Abstract
Twenty-eight different tea samples sold in the United States were evaluated using high-performance liquid chromatography (HPLC) with fluorescence detection (FLD) for their contamination with polycyclic aromatic hydrocarbons (PAHs). Many PAHs exhibit carcinogenic, mutagenic, and teratogenic properties and have been related to several kinds of cancer in man and experimental animals. The presence of PAHs in environmental samples such as water, sediments, and particulate air has been extensively studied, but food samples have received little attention. Eighteen PAHs congeners were analyzed, with percentage recovery higher than 85%. Contamination expressed as the sum of the 18 analyzed PAHs was between 101 and 1337 μg/kg on dry mass and the average contents in all of the 28 examined samples was 300 μg/kg on dry mass. Seven of the congeners were found in all samples with wide ranges of concentrations as follows: fluorene (7–48 μg/kg), anthracene (1–31 μg/kg), pyrene (1–970 μg/kg), benzo(a)anthracene (1–18 μg/kg) chrysene (17–365 μg/kg), benzo(a)pyrene (1–29 μg/kg), and indeno(1,2,3-cd)pyrene (4–119 μg/kg). The two most toxic congeners benzo(a)pyrene and dibenzo(a,h)anthracene were found at high concentrations only in Earl Grey Twinnings, Earl Grey Harney& Sons Fine Teas, and Chai Ultra Spice Black Tea Twinnings. Six PAH congeners are considered as suspected carcinogens (U.S.EPA), formed the basis of the estimation of the toxic equivalent (TEQ), Chai Ultra-Spice Black Tea Twinnings had the highest TEQ (110.9) followed by two grey tea samples, Earl Grey Harney & Sons Fine Tea (57.7) and Earl Grey Twinnings (54.5). Decaffeinated grey teas had the lowest TEQs, decaffeinated Earl Grey Bigelow (9.4) and Green Tea Honey Lemon Decaffeinated Lipton (9.6).
Keywords: Beverages, carcinogens, food contaminants, HPLC, PAH, toxic equivalent factor
Introduction
Tea (Camellia sinensis) is the most popular beverage in the world. Most of the tea consumed worldwide is made from young leaves which may be contaminated during the field drying, fermenting, and processing with chemicals that can be released into infusions and might be harmful to human health such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated dibenzodioxins (PCDD), polychlorinated dibenzofurans (PCDF), and pesticides.[1–3] Most PAHs are toxic, and some of them have been proven genotoxic or carcinogenic such as benzo(b)fluoranthene, benzo(k)fluoranthene, and benzo(a)pyrene.[4] They also exhibit mutagenic and teratogenic properties and have been related to several kinds of cancer in man and experimental animals.[5–7]
Concentrations of PAH in black teas measured in previous studies outside of the United States varied from 4.9 to 103.6 μg/kg,[8] from 9.0 to 44.6 μg/kg,[9] from 6.4 to 70.0 μg/kg,[10] and from 21.6 to 65.8 μg/kg.[11] Even higher concentrations were measured on Mate teas, with concentrations of PAH ranging from 184.6 to 1615 μg/kg.[12] The US Environmental Protection Agency (EPA) has identified 16 PAHs as priority environmental pollutants[13] to include benzo[a]-anthracene (BaA), benzo[b]fluoranthene (BbF), indeno[1,2,3-cd]pyrene (IND), benzo[k]fluoranthene(BkF), benzo[a]pyrene (BaP), dibenzo-[a,h] anthracene (DBA), naphthalene (Nap), acenaphthene (Acp), acenaphthylene (AcPy), fluorene (Flu), phenanthrene (PA), anthracene (Ant), fluoranthene (FL), pyrene (Pyr), chrysene (CHR), and benzo(g,h,i)perylene (BghiP).
Black tea is produced by fermenting the slightly withered leaves for many hours before being either smoke fired, flame fired, or steamed. In contrast, green tea is not fermented, but the leaves are steamed or pan fired to inactivate the polyphenol oxidase, thus avoiding oxidation. White tea is produced by air drying the unopened leaf buds followed by short heating to dryness. Oolong tea is prepared by withering the fresh leaves in the sun, then bruising them slightly, and partially fermenting. The color of oolong tea is intermediate between that of green and black tea. Orthodox black tea is made by drying the fermented tea leaves.[14] Since, many of them, especially orthodox black tea, are dried using combustion gases from burning wood, oil, or coal thus tea leaves may also be contaminated with PAHs.[15] The presence of PAHs in environmental samples such as water, sediments, and particulate air has been extensively studied, but food samples have received less attention.[16–21] The present work was undertaken to identify and determine the concentration of PAHs in different types of tea sold in the US markets.
