Abstract
Background:
Small round cell tumors (SRCTs) are a group of tumors composed of small, round, and uniform cells with high nuclear/cytoplasmic (N/C) ratios. The appearance of SRCT neoplastic cells in the effusion fluid is very rare. We reported the cytomorphological findings of SRCTs in effusion cytology, and performed statistical and mathematical analyses for a purpose to distinguish SRCTs.
Materials and Methods:
We analyzed the cytologic findings of effusion samples from 40 SRCT cases and measured the lengths of the nuclei, cytoplasms, and the cell cluster areas. The SRCT cases included 14 Ewing sarcoma (EWS)/primitive neuroectodermal tumor cases, 5 synovial sarcoma cases, 6 rhabdomyosarcoma cases, 9 small cell lung carcinoma (SCLC) cases, and 6 diffuse large B-cell lymphoma (DLBL) cases.
Results:
Morphologically, there were no significant differences in the nuclear and cytoplasmic lengths in cases of EWS, synovial sarcoma, and rhabdomyosarcoma. The cytoplasmic lengths in cases of SCLC and DLBL were smaller than those of EWS, synovial sarcoma, and rhabdomyosarcoma. The nuclear density of the cluster in SCLC was higher than that in other SRCTs, and cases of DLBL showed a lack of anisokaryosis and anisocytosis.
Conclusion:
We believe that it might be possible to diagnose DLBL and SCLC from cytologic analysis of effusion samples but it is very difficult to use this method to distinguish EWS, synovial sarcoma, and rhabdomyosarcoma. Statistical and mathematical analyses indicated that nuclear density and dispersion of nuclear and cytoplasmic sizes are useful adjuncts to conventional cytologic diagnostic criteria, which are acquired from experience.
Keywords: Cytology, effusion, small round cell tumors (SRCTs)
Introduction
Small round cell tumors (SRCTs) are a group of tumors composed of small, round, and uniform cells with high nuclear/cytoplasmic (N/C) ratios. SRCTs include Ewing sarcoma (EWS)/primitive neuroectodermal tumor, small cell variant of synovial sarcoma, rhabdomyosarcoma, non-Hodgkin's lymphoma, neuroblastoma, Wilms’ tumor, desmoplastic small round cell tumor, and others. SRCTs often occur during childhood, and accurate diagnosis has become increasingly crucial, as disparate approaches to therapy are used for distinct tumor types.[1] The diagnosis of these tumors is difficult using morphologic features alone. Rather, the diagnosis should be based on standard clinical and laboratory diagnostic modalities, immunohistochemistry, cytogenetics, and molecular studies.[1,2] In diagnosing an SRCT by effusion cytology, it is first important to identify the tumor cells as malignant.[3] To date, numerous immunocytochemical analyses of effusion samples have been reported using various primary antibodies and fluorescence in situ hybridization (FISH) to detect malignant cells in the effusion fluid.[4,5] These techniques can be used in combination with effusion cytology to help distinguish benign cells from malignant cells. However, it might be possible to diagnose SRCT malignancies by cytology alone because these tumors have the characteristic cytologic finding of small round cells with high N/C ratios.
In cytological diagnosis, most pathologists and cytotechnologists use diagnostic criteria acquired from experience such as nuclear size, cell size, anisokaryosis, and cellularity. These cytologic findings in general are very useful for histologic classification and distinction of malignancy. In this study, we measured the length of the nucleus and cytoplasm and the cell cluster area of cells from the effusion fluid of 40 SRCT cases, and we performed statistical and mathematical analyses in order to compare with the conventional cytologic diagnostic criteria acquired from experience, and we describe the cytologic characteristics of SRCTs including EWS, synovial sarcoma, rhabdomyosarcoma, small cell lung carcinoma (SCLC), and diffuse large B-cell lymphoma (DLBL). Although SCLC and DLBL are not strictly SRCTs, we included these tumors because it is necessary to distinguish SRCTs.
