Abstract
Mouse B-1 cells are major producers of steady-state natural antibodies but also rapid responders to infections and inflammation. These discrete functions may be the outcomes of distinct environmental or developmental triggers that drive B-1 cells toward IgM production or an effector cell fate. Alternatively, distinct B-1 cell subsets may exist, which differ in their functional plasticity. In this paper, we summarize existing data suggesting that B-1 cells form a heterogeneous group of cells with distinct developmental requirements and non-overlapping functions. Most spleen B-1 cells differ in development from that of bone marrow and peritoneal cavity B-1 cells, in that they develop in the absence of natural IgM. Functional heterogeneity is revealed by findings that B-1 cells in the bone marrow and spleen, but not the peritoneal cavity, generate natural serum IgM, while the latter are rapid responders to inflammatory and infectious insults, resulting in their relocation to secondary lymphoid tissues. A clearer understanding of the developmental and functional differences within the B-1 cell pool may reveal how they might be harnessed for prophylaxis or therapy.
Keywords: B cell subsets, IgM, influenza virus infections, natural antibodies
Introduction
Mouse B-1 cells are known as major producers of natural antibodies, that is, antibodies that are generated in the absence of prior exposure to foreign antigens.1, 2 This includes exposure to the normal microbiota, as spontaneous IgM production has been shown to be very similar in gnotobiotic and conventionally reared mice.3 Natural antibodies provide immune protection from pathogens,4-6 thus functioning as an evolutionarily conserved set of innate-like pathogen-binding effector molecules. By virtue of their ability to bind self-antigens, such as apoptotic and dying cells, they also seem to contribute to tissue homeostasis.7, 8 Natural serum antibody titers are maintained throughout life and at tightly controlled levels. Given the short half-life (0.5 days) of IgM,9 a major natural antibody isotype, this suggests a very strict regulation and maintenance of natural antibody-secreting B-1 cells over the life of the animal.
Increasingly, however, B-1 cells are also identified as rapid responders to infections and other insults. Earlier work demonstrated that injection of cytokines, lipopolysaccharide (LPS), or bacteria into the peritoneal cavity causes rapid activation of B-1 cells, resulting in their migration to the spleen and subsequent differentiation to IgM and cytokine-producing cells.10-12 Other studies showed activation of B-1 cells following infection with Streptococcus pneumonia,13 Borrelia hermsii,14 and Francisella tularensis,15 resulting in increased frequencies of pathogen-specific IgM-secreting and/or memory B-1 cells. Our studies on influenza virus infections demonstrated that B-1 cells rapidly accumulate in the draining lymph nodes of the respiratory tract.16 But even though this rapid B-1 cell mobilization and accumulation in secondary lymphoid tissues resulted in increased local IgM production, the B-1 cell–derived virus-binding IgM levels in the serum remained unchanged.16, 17
The distinct functions of B-1 cells—the provision of steady-state natural serum IgM levels on the one hand, and the rapid local response to inflammatory stimuli on the other—suggests either the existence of B-1 cell subsets differing in function and responsiveness to environmental triggers or the presence of unique stimuli that drive a proportion of B-1 cells toward natural IgM production. In addition, with regard to the B-1 responder cell populations, the question arises as to what extent antigen recognition and B cell receptor (BCR)–signaling contribute to their activation.
In this paper, we summarize recent findings demonstrating that B-1 cell populations in the spleen and bone marrow are the major secretors of natural IgM and have developmental requirements that are distinct from peritoneal cavity B-1 cells. In contrast, the well-studied B-1 cells in the body cavities provide a population of rapid B-1a cell responders to influenza infection that relocate to lymphoid tissues where they secrete IgM and might fulfill other functions. Such influenza infection–induced B-1a cell activation appears to be antigen independent but dependent on innate signals, which promote integrin-mediated sequestration of B-1 cells into regional lymph nodes of the respiratory tract. Together with the findings of other studies showing that chemokines and other innate factors are important in guiding the tissue relocation of activated B-1 cells, the data demonstrate that environmental cues strongly influence the B-1 cell response to pathogens.
B-1 cell distribution and phenotype
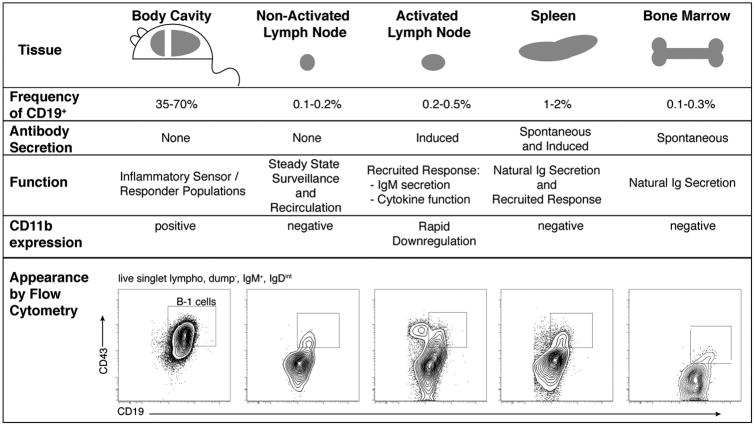
An often-stated misconception is that B-1 cells are present mostly in the body cavities of mice and are absent from lymphoid tissues, other than the spleen. Flow cytometric analysis of various mouse tissues shows the presence of B-1 cells in small frequencies in nearly all tissues studied, including resting lymph nodes and bone marrow (Fig. 1). We calculated the total number of bone marrow B-1 cells as roughly 1.6 × 106 per mouse and spleen B-1 cells as 1.8 × 106, which together account for about the same number of B-1 cells as found in the peritoneal cavity: 3.7 × 106 cells per 8- to 12-week-old female BALB/c or C57BL/6 mouse.18 In addition, B-1 cells are also found in blood (roughly 0.5% of B cells), adding significant numbers of B-1 cells to their total amounts per mouse. The contributions of B-1 cells to the pool of IgA-producing plasma cells in the lamina propria of the intestinal tract, which are dependent on the presence of microbiota, may add another large number of cells to the overall B-1 cell pool.19, 20 Rosado et al.20 recently suggested that these IgA-producing B-1 cells were derived from B-1–restricted precursors found in the fetal liver and then in the spleen of mice early after birth. Thus, B-1 cells contribute significantly to the B cell pool of lymphoid and non-lymphoid tissues.