Materials and methods
Reagents and materials
HPLC acetonitrile and water were obtained from VWR International LLC (Sugar Land, Texas, USA). PAH calibration mixtures were obtained from Restek Corporation (Bellefonte, PA, USA). Twenty eight different brands of tea were collected from the local markets in Houston, Texas. Each sample was given a code number according to their type as shown in Table 1.
Table 1.
Tea type, brand names, and their code numbers.
| Sample # | Tea samples |
|---|---|
| 1 | Earl Grey Decaf Bigelow |
| 2 | Earl Grey Bigelow |
| 3 | Earl Grey Twinnings |
| 4 | Earl Grey Harney & Sons Fine Teas |
| 5 | Earl Grey Tazo |
| Fine Teas | |
| 6 | English Breakfast Decaf Twinnings |
| 7 | English Breakfast Harney & Sons Fine Teas |
| 8 | English Breakfast Tazo |
| Black Teas | |
| 9 | Black Tea Teatulia |
| 10 | Black Tea Bigelow |
| 11 | Black Tea Breakfast Blend (organic) |
| 12 | Black Tea Tazo |
| 13 | Chai Ultra Spice Black Tea Twinnings |
| 14 | Traditional Chai (Organic Black Tea blend with Cinnamon, Cardamom & Nutmeg |
| 15 | Chai Tea Spiced Cinnamon Chai Black Tea Lipton |
| 16 | Black Tea & Linden Blossom Hyleys |
| 17 | Black Tea with Rosehip & Hibiscus Hyleys |
| 18 | Black Tea & Lemon Hyleys |
| 19 | Black Tea with Melissa & Mint Hyleys |
| Green Teas | |
| 20 | Green Tea & Chamomile Hyleys |
| 21 | Green Tea & Lemon Hyleys |
| 22 | Green Tea & Mint Hyleys |
| 23 | Green Tea Oriental Rituals |
| 24 | Green Tea Kirkland Signature |
| 25 | Green Earl Grey Tea Revolution |
| 26 | Green Tea Honey-Lemon Decaf Lipton |
| 27 | 100% Natural America Favorite Tea Lipton |
| 28 | Ahmad Tea |
Extraction of PAHs
One gram of each sample was extracted with 15 mL of HPLC grade hexane for 60 minutes using rotor mixer (Labnet labroller II, Woodbridge, NJ. USA) rotating at 25-revolutions per minute. Extracts were then centrifuged and supernatants were cleaned up using silica gel cartridge and passing additional 7 mL hexane through the cartridge to complete PAHs elution. The collected hexane solution was taken to dryness using Centrifan™ PE—Personal Evaporator (Sorbent Technologies, Inc., Norcross, GA, USA) and the residue obtained was dissolved in 1 mL acetonitrile filtered using 0.2 micron syringe filter and transferred to HPLC vials for analysis. All extractions were performed in 3 replicate.
