[17] This also explains the cleaner background seen in LBC smears. Despite the cleaner background and reduced neutrophils, usually inflammation is not missed out in LBC because as noted by other authors, the neutrophils (although reduced) are clearly seen in LBC.[17] A difference in the hemorrhagic background was also significant between CPS and LBC; a lesser number of RBCs in LBC makes screening easier.
Figure 1.
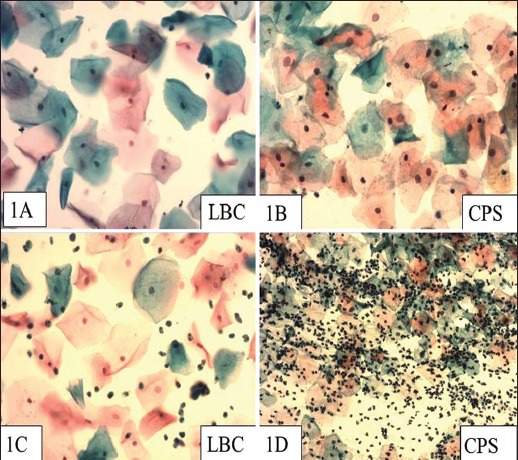
(a) A negative LBC smear with a clean background (Pap, ×200) (b) A negative CPS (Pap, ×200) (c) Inflammatory LBC smear (Pap, ×200) (d) Inflammation in a CPS (Pap, ×100)
The reactive changes observed in our cases showed no significant difference between the two techniques. The organisms inclusive of commensals and pathogenic species were better appreciated in CPS with a statistically significant difference. In the Sherwani et al., study where only the infectious organisms were taken into account, CPS detected organisms only in 3.1% smears while LBC detected them in 8.7% of the cases.[1] If only infectious organisms are considered in our study, the difference between CPS vs. LBC techniques in organism detection rate accounts to (55) 48 vs. 48 (37%).
There are some conflicting reports with regard to the interpretation of epithelial abnormalities between CPS and LBC. Obwegeser et al.[9] and Sykes et al.[15] showed no statistically significant difference between the two techniques in detecting cells ranging from normal to HSIL.[10,16] However, according to Abulafia et al.,[3] Obwegeser et al.,[9] Vincenzo et al.,[12] and Davey et al.,[14] an interpretation of ASCUS is more frequent with CPS although no significant difference is seen in LSIL/HSIL detection. The possible reason for a lesser number of ASCUS in some of the studies is perhaps because the cells, which appear normal on LBC, may appear abnormal with CPS. Generally, most studies claim LBC to be superior to CPS in identifying LSIL and HSIL. According to these authors, the frequency of interpretation of ASCUS is low with LBC.[3] Bergerone et al.[10] and Ilter et al.[13] claim an improvement in the detection rates of ASCUS with LBC. In the study of Strander et al.,[11] CPS could detect high grade lesions in as high as 93% of the smears as compared to 83% of LBC smears; the study considered histopathology as the gold standard.[11] In the our study, LSIL, HSIL, ASCUS, and ASCH [Figures 2a–d] were seen in four (3%) CPSs. LBC could pick up LSIL and HSIL in two (2%) cases but was unable to comment on the borderline categories, i.e., ASCUS and ASCH. The present work is a short-term study of 2 months’ duration, which is the reason for the limited number of epithelial abnormalities. This is one of the major limitations of our study. Another notable finding in our study was that LBC was associated with significant artifactual changes such as rounded/folded squamous cells and pseudo-koilocytic change.
Figure 2.
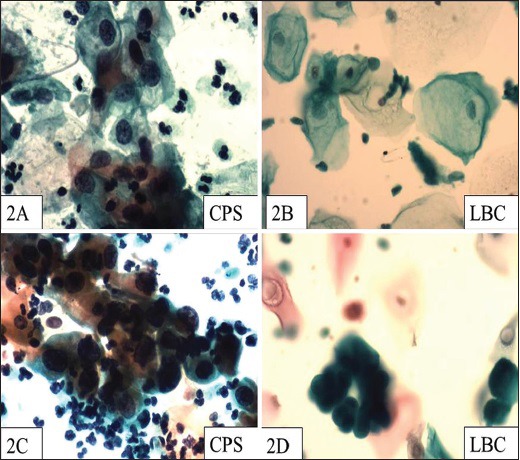
(a) A CPS showing LSIL change (Pap, ×400) (b) LBC smear from the same patient as Figure 2a, showing LSIL changes that are not so prominent (Pap, ×400) (c) HSIL in CPS (Pap, ×400) (d) HSIL in LBC (Pap, ×400) preparation of the same case as Figure 2c
In the present study, SurePath technology was used for LBC although ThinPrep technology is also utilized in various laboratories. No data is available in the indexed literature on direct comparison between the two technologies. Nonetheless, according to the information provided in the textbook edited by Bibbo and Wilbur, some preliminary studies (nonpeer-reviewed) have shown ThinPrep to be associated with higher unsatisfactory rates than SurePath, which is attributed to the interference of blood in ThinPrep specimens.[18]
Conclusion
In conclusion, despite the smaller sample size, some of the major advantages of LBC noted in our study were lesser screening time, cleaner background, and the better spread of cellular elements. Although a longer screening time is one of the major drawbacks of CPS, considering the lack of any significant difference in the final screening outcome and also the high cost associated with LBC, we feel that CPS still remains a better option in the context of countries with a resource-poor setting but with trained personnel for Pap screening.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to thank the Indian Council of Medical Research (ICMR) in New Delhi, Delhi, India and our institute's Scientific Advisory Committee for allowing us to conduct this study as a Bachelor of Medicine, Bachelor of Surgery (MBBS) student's short-term study (STS).