Figure 1.
B-1 cells harbor distinct functions at different tissue sites. Cells in the spleen and bone marrow spontaneously contribute to natural antibody in serum. Cells in the body cavities and resting lymph nodes are poised for activation upon sensing inflammation and are recruited/retained in local lymphoid organs where they contribute to the immune response through effector functions. B-1 cells in the body cavities express the integrin CD11b (Mac-1), which is rapidly downregulated upon recruitment to activated lymphoid sites during their response. FACS plots show expression of CD19 and CD43 among cells gated for live, non-dump (CD3+, CD4+, CD8+, F4/80, and GR-1) cells. Boxes indicate B-1 cells and the numbers indicate their frequencies among CD19+ non-dump live cells. Note the relatively higher expression of CD19 on B-1 cells. In activated lymph nodes, plasma blasts exist, which also express CD43, but show distinctly lower expression of CD19. FACS plots were obtained from different experiments. Activated lymph nodes were from mice infected for 7 days with influenza A/Mem/71.
A notable distinction between B-1 cells in the body cavity and B-1 cells in other tissues is the expression of CD11b (MAC-1/complement receptor (CR) 3)). CD11b is expressed on most B-1 cells in the body cavities21 but is not expressed on B-1 cells in spleen or other lymphoid tissues in steady state. Although, some researchers used expression of CD11b to identify the presence of CD5−CD11b+ B-1 cells in various tissues after infection or inflammatory insult,13, 22 activated peritoneal cavity B-1 cells rapidly lose CD11b expression after migration to the spleen.11, 23 Also, studies in C3-deficient mice suggested that complement levels regulate surface CD11b expression.24 Thus, CD11b expression is dynamically controlled by environmental clues.
Like other integrins, CD11b is expressed on the cell surface in a non-active conformation. It requires activation-induced change to an active conformation in order to mediate leukocyte adhesion, which is induced by inflammatory stimuli.25 This might explain why CD11b gene–targeted mice showed no defect in B-1 cell numbers in the body cavities in steady state24 and indicates that CD11b might be important during the mobilization of body cavity B-1 cells. Indeed, our recent data suggest a crucial role for CD11b in the accumulation of B-1 cells from the body cavities to the regional lymph nodes after influenza infection (discussed further below). Chemokine signaling has been shown to coordinate the steady-state migration of B-1 cells to and from the body cavities, as Cxcl13−/− mice fail to accumulate B-1 cells in their body cavities.26, 27
In addition to the phenotypic differences between spleen and body cavity B-1 cells, these cells also express distinct repertoires (i.e., specificities28) (Fig. 2E/F), and global gene array analysis revealed additional substantial differences in gene expression when comparing these cell populations.29 Using adoptive transfer experiments of peritoneal cavity B-1 cells into wild-type mice, similar to those outlined above, Weiss and colleagues concluded that at least some of these gene expression differences must be the result of tissue-induced changes, as peritoneal cavity–origin B-1 cells that reconstituted the splenic environment showed a gene expression profile similar to that of spleen B-1 cells in wild-type mice.30 However, since these studies have not been done with a transfer of single cells, it remains possible that the reconstitution of each tissue compartment is facilitated by the preferential expansion of certain B-1 cell subsets. Alternatively, differences in gene expression might be the outcome of a combination of distinct developmental origins and tissue-specific signals. Importantly, the large differences in gene expression between body cavity and spleen B-1 cells further support the observed functional differences of these B-1 cell populations (Fig. 1).
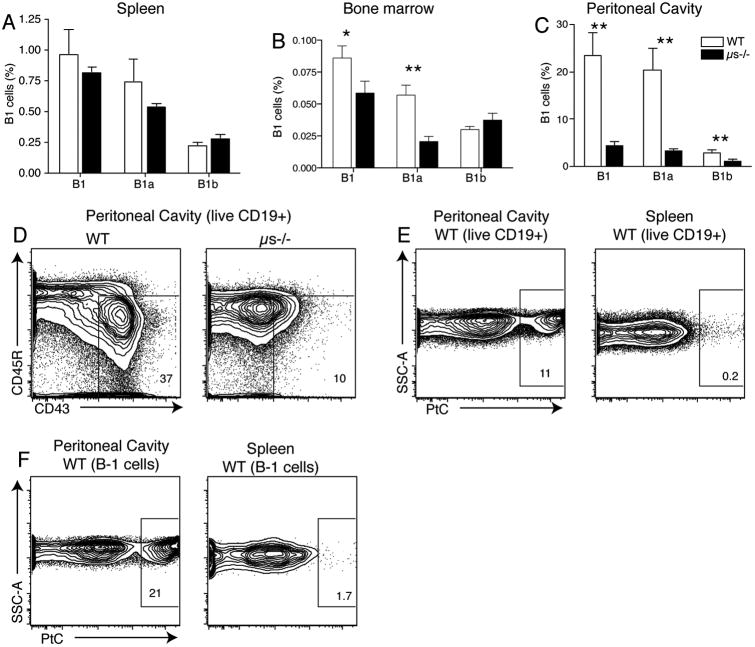
Figure 2.
Splenic B-1 cells differ in development from body cavity B-1 cells. Shown are bar charts indicating mean frequencies +/− SD of B-1 cells, including B-1a and B-1b, in (A) spleen, (B) bone marrow, and (C) peritoneal cavities of wild-type (WT) and sIgM−/− (μs−/−) mice (n = 4/group). Group-wise comparisons were done using Student's t test: *P < 0.05, **P < 0.005. (D) Contour plots identify B-1 cells (CD45Rlow CD43+) in WT and μs−/− peritoneal cavities after gating on CD19+ B cells. Note the near absence of B-1 cells in the peritoneal cavity of μs−/− mice. (E) Contour plots showing CD19+ live B cells from pleural cavity and spleen of wild-type mice binding to the fluorescent-labeled phophatidylcholine-containing liposomes (PtC+). (F) Similar to E but gated in addition for B-1 cell markers: IgMhi IgDlo CD43+. Note the large difference in the frequencies of Ptc binders among spleen and peritoneal cavity B and B-1 cells.