High-performance liquid chromatography (HPLC) analysis
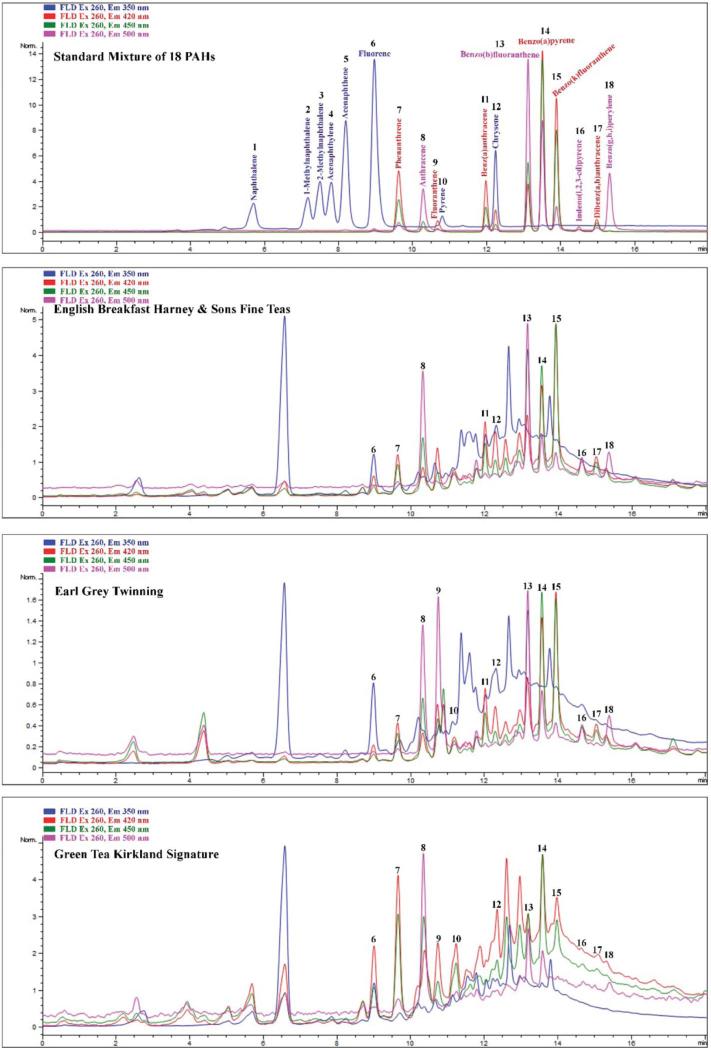
HPLC analysis was performed using an Agilent 1100 (Santa Clara, CA, USA) LC system consisting of quaternary pump, autosampler, thermostatted column compartment, and fluorescence detector with standard FLD flow cell using Agilent software Chemstation B.04.03. Pinnacle→ II PAH column (RESTEK, Restek Corporation, Bellefonte, PA, USA) 150 mm × 3.0 mm ID, Particle Size: 4 μm, Pore Size: 110 Å running at 30°C, mobile Phase was A: water, B: acetonitrile. Separation was carried out at a flow rate of 1 mL/min, starting at 45% B and increasing to 100% B at 12 min and kept at 100% B for 5 min before it recycled and equilibrated back to the original 45% B. Column was equilibrated for 5 min between samples. Samples were detected using multiple wavelength fluorescence at excitation wave lengths of 260 nm and emission at 350, 420, 450, and 500 nm. Peaks identification was based on the standard peak's retention time. The HPLC elution profile of the PAH compounds analyzed in this investigation are shown in Figure 1. External standard method was used to determine PAH concentration in the samples.
Fig. 1.
HPLC chromatograms of calibrated PAH standard and three examples of the tested tea samples. Fluorescent excitation at 260 nm and emission at 350 nm, 420 nm, 440 nm, and 500 nm. Retention times (minutes) are shown in Figure 2.
Quality assurance and control (QA/QC)
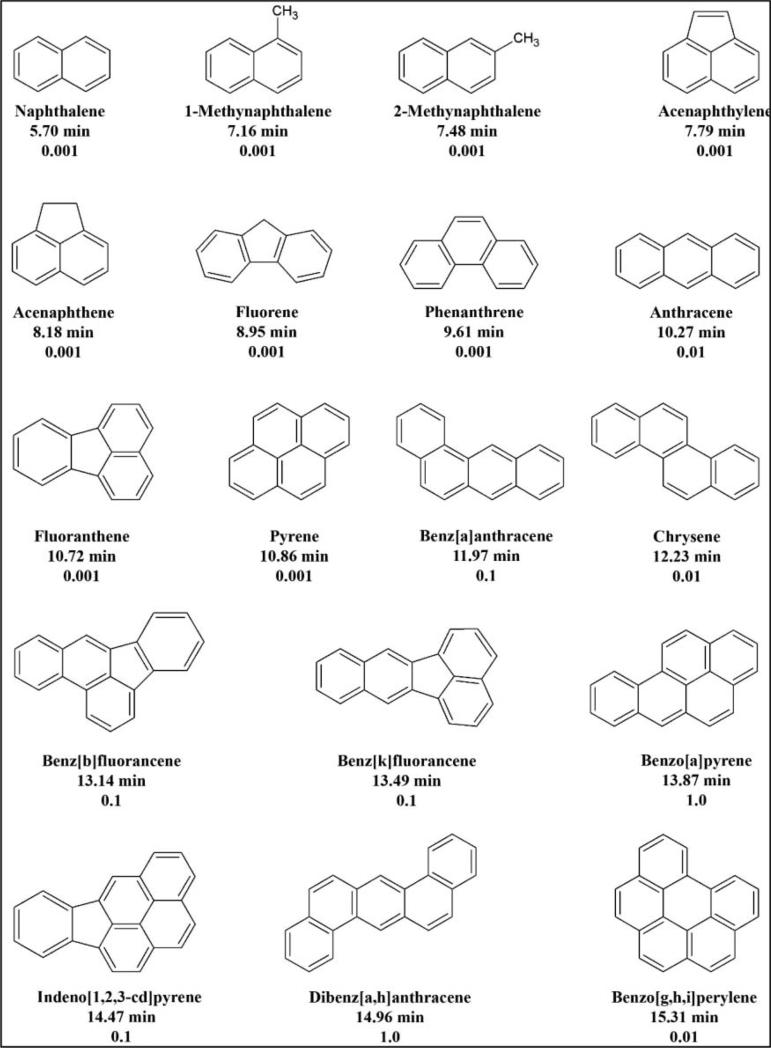
Linearity and precision were determined by analysis of a dilution series of the standard PAH mixtures in acetonitrile, ranging from 1 ng/mL to 50 ng/mL of individual PAHs in 6 dilution steps. Blank and low-spiked samples were analyzed directly, and limits of detection and quantification were evaluated from the concentration of PAHs required to give at least a signal to noise ratio of 3 and are shown in Table 2. The recoveries for all three spiked