Materials and Methods
Cases
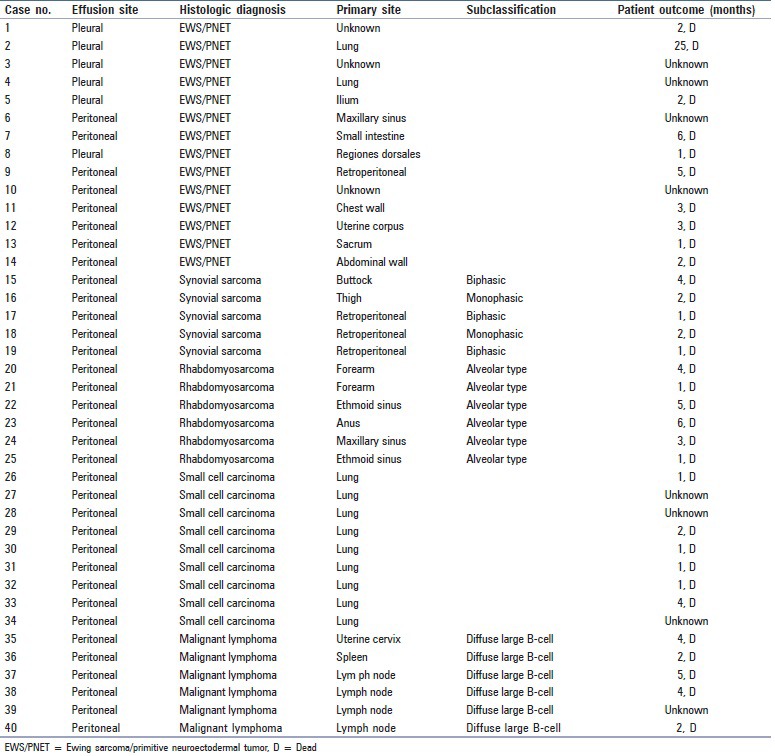
Effusion specimens were obtained from a cohort of patients with SRCTs and malignant effusions between January 2002 and December 2011 at the National Cancer Center Hospital. All cases of SRCT were confirmed by surgical biopsy or resection of the primary site. A total of 40 effusion specimens were evaluated and included pleural (n = 24), peritoneal (n = 14), and pericardial (n = 2) effusions. The 40 cases of SRCT included 14 EWS cases (males: 5; females: 9), 5 synovial sarcoma cases (male: 1; female: 4), 6 rhabdomyosarcoma cases (male: 2; female: 4), 9 SCLC cases (male: 7; female: 2), and 6 DLBL cases (male: 2; female: 4). The clinical features of the 40 patients with SRCT are listed in Table 1.
Table 1.
Clinicopathologic features of the 40 patients with small round cell tumors
For the cytological examinations, each sample of effusion fluid was centrifuged at 1,500 rpm for 60 s using Autosmear (Sakura Seiki Co., Tokyo, Japan), and the smears were fixed with 95% ethanol and stained using the Papanicolaou method.
Measurements and statistical analysis
For all 40 cases, the nuclear size, cytoplasmic size, and cellular cluster size of the stained specimens were measured using a microscope digital camera/measuring device (DS-Fi2-L3; NIKON Co., Tokyo, Japan). The diameters of the nucleus and cytoplasm were determined from 20 randomly selected and singly scattered cells at a magnification of ×1000. The cell cluster area was determined from 20 randomly selected clusters at a magnification of ×600 (data not shown). When fewer than 20 scattered cells and/or cellular clusters were observed, all scattered cells and clusters were measured.
The cluster area, the number of nuclei in the cluster, and the mean nuclear diameter were used to calculate the cluster's nuclear density, which is the ratio of all nuclear areas in the cluster. The equation used to determine the cluster's nuclear density is as follows: Cluster's nuclear density = π × r × r × (the number of nuclei in the cluster)/(the cluster area), where r is the radius of the mean nuclear length.
In the statistical analysis, P values for nuclear length, cytoplasmic length, and nuclear density were calculated using the Tukey–Kramer method for pairwise comparisons between each histologic classification. The standard deviations of these measurements were calculated from a weighted average and analyzed by hierarchical modeling. In all analyses, a P value of <0.05 was considered to be statistically significant.
Results
Ewing sarcoma/primitive neuroectodermal tumor
The average age of the 14 patients with EWS was 32 years (range: 3-67 years). The ranges of the mean nuclear lengths and cytoplasmic lengths of each case were 9.4-14.1 μm and 11.4-16.7 μm, respectively [Table 2]. The nuclear length was significantly different among all the cases and within each case [Figure 1]. The range of the mean nuclear density of each case was 0.64-1.17 [Table 2]. Two cases showed numerous tumor cells in the effusion specimen, two cases showed nuclear molding, five cases had conspicuous nucleoli, and three cases had prominent irregular nuclei. Figures 2a and b show the effusion cytology from a representative case of EWS, with singly scattered neoplastic cells (A) and cellular clusters (B). For one case of EWS, a different cytologic form was observed [Figure 3].