References
- 1.Sherwani RK, Khan T, Akhtar K, Zeba A, Siddiqui FA, Rahman K, et al. A comparative study of conventional pap smears and liquid based cytology. J Cytol. 2007;24:167–72. [Google Scholar]
- 2.Nandini NM, Nandish SM, Pallavi P, Akshatha SK, Chandrashekhar AP, Anjali S, et al. Manual liquid based cytology in primary screening for cervical cancer-a cost effective preposition for scarce resource settings. Asian Pac J Cancer Prev. 2012;13:3645–51. doi: 10.7314/apjcp.2012.13.8.3645. [DOI] [PubMed] [Google Scholar]
- 3.Abulafia O, Pezzullo JC, Sherer DM. Performance of ThinPrep liquid-based cervical cytology in comparison with conventionally prepared papanicolaou smears: A quantitative survey. Gynecol Oncol. 2003:137–44. doi: 10.1016/s0090-8258(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Norman I, Elfgren K, Gaberi V, Hagmar B, Hjerpe A, et al. A comparison of liquid based cytology and pap smear as a screening method for cervical cancer. Oncol Rep. 2007;18:157–60. [PubMed] [Google Scholar]
- 5.Siebers AG, Klinkhamer PJ, Grefte JM, Massuger LF, Vedder JE, Beijers-Broos A, et al. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors: A randomized controlled trial. JAMA. 2009;302:1757–64. doi: 10.1001/jama.2009.1569. [DOI] [PubMed] [Google Scholar]
- 6.Confortini M, Bulgaresi P, Cariaggi MP, Carozzi FM, Cecchini S, Cipparrone I, et al. Conventional pap smear and liquid-based cervical cytology smear: Comparison from the same patient. Tumori. 2002;88:288–90. doi: 10.1177/030089160208800409. [DOI] [PubMed] [Google Scholar]
- 7.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, et al. The 2001 Bethesda system: Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 8.Park IA, Lee SN, Chae SW, Park KH, Kim JW, Lee HP. Comparing the accuracy of ThinPrep Pap tests and conventional Papanicolaou smears on the basis of the histologic diagnosis: A clinical study of women with cervical abnormalities. Acta Cytol. 2001;45:525–31. doi: 10.1159/000327859. [DOI] [PubMed] [Google Scholar]
- 9.Obwegeser JH, Brack S. Does liquid-based technology really improve detection of cervical neoplasia? A prospective, randomized trial comparing the ThinPrep Pap test with the conventional Pap test, including follow-up of HSIL cases. Acta Cytol. 2001;45:709–14. doi: 10.1159/000328292. [DOI] [PubMed] [Google Scholar]
- 10.Bergeron C, Fagnani F. Performance of a new, liquid-based cervical screening technique in the clinical setting of a large French laboratory. Acta Cytol. 2003;47:753–61. doi: 10.1159/000326601. [DOI] [PubMed] [Google Scholar]
- 11.Strander B, Andersson-Ellström A, Milsom I, Rådberg T, Ryd W. Liquid-based cytology versus conventional Papanicolaou smear in an organized screening program: A prospective randomized study. Cancer. 2007;111:285–91. doi: 10.1002/cncr.22953. [DOI] [PubMed] [Google Scholar]
- 12.Maccallini V, Angeloni C, Caraceni D, Fortunato C, Venditti MA, Di Gabriele G, et al. Comparison of the conventional cervical smear and liquid-based cytology: Results of a controlled, prospective study in the Abruzzo Region of Italy. Acta cytol. 2008;52:568–74. doi: 10.1159/000325599. [DOI] [PubMed] [Google Scholar]
- 13.Ilter E, Midi A, Haliloglu B, Celik A, Yener A, Ulu I, et al. Comparison of conventional and liquid based cytology: Do the diagnosis benefits outweigh the financial aspect? Turk J Med SCI. 2012;42:1200–6. [Google Scholar]
- 14.Davey E, Barratt A, Irwig L, Chan SF, Macaskill P, Mannes P, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: A systemic review. Lancet. 2006;367:122–32. doi: 10.1016/S0140-6736(06)67961-0. [DOI] [PubMed] [Google Scholar]
- 15.Sykes PH, Harker DY, Miller A, Whitehead M, Neal H, Wells JE, et al. A randomized comparison of SurePath liquid-based cytology and conventional smear cytology in a colposcopy clinic setting. BJOG. 2008;115:1375–81. doi: 10.1111/j.1471-0528.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- 16.Cheung A, Szeto E, Leung B, Khoo US, Anita N. Liquid-based cytology and conventional cervical Smears: A Comparison study in an Asian screening population. Cancer. 2003;99:331–3. doi: 10.1002/cncr.11786. [DOI] [PubMed] [Google Scholar]
- 17.Roghaei MA, Afshar Moghaddam N, Pooladkhan SH, Roghaie SH. Adequacy criteria and cytomorphological changes in Liquid-Prep TM versus conventional cervical cytology. SEJM. 2010;11:173–82. [Google Scholar]
- 18.Wilbur DC, Bibbo M. Automation in cervical cytology. In: Bibbo M, Wilbur DC, editors. Comprehensive Cytopathology. 3rd ed. China: Saunders; 2008. pp. 1028–4. [Google Scholar]