samples were greater than 80%. Linear regression was applied to construct a calibration curve reporting peak area vs. PAH concentration. A calibration curve was made for every sequence of analysis and were found to have an R2 higher than 0.99. Chemical structures of the 18 tested PAHs, their HPLC retention times and toxicity factor equivalent are shown in Figure 2.
Table 2.
Limits of detection (LOD) and quantification (LOQ), linearity of each PAHs.
| PAH | LOD (μg/kg) | LOQ (μ/kg) | R2 | Equation for calibration line |
|---|---|---|---|---|
| Naphthalene | 0.18 | 0.61 | 0.99965 | Area = 0.1185 × Amount – 0.0195 |
| 1-Methylnaphthalene | 0.14 | 0.47 | 0.99988 | Area = 0.1185 × Amount – 0.0195 |
| 2-Methylnaphthalene | 0.11 | 0.36 | 0.99195 | Area = 0.1606 × Amount + 0.0177 |
| Acenaphthylene | 0.11 | 0.38 | 0.99876 | Area = 0.1492 × Amount + 0.0895 |
| Acenaphthene | 0.11 | 0.37 | 0.99932 | Area = 0.3332 × Amount + 0.1612 |
| Fluorene | 0.03 | 0.11 | 0.99546 | Area = 0.0520 × Amount + 0.9594 |
| Phenanthrene | 0.01 | 0.03 | 0.99983 | Area = 1.2824 × Amount + 0.5322 |
| Anthracene | 0.07 | 0.24 | 0.99970 | Area = 0.1048 × Amount + 0.0734 |
| Fluoranthene | 0.07 | 0.24 | 0.99504 | Area = 0.2085 × Amount + 0.4726 |
| Pyrene | 0.10 | 0.35 | 0.99410 | Area = 0.0403 × Amount + 0.4966 |
| Benz(a)anthracene | 0.01 | 0.04 | 0.99907 | Area = 0.7171 × Amount + 0.5026 |
| Chrysene | 0.21 | 0.71 | 0.99907 | Area = 0.1565 × Amount + 0.7083 |
| Benzo(b)fluoranthene | 0.01 | 0.04 | 0.99971 | Area = 0.3139 × Amount + 0.1467 |
| Benzo(k)fluoranthene | 0.01 | 0.04 | 0.99965 | Area = 2.4442 × Amount + 1.3522 |
| Benzo(a)pyrene | 0.01 | 0.04 | 0.99968 | Area = 2.0054 × Amount + 1.2464 |
| Indeno(l,2,3-cd)pyrene | 0.15 | 0.50 | 0.99440 | Area = 0.0972 × Amount + 0.1834 |
| Dibenz(a,h)anthracene | 0.09 | 0.31 | 0.99697 | Area = 0.2351 × Amount + 0.3088 |
| Benzo(g,h,i)perylene | 0.07 | 0.22 | 0.99974 | Area = 0.1556 × Amount + 0.0566 |
Fig. 2.