Table 2.
The measured lengths and data of the 40 cases of small round cell tumors
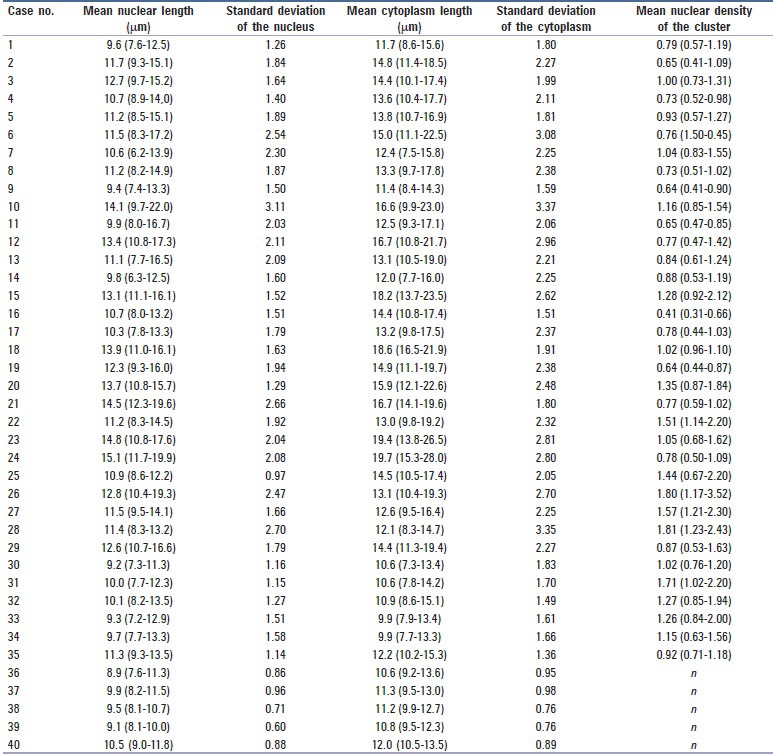
Figure 1.
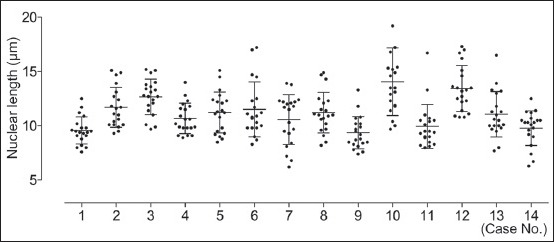
The nuclear length of 14 cases of Ewing sarcoma/primitive neuroectodermal tumor (mean with standard deviations). The dot plot is the nuclear length of each nucleus counted. The nuclear length was significantly different among all cases and within each case
Figure 2.
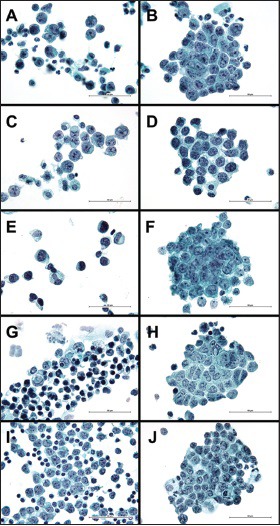
Cytologic features of effusion fluid samples from patients with small round cell tumors (Pap, ×1000). Left panel: Singly scattered neoplastic cells with a high nuclear-cytoplasmic ratio. Right panel: Neoplastic cells in a loosely cohesive two-dimensional cluster. (a and b) Effusion cytology of Ewing sarcoma/primiti ve neuroectodermal tumor from case No. 10 (b) This cluster consists of 39 neoplastic cells with a cluster area of 7129 μm2. The mean nuclear radius is 7.05 μm. The nuclear density of the cluster is 0.85 (c and d) Effusion cytomorphology of the synovial sarcoma (C: Case No. 19; D: Case No. 17) (d) This cluster consists of 32 neoplastic cells with a cluster area of 5915 μm2. The mean nuclear radius is 5.15 μm. The nuclear density of the cluster is 0.45 (e and f) Effusion cytomorphology of rhabdomyosarcoma (E: Case No. 20; F: Case No. 23) (f) This cluster consists of 71 neoplastic cells with a cluster area of 8474 μm2. The mean nuclear radius is 7.40 μm. The nuclear density of the cluster is 1.14 (g and h) Effusion cytomorphology of small cell lung carcinoma from case No. 29 (h) This cluster consists of 45 neoplastic cells with a cluster area of 6891 μm2. The mean nuclear radius is 6.30 μm. The nuclear density of the cluster is 0.81 (i and j) Effusion cytomorphology of DLBL (I: Case No. 40; J: Case No. 35) (j) This cluster consists of 74 neoplastic cells with a cluster area of 8584 μm2. The mean nuclear radius of this case is 5.65 μm. The nuclear density of the cluster is 0.86
Figure 3.