The apparent heterogeneity between B-1 cell populations of secondary lymphoid tissues and the body cavity is in contrast to findings from our and others' studies, outlined above, which showed that the transfer of peritoneal cavity B-1 cells into newborn or lethally irradiated mice can reconstitute all B-1 cell compartments, including those of the spleen, bone marrow, lymph nodes, blood, and body cavities. The transfer also fully reconstitutes natural serum IgM levels. Thus, non-IgM-secreting body cavity B-1 cells seem to have the functional plasticity to differentiate to natural IgM–producing cells, not only in response to an insult, but also in response to unknown homeostatic signals. In addition, B-1 cells seem to continuously recirculate from the body cavities to the blood,26 suggesting that they contribute to the pool of B-1 cells present in the spleen, even under steady-state conditions. Further work is required to fully understand the likely multifaceted origins, roles, and functions of B-1 cells in different tissues.
Bone marrow and spleen, but not peritoneal cavity, B-1 cells are major sources of protective natural IgM
Following the identification of B-1a cells first in the spleen31 and then in the peritoneal cavity of laboratory mice, various investigators performed adoptive-transfer experiments that exploited the availability of Ig-allotypic markers, and congenic but allotype-disparate strains of mice (such as BALB/c and C.B-17 mice expressing Igh-a and Igh-b, respectively), to distinguish B-1 and B-2 cells and their secreted products.32-34 These studies demonstrated that, after their adoptive transfer into neonatal or lethally-irradiated adult mice, peritoneal cavity–derived B-1a cells become the major producers of natural IgM in serum,17, 35 intestinal fluids,19 and the respiratory tract.16 Indeed, as analyzed by flow cytometry, B-1 cell populations in all tissues seem to be fully reconstituted in frequency and phenotype by adult peritoneal cavity B cell transfer16, 32-34 (and Baumgarth, unpublished data).
Independent studies by Benner and colleagues who were studying natural IgM production in wild-type mice around the same time, but did not analyze the body cavities of mice, demonstrated that spleen and bone marrow are the tissue locations with the highest numbers of spontaneously IgM-secreting cells and that these frequencies were unaffected by establishment of the microbiota, as similar frequencies of IgM-secreting cells were found in mice held under germ-free conditions.3, 36
Since the spleen, but not the bone marrow, had been shown to contain B-1 cells, the question arose as to whether bone marrow IgM-secreting cells were B-1 cells. Using multicolor flow cytometry on bone marrow from wild-type mice, we indeed were able to demonstrate the presence of a small frequency (0.7% of CD19+ cells) of both CD5+ and CD5– B-1 cells, which resembled B-1 cells in the spleen with respect to phenotype (CD19hi IgM+ IgDlo/− CD23− CD43+ CD138−) (Fig. 1). Generation of neonatal allotype chimeras confirmed that IgM production in the bone marrow (and the spleen) was derived from peritoneal cavity donor cells,18 and more recent studies showed that FACS-purified B-1 cells indeed secrete IgM.a
In contrast, the body cavitiy B-1 cells are not a major source of serum IgM10,37,38 (Fig. 3). The process of isolating B-1 cells, however, either by flow cytometry or MACS does appear to stimulate B-1 cells for increased IgM production. It is also possible that it is not the actual stresses applied to the cells by their isolation, but that the isolation from other cells or factors in the body cavities causes de-repression of IgM secretion. Consistent with this, early studies identified prostaglandin E2 released from LPS-activated macrophages as an inhibitor of IgM secretion by peritoneal cavity B-1 cells.39 This might explain why some investigators concluded that B-1 cells in body cavities are the source of natural IgM secretion.2, 40-42 However, studies by other laboratories are consistent with our findings that peritoneal cavity B-1 cells do not spontaneously produce natural IgM, either in vivo or ex vivo.11, 27, 38, 43, 44 Together, the data indicate that, similar to conventional B cells, B-1 cells require spleen and bone marrow tissue niches for antibody production. Furthermore, they suggest that body cavity B-1 cells are uniquely regulated by their tissue environment and serve a distinct function from that of their spleen and bone marrow counterparts.
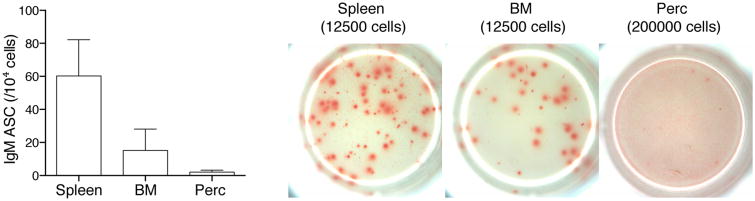
Figure 3.
Bone marrow and spleen, but not peritoneal cavity, B-1 cells are major sources of protective natural IgM. Bar graphs summarize levels of IgM-secreting cells in spleen, bone marrow, and peritoneal cavities from wild-type (WT) C57BL/6 mice (n = 4). Shown on the right are representative ELISPOT images of IgM-secreting cells in spleen, bone marrow (BM), and peritoneal cavity (Perc). The number in parentheses indicates the total number of input cells per well.