PAHs chemical structures, HPLC retention times, and toxicity equivalent factor.
Results and discussion
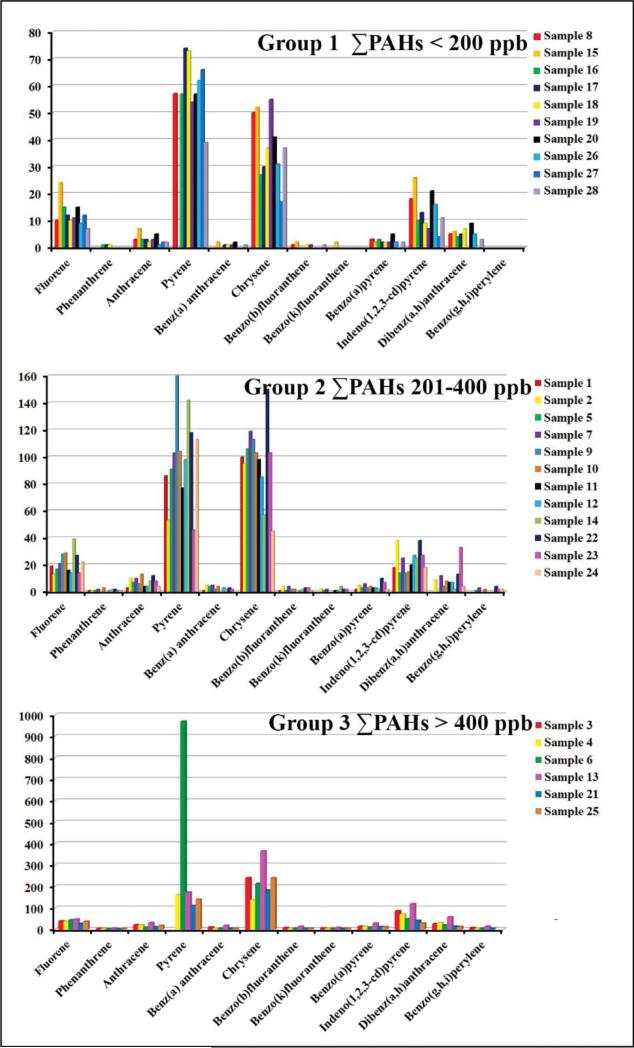
PAHs concentration of the individual compounds in the tested tea samples as well as the sum of the concentration of the identified PAHs are shown in Table 3. Naphthalene and 1-methylnaphthalene, acenaphthylene and fluoranthene were not detected in any of the examined tea samples. Ten of the tested tea samples were found to contain less than 200 ng/g (ppb) of total PAHs, those were: English Breakfast Tazo (sample # 8), Chai Tea Spiced Cinnamon Chai Black Tea Lipton (sample #15), Black Tea & Linden Blossom Hyleys (sample #16), Black Tea with Rosehip & Hibiscus Hyleys (sample #17), Black Tea & Lemon Hyleys (sample #18), Black Tea with Melissa & Mint Hyleys (sample #19), Green Tea & Chamomile Hyleys (sample #20), Green Tea Honey-Lemon Decaf Lipton (sample #26), 100% Natural America Favorite Tea Lipton (sample #27) and Ahmad Tea (sample # 28). Twelve samples contained total PAHs at concentration between 201 and 400 ppb, those were: Earl Grey Decaf Bigelow (sample #1), Earl Grey Bigelow (sample #2), Earl Grey Tazo (sample #5), English Breakfast Harney & Sons Fine Teas (sample #7), Black Tea Teatulia (sample #9), Black Tea Bigelow(sample #10), Black Tea Breakfast Blend organic (sample #11), Black Tea Tazo (sample #12), Traditional Chai Organic Black Tea blend with Cinnamon, Cardamom & Nutmeg (sample #14), Green Tea & Mint Hyleys (sample #22), Green Tea Oriental Rituals (sample #23) and Green Tea Kirkland Signature (sample #24). Six samples contain total PAHs higher than 400 ppb such as, Earl Grey Twinnings (sample #3), Earl Grey Harney& Sons Fine Teas (sample #4), English Breakfast Decaf Twinnings (sample #6), Chai Ultra Spice Black Tea Twinnings (sample #13), Green Tea & Lemon Hyleys (sample #21) and Green Earl Grey Tea Revolution (sample #25). Chemical composition of each concentration groups listed above (<200 ppb, 201 to 400 ppb, and >400 ppb) is shown in Figure 3. It appears from Figure 3 that regardless of the concentration level of the PAHs sum, pyrene and chrysene were the most abundant in all samples. However, in the low concentration group (ΣPAHs of less than 200 ppb) pyrene was more abundant than chrysene, but was about the same in the middle concentration group (ΣPAHs of 201–400 ppb) and chrysene was higher in the high concentration group ((ΣPAHs higher than 400 ppb). The two most toxic congeners benzo [a]pyrene and dibenzo[a,h]anthracene were found at high concentrations only in samples Earl Grey Twinnings (sample #3), Earl Grey Harney& Sons Fine Teas (sample #4) and Chai Ultra Spice Black Tea Twinnings (sample #13).