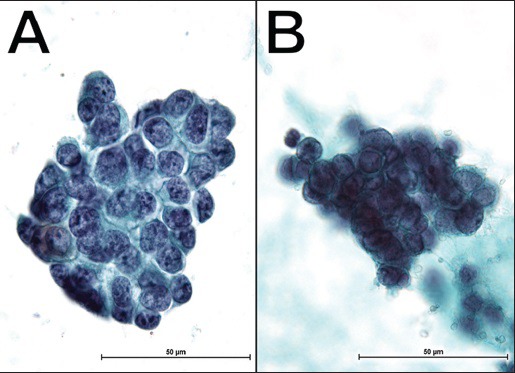
Effusion cytology of Ewing sarcoma/primitive neuroectodermal tumor (case No. 3; Pap, ×1000). A different cytologic form was observed in the same patient by the difference of the time, which extracted the effusion. (a) Neoplastic cells disposed in loose cohesive clusters, which consist of round-to-oval nuclei (b) The cellular cluster is three-dimensional and consists of smaller nuclei (leftpanel). The cluster's nuclear density is very high
Synovial sarcoma
The average age of the five patients with synovial sarcoma was 34 years (range: 22-60 years). Three cases had biphasic synovial sarcoma, and two cases had monophasic synovial sarcoma. The ranges of the mean nuclear lengths and cytoplasmic lengths of each case were 10.3-13.9 μm and 13.2-18.6 μm, respectively, and the range of the mean nuclear density was 0.41-1.28 [Table 2]. Spindle-shaped cells and epithelioid cells were not seen in all cases, and fine chromatin and remarkable irregular nuclei were seen in most cases. Figures 2c and d show the effusion cytology from representative cases of synovial sarcoma, with singly scattered neoplastic cells (C) and cellular clusters (D).
Rhabdomyosarcoma
The average age of the six patients with rhabdomyosarcoma was 23 years (range: 15-35 years). All cases were of the alveolar type. The ranges of the mean nuclear lengths and cytoplasmic lengths of each case were 10.9-15.1 μm and 13.0-19.7 μm, respectively, and the range of the mean nuclear density was 0.77-1.51 [Table 2]. Rhabdoid cells with abundant, dense cytoplasm and eccentrically located nuclei were seen in two cases. Figures 2e and f show the effusion cytology from the representative cases of rhabdomyosarcoma, with singly scattered neoplastic cells (E) and cellular clusters (F).
Small cell lung carcinoma
The primary site of all cases of SCLC was the lung. The average age of the nine patients with SCLC was 69 years (range: 48-79 years). The ranges of the mean nuclear lengths and cytoplasmic lengths were 9.2-12.8 μm and 9.9-14.4 μm, respectively, and the range of the mean nuclear density was 0.87-1.81 [Table 2]. Seven out of nine cases showed nuclear molding, six cases showed the appearance of many cellular clusters with scanty isolated cells, and one case showed a necrotic background. Compared to the other histologic classifications of SRCT, cases of SCLC showed fewer isolated cells. Figures 2g and h show the effusion cytology from a representative case of SCLC, with singly scattered neoplastic cells (G) and cellular clusters (H).
Diffuse large B-cell lymphoma
The average age of the six patients with DLBL was 65 years (range: 50-79). The ranges of the mean nuclear lengths and cytoplasmic lengths were 8.9-11.3 μm and 10.6-12.2 μm, respectively [Table 2]. In five of the six cases, neoplastic cells consisted entirely of singly scattered cells. In the single case with a cell cluster, case number 35, there were numerous isolated neoplastic cells in the background. The mean nuclear density of this case was 0.92. Figures 2i and j show the effusion cytology from a representative case of DLBL, with singly scattered neoplastic cells (I) and cellular clusters (J).