Natural IgM is required for normal B-1 and B-2 cell development
Recent studies with mice that lack secreted IgM (sIgM) but have membrane-bound IgM and can undergo isotype switching identified a previously unappreciated role for sIgM in the development of B-1 and B-2 cells.45 The initial description of these mice suggested that overall B cell development is only modesty affected by the absence of sIgM, with some increases in marginal zone B cells and peritoneal cavity B-1a cells.46,47 The mice were also reported to have increased serum autoantibody levels,48 a finding that we confirmed.45 It was suggested that this demonstrates a crucial role for natural sIgM in the maintenance of tissue homeostasis, as the removal of dead and dying cells by IgM might increase the risk of inadvertent autoreactive B cell activation in the absence of sIgM.48
However, our analysis of peripheral B cell subsets and bone marrow B cell development showed a much more profound effect of sIgM on B cell development and selection. Secreted IgM-deficient (Ighm−/−; hereafter, sIgM−/−) mice had significant reductions in bone marrow B cell output at both the pre- and immature B cell stages.45 This resulted in reduced overall B cell numbers in the periphery and strong changes in the repertoire of the B-2 cell pool. Follicular B cells showed a strong reduction in their ability to respond to BCR cross linking. While we confirmed the relative increase of the marginal zone B cell compartment in the spleen of sIgM−/− mice, an analysis of B-1 cells in the body cavities showed that the cavities almost completely lacked B-1 cells45 (Fig. 2A–C). Instead, as previously interpreted as an increase in B-1a cells, the mice harbored a population of CD5+ CD19int CD23− B220hi IgD+ IgM+ anergic conventional B cells both in the body cavities and the spleen.45 In addition to their phenotypic similarities to anergic B cells, they lacked classical markers of B-1 cells, such as low expression of CD45R (B220) and expression of CD43 (Fig. 2D). These cells showed a rapid cell turnover and were unable to proliferate in response to BCR-stimulation in vitro. Furthermore, sIgM−/− mice almost completely lacked VH11-expressing B cells in the body cavities, resulting in a lack of phophatidylcholine liposome-binding cells (Fig. 2E), a specificity strongly associated with CD5+ B-1 cells. Polyclonal serum IgM, but not monoclonal IgM, was able to rescue or at least ameliorate these developmental defects.45
Together, our data showed that the lack of natural IgM causes severe changes in B cell selection. Given the strong increases in autoantibody production and the presence of large numbers of anergic B cells in sIgM−/− mice, we conclude that natural IgM is required for the development of both B-1 and B-2 cells, revealing a new function for this enigmatic immunoglobulin.
Differential requirement of lymph tissue and body cavity B-1 cell development for the presence of natural sIgM
Surprisingly, however, while B-1a cell populations in the body cavity and bone marrow were severely reduced in sIgM−/− mice, B-1 cell frequencies in the spleen were unaffected45 (Fig. 3A-C). This indicates that peritoneal cavity and bone marrow B-1 cells develop by mechanisms other than B-1 cells in spleen or that the lack of sIgM leads to compensatory increases in spleen but not in the body cavity or bone marrow. Whatever the reason, the data demonstrate profound differences between peritoneal cavity and splenic B-1 cell populations and their dependence on natural IgM for their development/maintenance.
The data also raise the question of what population of B cells is responsible for secretion of the initial IgM during ontogeny, as B cell development is so importantly affected by its absence. Given that B-1 cells are known to develop early in ontogeny, it is tempting to speculate that a first wave of B-1 cell development populates spleen compartments and leads to the cell-autonomous development of natural IgM–secreting cells. In subsequent waves, additional B-1 cells and eventually B- 2 cells develop, which are reliant on the presence of natural IgM for normal selection/expansion. Studies by Carsetti and colleagues demonstrated that the removal of the spleen leads to a loss of peritoneal cavity B-1a cells, further suggesting a dependence on the spleen or spleen-derived cells, antibodies, or other factors, on the maintenance of the B-1a cell pool in the body cavities.49 Since B-1 cells appear to continuously circulate through the body (see discussion below), the splenic environment might provide non-redundant signals, which could promote the maintenance of the self-renewing B-1 cell pool. Alternatively, natural IgM production by splenic B-1 cells might be required for the development and/or selection of B-1a cells even after birth.
A staggered development of B-1 cell populations would be consistent with the delayed appearance of peritoneal cavity compared to splenic B-1 cells in ontogeny50. It is also consistent with a working model articulated most recently by Montecino-Rodriguez and Dorshkind, who suggested that B-1 cells indeed develop in waves during ontogeny, with the earliest precursors possibly arising from the yolk sac.51 Interestingly, work by Flajnik and colleagues recently demonstrated that sharks have at least two distinct populations of IgM-secreting cells.52 Heterogeneity among spontaneous IgM-secreting cells, staggered development of B-1 cells, and our finding of the differential dependence on natural IgM for development, further indicate profound differences between body cavity and lymph tissue B-1 cells. The existing literature provides no clear resolution on the question of whether the functional differences of B-1 cells are due mainly or in part to distinct environmental cues that drive B-1 cells into one or the other niche, or to the existence of distinct B-1 cell subsets. Resolving this question would greatly enhance our understanding and eventual exploitation of B-1 cells with their multifaceted functions.
B-1 cells in the body cavity are responder B-1 cells
Our early studies indicated that the generation of polyreactive natural IgM by B-1 cells contributes to immune protection from influenza virus infection, lowering lung viral loads and mortality rates after infection.5 Others found similar protective effects of natural IgM after infection with vesicular stomatitis virus (VSV), lymphocytic choriomeningitis virus (LCMV), and Listeria monocytogenes.6 Importantly, germ-free mice harbor similar levels of natural IgM as do conventionally reared or SPF mice,53 clearly demonstrating a non-redundant function of antigen-independent natural IgM, which seems to provide an innate-like first line of immune protection.54, 55
Our more recent studies showed that B-1 cells are also activated by infection. They contribute strongly to the production of IgM locally in the respiratory tract;16 B-1 cell–derived IgM levels in bronchoalveolar lavage fluid as well as local B-1 cell production of IgM in the draining MedLN were strongly and rapidly enhanced by the infection, while serum IgM levels remained unchanged,16,17 which correlated with increases in B-1 cell frequencies and absolute numbers in the MedLN. These lymph nodes are barely visible prior to infection and increase rapidly to a cellularity of about 1 × 107 cells by day 7 after influenza infection. B-1 cells make roughly 0.3–0.5% of the B cell pool in that tissue (Fig. 1). Thus, within a few days after influenza infection, approximately 50,000 B-1 cells accumulate in the MedLN.