Table 3.
PAHs concentration in the different types of tea.
| PAHs / Sample Code # | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Fluorene | 19 ± 5 | 13 ± 1 | 39 ± 15 | 37 ± 1 | 17 ± 5 | 44 ± 0.7 | 21 ± 0.3 |
| Phenanthrene | 1 ± 0 | 1 ± 0 | 3 ± 1 | 3 ± 0 | 1 ± 1 | 2 ± 0.1 | 2 ± 0.1 |
| Anthracene | 3 ± 2 | 10 ± 1 | 21 ± 8 | 20 ± 1 | 7 ± 2 | 10 ± 0.3 | 10 ± 0.3 |
| Pyrene | 86 ± 13 | 53 ± 2 | 161 ± 18 | 91 ± 51 | 970 ± 25.3 | 103 ± 3.8 | |
| Benz(a) anthracene | 1 ± 0 | 5 ± 0 | 11 ± 2 | 6 ± 0 | 4 ± 2 | 5 ± 0.1 | 5 ± 0.2 |
| Chrysene | 100 ± 44 | 95 ± 1 | 240 ± 42 | 138 ± 11 | 106 ± 78 | 214 ± 112 | 119 ± 7.3 |
| Benzo(b)fluoranthene | 1 ± 0 | 4 ± 0 | 8 ± 1 | 5 ± 0 | 1 ± 0 | 5 ± 1 | 4 ± 0.1 |
| Benzo(k)fluoranthene | 2 ± 0 | 6 ± 1 | 4 ± 2 | 1 ± 0 | 3 ± 1 | 2 ± 0.1 | |
| Benzo(a)pyrene | 2 ± 0 | 5 ± 0 | 14 ± 2 | 14 ± 5 | 3 ± 2 | 10 ± 4 | 6 ± 0.3 |
| Indeno(1,2,3-cd)pyrene | 18 ± 6 | 38 ± 0 | 86 ± 21 | 71 ± 28 | 14 ± 11 | 49 ± 11 | 25 ± 1.4 |
| Dibenz(a,h)anthracene | 9 ± 0 | 26 ± 3 | 31 ± 15 | 20 ± 3 | 12 ± 0.7 | ||
| Benzo(g,h,i)perylene | 8 ± 4 | 5 ± 2 | 1 ± 0 | 5 ± 2 | 3 ± 0.2 | ||
| Total | 231 | 235 | 462 | 495 | 246 | 1337 | 312 |
| PAHs / Sample Code # | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|
| 2-Methylnaphthalene | 24 ± 3.0 | ||||||
| Acenaphthene | 12 ± 1.0 | ||||||
| Fluorene | 10 ± 0.2 | 28 ± 0.4 | 29 ± 0.8 | 16 ± 0.4 | 14 ± 0.6 | 48 ± 0.2 | 39 ± 1.0 |
| Phenanthrene | 3 ± 0.1 | 1 ± 0.0 | 4 ± 0 | 2 ± 0 | |||
| Anthracene | 3 ± 0.1 | 6 ± 0.2 | 13 ± 0.4 | 4 ± 0.3 | 4 ± 0.0 | 31 ± 0.2 | 8 ± 1.0 |
| Pyrene | 57 ± 1.3 | 160 ± 4.8 | 104 ± 3.8 | 77 ± 1.7 | 98 ± 1.4 | 173 ± 4.6 | 142 ± 15.0 |
| Benz(a) anthracene | 2 ± 0.1 | 4 ± 0.1 | 3 ± 0.2 | 18 ± 1.0 | 2 ± 0 | ||
| Chrysene | 50 ± 2.5 | 113 ± 9.0 | 103 ± 7.1 | 98 ± 6.6 | 85 ± 5.0 | 365 ± 9.0 | 57 ± 18 |
| Benzo(b)fluoranthene | 1 ± 0 | 2 ± 0.1 | 2 ± 0.1 | 1 ± 0.0 | 14 ± 1.0 | 2 ± 0 | |
| Benzo(k)fluoranthene | 1 ± 0.0 | 1 ± 0.0 | 9 ± 0.3 | 4 ± 0.1 | |||
| Benzo(a)pyrene | 3 ± 0.1 | 3 ± 0.2 | 4 ± 0.2 | 3 ± 0.1 | 3 ± 0.1 | 29 ± 0.1 | 2 ± 1 |
| Indeno(1,2,3-cd)pyrene | 18 ± 0.8 | 14 ± 0.6 | 15 ± 0.7 | 20 ± 0.4 | 27 ± 0.4 | 119 ± 0.2 | 25 ± 0 |