Statistical analysis
For cases of EWS, synovial sarcoma, and rhabdomyosarcoma, there were no significant differences in nuclear and cytoplasmic morphologies including chromatin pattern, nuclear shape, presence of nucleoli, quality of the cytoplasm, and type of background cells.
Figure 4 shows the distribution of cytoplasmic lengths for each histologic tumor type. Figure 4 shows that the cytoplasmic length of effusion cells in SCLC and DLBL were significantly smaller than those in EWS, synovial sarcoma, and rhabdomyosarcoma.
Figure 4.
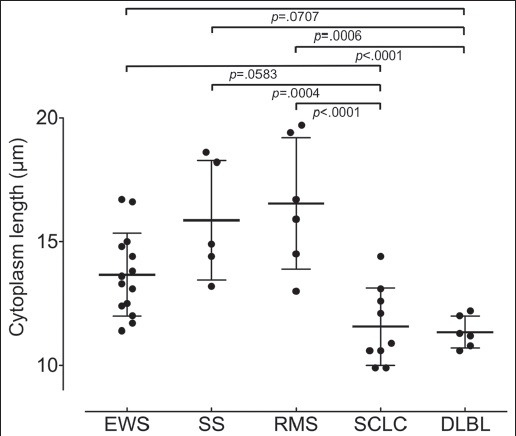
The cytoplasmic length of all 40 SRCT cases. The dot plot is the mean cytoplasmic length. Cytoplasmic lengths of the small cell lung carcinoma (SCLC) and DLBL are significantly smaller than those of synovial sarcoma (SS) and rhabdomyosarcoma (RMS)
As seen in Figure 5, the nuclear density of the cell cluster for SCLC was significantly higher than those in EWS (P <.0001) and synovial sarcoma (P = .0026).
Figure 5.
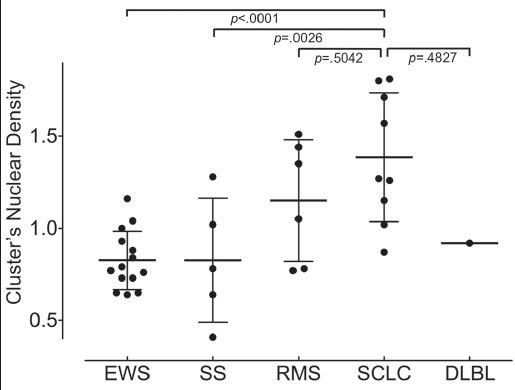
The cell cluster nuclear density. The dot plot is the mean nuclear density of the cluster in a case. The nuclear density of the cluster for small cell lung carcinoma (SCLC) is significantly higher than that of the Ewing sarcoma/primitive neuroectodermal tumor (EWS) and synovial sarcoma (SS). A significant difference in cluster nuclear density is not seen between rhabdomyosarcoma (RMS) and DLBL.
A significant difference in the standard deviation of nuclear length (data not shown) and cytoplasmic length were shown between DLBL and EWS, rhabdomyosarcoma, synovial sarcoma and SCLC [Figure 6]. With a significantly smaller standard deviation, DLBL cells lack anisokaryosis and anisocytosis compared to cells from the other SRCTs.
Figure 6.
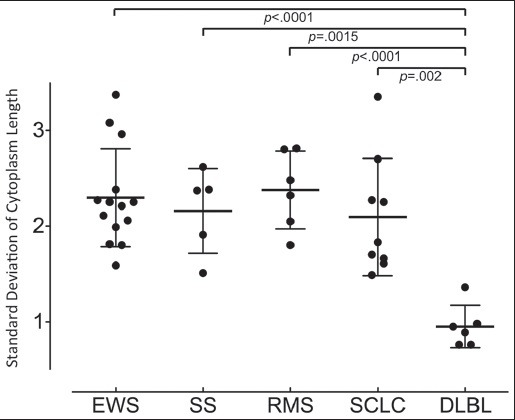
The standard deviation of cytoplasmic length represents the dispersion of size. A significant difference in the standard deviation of cytoplasmic length was observed between diffuse large B-cell lymphoma (DLBL) on the one hand and Ewing sarcoma/primitive neuroectodermal tumor (EWS), synovial sarcoma (SS), rhabdomyosarcoma (RMS), and small cell lung carcinoma (SCLC) on the other hand, showing that the effusion cells from cases of DLBL had more uniform cytologic features
Discussion
In fine-needle aspiration cytology, various cytologic forms are observed in the nucleus, cytoplasm, cluster, background, and other areas. These cytologic forms are sometimes able to support a specific histologic classification. However, these findings are less reliable in fluid specimens.[3] In effusion cytology, descriptions of the cytomorphologic features of SRCTs are scarce, and the features that exist are not useful for morphologic comparisons among the various types of SRCTs.[3,6,7,8,9] In a broad study of 226 specimens from 146 patients over a 40-year period, Wong et al. studied the effusion cytology of children and reported 88 positive specimens including lymphoma and leukemia (52%), bone and soft tissue sarcomas (7%), and Ewing sarcoma (2%).[6] In the present study, we describe the cytomorphologic features of effusion samples from five histologic types of SRCT.