Such antigen nonspecific activation and redistribution of B-1 cells to secondary lymphoid tissues is consistent with the findings of numerous other studies that investigated B-1 cell activation to inflammatory stimuli. A rapid migration of B-1 cells from the body cavities to the spleen has been reported following activation via mitogens,11,12,23,38 microbial antigens, and cytokines such as interleukin (IL)-5 and IL-10.10, 37 Following LPS injection into the peritoneal cavity, B-1 cells were found to upregulate CXCR4 and correspondingly showed increased CXCL12 responsiveness, which induced efflux of B-1 cells from the peritoneal cavity.56 A redistribution of CD5+ but not CD5− B-1 cells from the pleural cavity to the lung was shown in response to influenza infection16 and intranasal instillation of LPS.57 Thus, body cavity B-1 cells represent a population of responder B-1 cells that, when stimulated, will migrate to the anatomical closely-aligned secondary lymphoid organs (i.e., mediastinal lymph nodes for pleural cavity B-1 cells and spleen and mesenteric lymph nodes for peritoneal cavity B-1 cells) (Fig. 4). The differentiation of body cavity B-1 cells to IgM-secreting cells at those sites is well documented. However, local elaboration of cytokines, such as IL-10 or granulocyte-macrophage colony-stimulating factor (GM-CSF) could mean that B-1 cells fulfill roles other than the production of IgM.
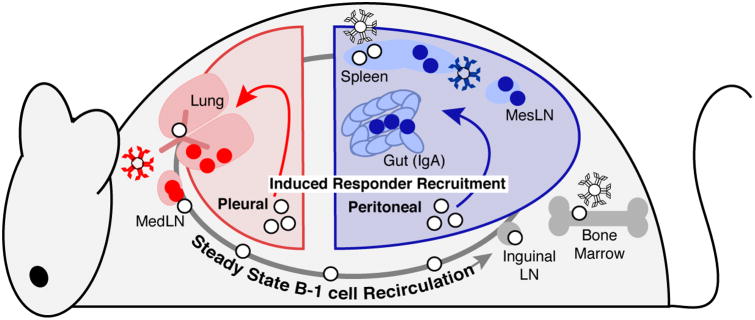
Figure 4.
Model of pathogen-induced B-1 cell responses. Body cavity B-1 cells circulate throughout the animal in the steady state. Following infection by pathogens, innate inflammatory signals contribute to induction of B-1 cells in the body cavities, and these responder populations migrate to neighboring lymphoid sites. B-1 cells activated by respiratory tract infection aggregate locally in the mediastinal lymph node and lung parenchyma, while B-1 cells stimulated in the peritoneal cavity are correspondingly recruited to the spleen, mesenteric lymph node, and lymphoid sites within the gastrointestinal tract. Responder B-1 cells contribute to induced antibody secretion and cytokine functions in local sites, thus contributing to pathogen defense as well as shaping the environment within the locally activated lymphoid site.
Regulation and specificity of the B-1 cell response to pathogens
Only about 10% of the B-1 cells that accumulate in the MedLN after influenza infection secrete IgM that binds to influenza virus, a frequency that is comparable to frequencies of influenza-binding IgM-secreting B-1 cells in the spleen of mice never exposed to the virus.16 Thus, BCR-mediated virus binding and signaling might not be the (primary) stimulus for body cavity B-1 cell activation and cell accumulation and differentiation in local tissues, consistent with their inability to respond to BCR cross linking in vitro.58 It also suggested that the accumulation of B-1 cells in the MedLN was largely a matter of B-1 cell recruitment, rather than clonal expansion, a notion that we confirmed with bromodeoxyuridine (BrdU) incorporation experiments.16
Other studies show that B-1 cells respond in an antigen-specific manner to certain pathogens. The classic model is the activation of T15 idiotype–bearing B-1 cells and their responses to S. pneumonia infection;40, 59 other models include the stimulation of peritoneal cavity B-1 cells with the non-mitogenic LPS of Francisella tularensis.15 Following stimulation by the latter antigen, antigen-binding B-1 cells were shown to redistribute from the peritoneal cavity to the spleen, where they underwent limited clonal expansion and differentiation to antibody-producing cells.15 Others suggested clonal expansion of B-1b cells following infection with Salmonella22 and B. hermsii,14 the latter leading to the accumulation of increased frequencies and/or protective functions of Borrelia-specific B-1b cells in the body cavities, which was considered an innate-like memory B cell response.4
Addressing the question of antigen specificity of the B-1 cell response is important, because it has implications on the likely function and impact of these cells on the immune response. Our studies and those of other laboratories on influenza virus infection demonstrated that the natural IgM of mice contained IgM that was able to neutralize the influenza virus.16 The ongoing secretion of natural IgM seems to provide a certain amount of immune protection by binding to and inactivating infectious virus.17, 60 Given that mice are not natural hosts to influenza infection, shared conformational epitopes with mouse pathogens, or self-antigens, might select for the influenza-binding antibody specificities. Because the local activation of B-1 cells results in the elaboration of mostly non-influenza-binding IgM antibodies, the question arises whether specific IgM production is less relevant, or whether B-1 cells have additional roles to play in the local lymph nodes at the site of infection. One possibility is that the continued production by B-1 cells of polyreactive IgM in the lymph nodes is important for the removal of cellular debris at the site of inflammation, suggesting that the local activation of B-1 cells plays a major role in tissue homeostasis rather than anti-pathogen responses. An alternative explanation that is not mutually exclusive is that the polyreactive IgM production at the site of injury might reduce the risk of secondary infections, such as with S. pneumonia, which frequently occur in humans infected with influenza virus.61
B-1 cells also generate cytokines and have long been recognized to secrete IL-10,62 which has important regulatory functions. More recently, Swirski and colleagues suggested a subset of B-1 cells identified by production of GM-CSF, which they termed “innate-response activator” cells.63 These cells were shown to produce both GM-CSF and IL-3, which had distinct protective and deleterious effects during sepsis, respectively. GM-CSF production was shown to enhance bacterial clearance,63 while IL-3 production was suggested to increase myelopoiesis, enhancing the developing uncontrolled cytokine storm during the same process.64 They also suggested that production of GM-CSF was an important autocrine factor for IgM secretion by B-1 cells leading to protection from pneumonia.57 We were unable, however, to confirm any effects of GM-CSF on enhancement of IgM production by B-1 cells in vivo and in vitro (Waffarn and Baumgarth, in preparation). Finally, some studies have suggested that B-1 cells can promote CD4+ T cell activation,65, 66 possibly presenting phagocytosed antigen to these cells.67 The presentation of antigen by B-1 cells may significantly affect the quality of the ensuing CD4+ T cell response.66 Thus, B-1 cells accumulating in secondary lymphoid tissues after immune activation may regulate adaptive immune responses by producing cytokines or other immune mediators.