| Dibenz(a,h)anthracene | 5 ± 0.1 | 4 ± 0.3 | 8 ± 0.5 | 7 ± 0.2 | 7 ± 0.1 | 58 ± 2.1 | 2 ± 0.1 |
| Benzo(g,h,i)perylene | 2 ± 0.1 | 15 ± 0.1 | |||||
| Total | 147 | 332 | 287 | 226 | 246 | 883 | 321 |
| PAHs / Sample Code # | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
|---|---|---|---|---|---|---|---|
| Fluorene | 24 ± 0.3 | 15 ± 0.2 | 12 ± 0 | 10 ± 0.2 | 11 ± 0.1 | 15 ± 0.4 | 27 ± 0.6 |
| Phenanthrene | 1 ± 0 | 1 ± 0 | 1 ± 0 | 2 ± 0 | |||
| Anthracene | 7 ± 0.3 | 3 ± 0.2 | 3 ± 0.1 | 2 ± 0.1 | 3 ± 0 | 5 ± 0.3 | 12 ± 0.4 |
| Pyrene | 57 ± 3.3 | 74 ± 1.3 | 73 ± 1.9 | 54 ± 0.3 | 57 ± 1.9 | 110 ± 1.2 | |
| Benz(a) anthracene | 2 ± 0.1 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 2 ± 0.1 | 4 ± 0.1 | |
| Chrysene | 52 ± 1.9 | 27 ± 3.0 | 30 ± 0.8 | 37 ± 1.2 | 55 ± 2.7 | 41 ± 2.0 | 183 ± 8.6 |
| Benzo(b)fluoranthene | 2 ± 0.1 | 1 ± 0 | 1 ± 0 | 4 ± 0.1 | |||
| Benzo(k)fluoranthene | 2 ± 0.1 | 3 ± 0.1 | |||||
| Benzo(a)pyrene | 2 ± 0.1 | 3 ± 0.1 | 2 ± 0 | 2 ± 0.1 | 2 ± 0.1 | 5 ± 0.2 | 12 ± 0.1 |
| Indeno(1,2,3-cd)pyrene | 26 ± 0.9 | 10 ± 1.0 | 13 ± 0.1 | 9 ± 0.5 | 7 ± 0.5 | 21 ± 0.7 | 42 ± 0.9 |
| Dibenz(a,h)anthracene | 6 ± 0.3 | 4 ± 0.2 | 5 ± 0 | 7 ± 0.2 | 8 ± 0.4 | 15 ± 0.6 | |
| Benzo(g,h,i)perylene | 4 ± 0.3 | ||||||
| Total | 123 | 120 | 141 | 143 | 134 | 154 | 418 |
| PAHs / Sample Code # | 22 | 23 | 24 | 25 | 26 | 27 | 28 |
|---|---|---|---|---|---|---|---|
| Fluorene | 27 ± 0.7 | 14 ± 0.8 | 22 ± 0.5 | 38 ± 0.5 | 9 ± 0.3 | 12 ± 0.1 | 7 ± 0.2 |
| Phenanthrene | 2 ± 0.1 | 1 ± 0 | 1 ± 0 | 3 ± 0.1 | |||
| Anthracene | 12 ± 0.3 | 8 ± 0.4 | 4 ± 0.1 | 18 ± 1.0 | 1 ± 0 | 2 ± 0.1 | 2 ± 0 |
| Pyrene | 118 ± 2.3 | 46 ± 1.7 | 113 ± 1.5 | 140 ± 3.7 | 62 ± 1.3 | 66 ± 0.4 | 39 ± 0.2 |
| Benz(a) anthracene | 3 ± 0.0 | 2 ± 0.1 | 6 ± 0.3 | 1 ± 0 | |||
| Chrysene | 150 ± 6.1 | 103 ± 4.3 | 45 ± 1.6 | 240 ± 19.9 | 31 ± 1.2 | 17 ± 0.1 | 37 ± 2.1 |
| Benzo(b)fluoranthene | 3 ± 0.1 | 3 ± 0.1 | 1 ± 0 | 5 ± 0.3 | 1 ± 0 | ||
| Benzo(k)fluoranthene | 2 ± 0.1 | 2 ± 0.1 | 4 ± 0.1 | ||||
| Benzo(a)pyrene | 10 ± 0.1 | 7 ± 0.2 | 2 ± 0.1 | 12 ± 0.3 | 2 ± 0 | 2 ± 0 | |