In the diagnosis of small cell carcinoma from cytologic specimens, it is well-known that the cytologic findings exhibit clusters with a high nuclear density. Our results showed that the mean nuclear density of SCLC effusions, computed from the cluster area, nuclear size, and the number of nuclei comprising the cluster was 1.38 [Figure 5 and Table 2]. This is a significantly higher value than the nuclear density of effusions due to EWS (mean nuclear density: 0.83), synovial sarcoma (0.83), and rhabdomyosarcoma (1.15), confirming that a higher nuclear density of the cluster is characteristic of small cell carcinoma.
The most important morphological feature that helps to distinguish lymphomas from other SRCTs is a total lack of cell clustering.[10] Of the six cases of DLBL from our study, only a single case had a cellular cluster; the remaining cases showed only singly scattered cells. The case with the cellular cluster, case number 35, was distinguishable from the other SRCTs because it was small, with many isolated neoplastic cells. In this study, we examined the dispersion of nuclear and cytoplasmic sizes by measuring the length and calculating the standard deviation using statistical analysis. Compared to the other SRCTs, the standard deviations of the DLBL cases were small for both, the nucleus (data not shown) and cytoplasm [Figure 6], showing that effusion cells of DLBL lack anisokaryosis and anisocytosis; we believe that lack of cell clustering and small size dispersion could be very useful in differentiating DLBL from other SRCTs.
Although our results suggest that it might be possible to diagnose DLBL and SCLC from cytomorphologic findings and image morphometry in effusion samples, it is very difficult to distinguish synovial sarcoma, rhabdomyosarcoma, and EWS. Characteristic effusion cytology findings to classify these three histologic types were lacking, and the significant values were not obtained in the data of measurements and statistical analysis because the size of the nucleus and cytoplasm varied within each case and among cases with the same histologic classification [Figure 4 and Table 2].
Theunissen et al. reported a case of the cytologic diagnosis of rhabdomyosarcoma in a patient with pleural effusion.[8] They described a Papanicolaou-stained smear of the pleural effusion that showed highly cellular fluid containing isolated and clustered cells with rather scanty cytoplasm and large, round, slightly polymorphous nuclei; the nuclei were accompanied by very large cells with abundant cytoplasm, conspicuous perinuclear clearing, and very prominent nucleoli.[8] Other articles have described the effusion cytomorphologic features of rhabdomyosarcoma as having scattered multinucleated tumor giant cells, eccentric nuclei, dense and opaque cytoplasm, multinucleation, coarse chromatin, and inconspicuous nucleoli.[3,9] In our study, we confirmed similar morphologic findings of abundant, dense cytoplasm, and eccentrically located nuclei but we were unable to find cytomorphologic features common to the six rhabdomyosarcoma cases.
Reports of effusion cytology for patients with EWS are scarce. Oliveira et al. reported two cases of EWS among the four SRCT cases studied; one of these cases showed neoplastic cells in cohesive groups with no superposition, favoring the hypothesis of a neuroendocrine origin.[11] Cytology of the second case showed poorly differentiated tumor cells suggestive of neuroendocrine lineage.[11] In our study, we examined pleural and peritoneal effusions from 14 cases of EWS but did not see cytologic findings suggestive of neuroendocrine differentiation, such as rosette formation, although small round monotonous cells with high N/C ratio were seen. In all four cases of SRCT studied by Oliveira, lymphatic cells were seen in the background.[11] In our study, there were no common findings in the background. We suspect that the background finding varies with the period for which the tumor cells are floating in a fluid, and/or that the background finding is affected by disease treatment.