Studies demonstrating antigen-specific B-1 cell activation provide evidence for B-1 cells as important contributors of adaptive immunity both during acute and recall responses. Given the current uncertainty about the extent to which antigen–BCR engagement shapes B-1 cells and their responses to pathogen encounter, we suggest that the term natural IgM production be restricted to the truly antigen-independent elaboration of IgM in the spleen and bone marrow and not be extended to antigen-induced responses by B-1 cells. Further work will be required to fully understand whether there is any antigen-specific selection of B-1 cells for activation and differentiation to IgM production, even by these apparently antigen-independent responses.
The specific signals required that differentially regulate B-1 cell responses to various pathogens have not been identified. It appears, however, that while BCR-engagement might drive B-1 cell responses under some circumstances, innate signals alone can be sufficient to activate body cavity B-1 cells to redistribute. Consistent with this, the redistribution of B-1 cells from the peritoneal cavity to the spleen after injection of LPS was shown to depend on MyD88-mediated signaling and was correlated with decreased surface expression of various integrins and CD9.12 It will be important to identify the precise mechanisms by which B-1 cells can be activated, both to test for their functionality in various disease states, but also to harness their protective capacity for prophylactic approaches.
Conclusions and future directions
We conclude that B-1 cells are heterogeneous in development and functions. Populations of natural IgM–secreting B-1 cells exist in the spleen and bone marrow that contribute most, if not all, of the serum natural IgM. Other B-1 cell populations, mainly those residing in the body cavities, act as sensitive sentries of the various organ systems, where they respond to inflammatory signals, such as type-I IFN, with rapid mobilization and redistribution to secondary lymphoid tissues (Fig. 4). This redistribution to secondary lymphoid tissues suggests that B-1 responders are in intimate contact with other cells of the adaptive immune response. Thus, in that regard, they are similar to the recently identified populations of innate-like lymphocytes, which seem to regulate adaptive immune responses by early elaboration of cytokines.70 Support in the literature exists for B-1 cells functioning via phagocytosis,67 antigen presentation,65, 67 secretion of cytokines such as IL-3, IL-10, and GM-CSF,62, 63 and secretion of antigen-specific IgM and IgG, as well as antimicrobial IgA. 13, 15, 19, 22, 71, 72 As is the case for other lymphocytes, it is likely that the functions and functional impacts of B-1 cells depend on the context of the insult. A focus on studying the functions of B-1 cells should ultimately also reveal the precise signals that drive B-1 cell responses, to exploit their multifaceted nature for therapeutic and prophylactic uses.
Materials and methods
Mice
Eight- to twelve-week-old female and male mice, C57BL/6J (The Jackson Laboratory, Bar Harbor, ME) and B6.129S-sIgM−/− (μs−/−, breeders were a kind gift from Dr. Frances Lund) were used for all experiments shown. All mice were kept in specific pathogen–free housing, in HVAC-filtered filter-top cages. Mice were euthanized by overexposure to carbon dioxide. Protocols using mice were approved by the UC Davis Institutional Committee on Animal Care and Use.
Flow cytometry
Spleen and lymph node cells were isolated by grinding the organ between frosted ends of glass slides. Bone marrow was obtained by flushing fibula and tibia with medium using a 23-gauge needle. Peritoneal cavity cells were obtained by flushing the peritoneal cavity three times with 5 ml of cold PBS. Single-cell suspensions were stained for flow cytometry. Briefly, after Fc receptor blocking (anti-CD16/32, 5μg/ml) for 20 min on ice, cells were stained with the following fluorochome conjugates: SA-Qdot 605, SA-APC, CD3e-Cy55APC, CD5-(biotin, Cy5PE), CD45R-(FITC, Cy7APC), FITC-labeled, phophatidyl-choline–containing liposoumes (PtC)-FITC, CD21-(FITC, biotin), CD23-(FITC, APC), CD43-(PE, Cy7APC), CD19-(Cy5PE, APC), CD21-Cy5.5PE, CD24-Cy5.5PE, IgD-Cy7PE, and IgM-(Cy7APC, Cy55APC) (all in-house generated). Dead cells were excluded by live/dead pacblue staining (Invitrogen, Grand Island, NY). To accurately set gates to identify CD5- and CD43-expressing cells, we used fluorescence minus one controls in which cells were stained with all reagents except anti-CD5 and anti-CD43, respectively.
FACS analysis was done using a 15-parameter FACS-Aria or a Fortessa (BD) equipped with four lasers and optics for 22-paramenter analysis. Analysis was done using FlowJo (a kind gift from Adam Treister).
ELISPOT
To probe for IgM-secreting cells, 96 well plates (Multiscreen HA filtration; Millipore, Billerica, MA) were coated overnight with anti-IgM (clone 331, in-house generated by purification from hybridoma supernatants via protein G column affinity chromatography). After blocking with PBS/4% BSA for 1 h, 5 × 105 cells in medium (RPMI 1640, 292 μg/mL L-glutamine, 100 μg/mL of penicillin and streptomycin, 10% heat inactivated FCS, and 0.03 M 2-ME) were placed in the starting well and twofold serially diluted. Cells were incubated at 37 °C overnight and then lysed with water. Antibody binding was revealed by adding biotin conjugated goat anti-IgM (Southern Biotech in PBS/2% BSA for 2 h, SA-HRP (Vector Laboratories, Burlingame, CA) in PBS/2% BSA for 1 h and revealed with 3-amino-9-ethylcarbazole (Sigma-Aldrich, St. Louis, MO) for 10 minutes. Plates were dried and spots were counted in all wells with dilutions resulting in visible and countable spots using a stereomicroscope. Mean numbers ± SD were calculated from cell counts of all wells with countable spots.
Statistical analysis
Statistical analysis was done using a two-tailed Student's t test. P < 0.05 was considered to indicate significant differences.
Acknowledgments
The authors would like to thank Zhang Luo and Jacqueline Lerche (UC Davis) for excellent technical support and Abigail Spinner for support with flow cytometry. The work by the authors summarized here was supported by NIH/NIAID R01 AI051354, AI085568, and U19 AI109962.
Footnotes
See Savage and Baumgarth in Ann. N.Y. Acad. Sci. XXXX: XX–XX (2015).
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 2.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 3.Hooijkaas H, Benner R, Pleasants J, Wostmann B. Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered “antigen-free” diet. Eur J Immunol. 1984;14:1127–1130. doi: 10.1002/eji.1830141212. [DOI] [PubMed] [Google Scholar]
- 4.Alugupalli KR, et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Baumgarth N, Chen J, Herman OC, Jager GC, Herzenberg LA. The role of B-1 and B-2 cells in immune protection from influenza virus infection. Curr Top Microbiol Immunol. 2000;252:163–169. doi: 10.1007/978-3-642-57284-5_17. [DOI] [PubMed] [Google Scholar]
- 6.Ochsenbein AF, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 8.Silverman GJ, Gronwall C, Vas J, Chen Y. Natural autoantibodies to apoptotic cell membranes regulate fundamental innate immune functions and suppress inflammation. Discov Med. 2009;8:151–156. [PubMed] [Google Scholar]
- 9.Fahey JL, Sell S. The Immunoglobulins of Mice. V. The Metabolic (Catabolic) Properties of Five Immunoglobulin Classes. J Exp Med. 1965;122:41–58. doi: 10.1084/jem.122.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nisitani S, Tsubata T, Murakami M, Honjo T. Administration of interleukin-5 or -10 activates peritoneal B-1 cells and induces autoimmune hemolytic anemia in anti-erythrocyte autoantibody-transgenic mice. Eur J Immunol. 1995;25:3047–3052. doi: 10.1002/eji.1830251110. [DOI] [PubMed] [Google Scholar]
- 11.Kawahara T, Ohdan H, Zhao G, Yang YG, Sykes M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J Immunol. 2003;171:5406–5414. doi: 10.4049/jimmunol.171.10.5406. [DOI] [PubMed] [Google Scholar]
- 12.Ha SA, et al. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Alugupalli KR, et al. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol. 2003;170:3819–3827. doi: 10.4049/jimmunol.170.7.3819. [DOI] [PubMed] [Google Scholar]
- 15.Cole LE, et al. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proc Natl Acad Sci USA. 2009;106:4343–4348. doi: 10.1073/pnas.0813411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci USA. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol. 2012;42:120–129. doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroese FG, et al. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1989;1:75–84. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- 20.Rosado MM, et al. From the fetal liver to spleen and gut: the highway to natural antibody. Mucosal Immunol. 2009;2:351–361. doi: 10.1038/mi.2009.15. [DOI] [PubMed] [Google Scholar]
- 21.Tung JW, Parks DR, Moore WA, Herzenberg LA, Herzenberg LA. Identification of B-cell subsets: an exposition of 11-color (Hi-D) FACS methods. Methods Mol Biol. 2004;271:37–58. doi: 10.1385/1-59259-796-3:037. [DOI] [PubMed] [Google Scholar]
- 22.Gil-Cruz C, et al. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc Natl Acad Sci USA. 2009;106:9803–9808. doi: 10.1073/pnas.0812431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Tung JW, Ghosn EE, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci USA. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson AC, Roundy KM, Weis JJ, Weis JH. Regulation of murine splenic B cell CR3 expression by complement component 3. J Immunol. 2009;183:3963–3970. doi: 10.4049/jimmunol.0900038. [DOI] [PubMed] [Google Scholar]
- 25.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe N, et al. Migration and differentiation of autoreactive B-1 cells induced by activated gamma/delta T cells in antierythrocyte immunoglobulin transgenic mice. J Exp Med. 2000;192:1577–1586. doi: 10.1084/jem.192.11.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klinman DM, Holmes KL. Differences in the repertoire expressed by peritoneal and splenic Ly-1 (CD5)+ B cells. J Immunol. 1990;144:4520–4525. [PubMed] [Google Scholar]
- 29.Kretschmer K, et al. Antibody repertoire and gene expression profile: implications for different developmental and functional traits of splenic and peritoneal B-1 lymphocytes. J Immunol. 2003;171:1192–1201. doi: 10.4049/jimmunol.171.3.1192. [DOI] [PubMed] [Google Scholar]
- 30.Stoermann B, Kretschmer K, Duber S, Weiss S. B-1a cells are imprinted by the microenvironment in spleen and peritoneum. Eur J Immunol. 2007;37:1613–1620. doi: 10.1002/eji.200636640. [DOI] [PubMed] [Google Scholar]
- 31.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayakawa K, Hardy RR, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lalor PA, Herzenberg LA, Adams S, Stall AM. Feedback regulation of murine Ly-1 B cell development. Eur J Immunol. 1989;19:507–513. doi: 10.1002/eji.1830190315. [DOI] [PubMed] [Google Scholar]
- 34.Lalor PA, Stall AM, Adams S, Herzenberg LA. Permanent alteration of the murine Ly-1 B repertoire due to selective depletion of Ly-1 B cells in neonatal animals. Eur J Immunol. 1989;19:501–506. doi: 10.1002/eji.1830190314. [DOI] [PubMed] [Google Scholar]
- 35.Stall AM, Wells SM, Lam KP. B-1 cells: unique origins and functions. Semin Immunol. 1996;8:45–59. doi: 10.1006/smim.1996.0007. [DOI] [PubMed] [Google Scholar]
- 36.Van Oudenaren A, Haaijman JJ, Benner R. Frequencies of background cytoplasmic Ig-containing cells in various lymphoid organs of athymic and euthymic mice as a function of age and immune status. Immunology. 1984;51:735–742. [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami M, et al. Oral administration of lipopolysaccharides activates B-1 cells in the peritoneal cavity and lamina propria of the gut and induces autoimmune symptoms in an autoantibody transgenic mouse. J Exp Med. 1994;180:111–121. doi: 10.1084/jem.180.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohdan H, et al. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Gal alpha 1,3Gal epitopes in alpha 1,3-galactosyltransferase-deficient mice. J Immunol. 2000;165:5518–5529. doi: 10.4049/jimmunol.165.10.5518. [DOI] [PubMed] [Google Scholar]