Interestingly, for one case of EWS (case number 3), an unexpected and different cytologic form was observed [Figure 3]; differences in nuclear size, chromatin, three-dimensional structure, and nuclear density were seen in the cluster of this case. In this sample, there was a difference in the time of extraction. We believe that this different cytologic form could have resulted from the influence of treatment, the duration of effusion in the body cavity, or the duration of the sample processing period, among other reasons. Although it is interesting to see the different cytologic forms in the same patient, this case exemplifies how difficult it is to determine disease classification in an effusion sample. In effusion specimens, it is important to realize that neoplastic cells change to a round shape and often form a cellular cluster in many cases, as is the case with effusions from SRCTs. Therefore, it is necessary to understand secondary variations of tumor cells in the diagnosis by effusion cytology.
Conclusion
In conclusion, patients with SRCTs who have tumor cells in effusion specimens have an extremely poor prognosis. Accordingly, it is very important to diagnose malignancy by cytology of the effusion specimens. In this study, statistical and mathematical analyses, including the nuclear density and the dispersion of nuclear size and cytoplasmic size, are novel methods in cytologic analysis. This analysis is a useful adjunct to conventional cytologic diagnostic criteria acquired from experience.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cohn SL. Diagnosis and classification of the small round-cell tumors of childhood. Am J Pathol. 1999;155:11–5. doi: 10.1016/S0002-9440(10)65092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson AC, Singh C, Pambuccian SE. Cytological diagnosis of metastatic alveolar rhabdomyosarcoma in the ascetic fluid: Report of a case highlighting the diagnostic difficulties. Cytojournal. 2012;9:9. doi: 10.4103/1742-6413.94569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abadi MA, Zakowski MF. Cytologic features of sarcomas in fluids. Cancer. 1998;84:71–6. doi: 10.1002/(sici)1097-0142(19980425)84:2<71::aid-cncr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Fiegl A, Massoner A, Haun M, Sturm W, Kaufmann H, Hack R, et al. Sensitive detection of tumour cells in effusions by combining cytology and fluorescence in situ hybridisation (FISH) Br J Cancer. 2004;91:558–63. doi: 10.1038/sj.bjc.6601942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda K, Tate G, Suzuki T, Kitamura T, Mitsuya T. Diagnostic usefulness of EMA, IMP3, and GLUT-1 for the immunocytochemical distinction of malignant cells from reactive mesothelial cells in effusion cytology using Cytospin preparations. Diagn Cytopathol. 2011;39:395–401. doi: 10.1002/dc.21398. [DOI] [PubMed] [Google Scholar]
- 6.Wong JW, Pitlik D, Abdul-Karim FW. Cytology of pleural, peritoneal and pericardial fluids in children. A 40-year summary. Acta Cytol. 1997;41:467–73. doi: 10.1159/000332540. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen GK, Jeannot A. Cytology of synovial sarcoma metastases in pleural fluid. Acta Cytol. 1982;26:517–20. [PubMed] [Google Scholar]
- 8.Theunissen P, Cremers M, van der Meer S, Bot F, Bras J. Cytologic diagnosis of rhabdomyosarcoma in a child with a pleural effusion: A case report. Acta Cytol. 2004;48:249–53. doi: 10.1159/000326326. [DOI] [PubMed] [Google Scholar]
- 9.Thiryayi SA, Rana DN, Roulson J, Crosbie P, Woodhead M, Eyden BP, et al. Diagnosis of alveolar rhabdomyosarcoma in effusion cytology: A diagnostic pitfall. Cytopathol. 2010;21:273–5. doi: 10.1111/j.1365-2303.2009.00700.x. [DOI] [PubMed] [Google Scholar]
- 10.Akhtar M, Iqbal MA, Mourad W, Ali MA. Fine-needle aspiration biopsy diagnosis of small round cell tumors of childhood: A comprehensive approach. Diagn Cytopathol. 1999;21:81–91. doi: 10.1002/(sici)1097-0339(199908)21:2<81::aid-dc2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira MJ, de Almeida LP, Wengerkievicz AC, Siqueira SA, Antonangelo L. From conventional fluid cytology to unusual histological diagnosis: Report of four cases. Diagn Cytopathol. 2013;41:348–53. doi: 10.1002/dc.21771. [DOI] [PubMed] [Google Scholar]