- 39.Chace JH, Fleming AL, Gordon JA, Perandones CE, Cowdery JS. Regulation of differentiation of peritoneal B-1a (CD5+) B cells. Activated peritoneal macrophages release prostaglandin E2, which inhibits IgM secretion by peritoneal B-1a cells. J Immunol. 1995;154:5630–5636. [PubMed] [Google Scholar]
- 40.Masmoudi H, Mota-Santos T, Huetz F, Coutinho A, Cazenave PA. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- 41.Tumang JR, Frances R, Yeo SG, Rothstein TL. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol. 2005;174:3173–3177. doi: 10.4049/jimmunol.174.6.3173. [DOI] [PubMed] [Google Scholar]
- 42.Holodick NE, Tumang JR, Rothstein TL. Immunoglobulin secretion by B1 cells: differential intensity and IRF4-dependence of spontaneous IgM secretion by peritoneal and splenic B1 cells. Eur J Immunol. 2010;40:3007–3016. doi: 10.1002/eji.201040545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fairfax KA, et al. Different kinetics of Blimp-1 induction in B cell subsets revealed by reporter gene. J Immunol. 2007;178:4104–4111. doi: 10.4049/jimmunol.178.7.4104. [DOI] [PubMed] [Google Scholar]
- 44.McIntyre TM, Holmes KL, Steinberg AD, Kastner DL. CD5+ peritoneal B cells express high levels of membrane, but not secretory, C mu mRNA. J Immunol. 1991;146:3639–3645. [PubMed] [Google Scholar]
- 45.Nguyen TT, Elsner RA, Baumgarth N. Natural IgM Prevents Autoimmunity by Enforcing B Cell Central Tolerance Induction. J Immunol. 2015;194:1489–1502. doi: 10.4049/jimmunol.1401880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boes M, et al. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 47.Ehrenstein MR, O'Keefe TL, Davies SL, Neuberger MS. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc Natl Acad Sci USA. 1998;95:10089–11093. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boes M, et al. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci USA. 2000;97:1184–1189. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195:771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kantor AB. The development and repertoire of B-1 cells (CD5 B cells) Immunol Today. 1991;12:389–391. doi: 10.1016/0167-5699(91)90136-H. [DOI] [PubMed] [Google Scholar]
- 51.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castro CD, Ohta Y, Dooley H, Flajnik MF. Noncoordinate expression of J-chain and Blimp-1 define nurse shark plasma cell populations during ontogeny. Eur J Immunol. 2013;43:3061–3075. doi: 10.1002/eji.201343416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hooijkaas H, Benner R, Pleasants JR, Wostmann BS. Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered “antigen-free” diet. Eur J Immunol. 1984;14:1127–1130. doi: 10.1002/eji.1830141212. [DOI] [PubMed] [Google Scholar]
- 54.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 55.Zhou ZH, et al. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe. 2007;1:51–61. doi: 10.1016/j.chom.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moon H, Lee JG, Shin SH, Kim TJ. LPS-induced migration of peritoneal B-1 cells is associated with upregulation of CXCR4 and increased migratory sensitivity to CXCL12. J Korean Med Sci. 2012;27:27–35. doi: 10.3346/jkms.2012.27.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber GF, et al. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J Exp Med. 2014;211:1243–1256. doi: 10.1084/jem.20131471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothstein TL, Kolber DL. Anti-Ig antibody inhibits the phorbol ester-induced stimulation of peritoneal B cells. J Immunol. 1988;141:4089–4093. [PubMed] [Google Scholar]
- 59.Claflin JL, Berry J. Genetics of the phosphocholine-specific antibody response to Streptococcus pneumoniae. Germ-line but not mutated T15 antibodies are dominantly selected. J Immunol. 1988;141:4012–4019. [PubMed] [Google Scholar]
- 60.Baumgarth N, et al. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nair N, Biswas R, Gotz F, Biswas L. Impact of Staphylococcus aureus on pathogenesis in polymicrobial infections. Infect Immun. 2014;82:2162–2169. doi: 10.1128/IAI.00059-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Garra A, et al. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 63.Rauch PJ, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weber GF, et al. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science. 2015;347:1260–1265. doi: 10.1126/science.aaa4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Margry B, Wieland WH, van Kooten PJ, van Eden W, Broere F. Peritoneal cavity B-1a cells promote peripheral CD4+ T-cell activation. Eur J Immunol. 2013;43:2317–2326. doi: 10.1002/eji.201343418. [DOI] [PubMed] [Google Scholar]
- 66.Zhong X, et al. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol. 2007;37:2400–2404. doi: 10.1002/eji.200737296. [DOI] [PubMed] [Google Scholar]
- 67.Parra D, et al. Pivotal advance: peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leukoc Biol. 2012;91:525–536. doi: 10.1189/jlb.0711372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang WL, et al. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J Immunol. 2007;178:1457–1467. doi: 10.4049/jimmunol.178.3.1457. [DOI] [PubMed] [Google Scholar]
- 69.Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol. 2006;176:4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 70.Gasteiger G, Rudensky AY. Interactions between innate and adaptive lymphocytes. Nat Rev Immunol. 2014;14:631–639. doi: 10.1038/nri3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Waard R, et al. Presence of germline and full-length IgA RNA transcripts among peritoneal B-1 cells. Dev Immunol. 1998;6:81–87. doi: 10.1155/1998/37576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kroese FG, de Waard R, Bos NA. B-1 cells and their reactivity with the murine intestinal microflora. Semin Immunol. 1996;8:11–18. doi: 10.1006/smim.1996.0003. [DOI] [PubMed] [Google Scholar]