| Indeno(1,2,3-cd)pyrene | 38 ± 0.5 | 27 ± 0.7 | 18 ± 1.2 | 29 ± 0.7 | 16 ± 0.4 | 4 ± 0.1 | 11 ± 0.5 |
| Dibenz(a,h)anthracene | 13 ± 0.5 | 33 ± 3.4 | 4 ± 0.3 | 13 ± 0.1 | 5 ± 0 | 3 ± 0 | |
| Benzo(g,h,i)perylene | 4 ± 0.1 | 2 ± 0.1 | 2 ± 0 | ||||
| Total | 382 | 248 | 212 | 508 | 126 | 101 | 103 |
Fig. 3.
Total PAHs concentrations in ppb for all of the tested samples and their relative abundances of individual congeners.
Carcinogenic potency of each collected sample was determined in terms of its B[a]P equivalent concentration (B[a]P eq). To calculate the B[a]P eq for each individual PAH species, the use of its toxic equivalent factor (TEF) is required for the given species relative to B[a]P carcinogenic potency. In this study, the list of TEFs reported by Nisbet and LaGoy[21] are shown in Figure 2. The toxic equivalents of ΣPAHs for each sample were calculated using the equation:
where TEQ is the toxic equivalents of reference compound; PAHi is concentration of PAH congeneri; TEFi is toxic equivalent factor for PAH congener i.
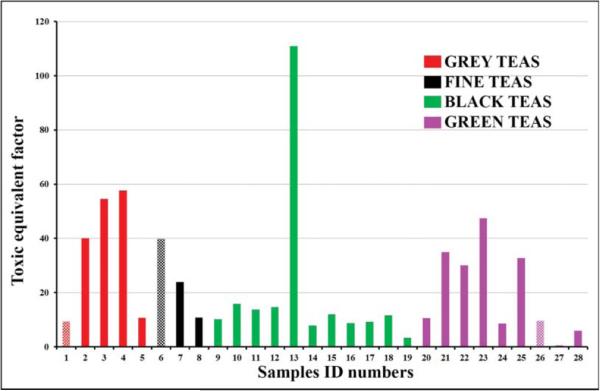
Figure 4 shows that Chai ultra-spice Black Tea Twinnings (Sample 13) was found to have the highest Toxic Equivalent (TEQ) of 110.9, followed by sample 3: 54.53 and sample 4: 57.65 (Grey Tea) respectively. Sample 1-Earl-grey decaffeinated tea was found to have the least toxic equivalent of 9.35; followed by sample 26-Green Tea Honey-Lemon Decaffeinated Lipton with a toxic equivalent of 9.64. Among the Green Tea brands, sample 23 was found to have the highest TEQ of 47.24; followed by sample 21 (TEQ = 34.94) and then sample 25 (TEQ = 32.77).
Fig. 4.
Toxic equivalent factor for tea groups as shown in Table 1.
Although no regulatory standard using the TEQ values have been established yet, these values are in direct correlation to the total concentration of the 6- Group.2A/2B carcinogenic PAHs found in the tea samples. Therefore, due to very limited regulatory guidelines concerning the PAHs found in beverages, these values enable us to make comparisons amongst the tea samples and make recommendations (as shown in the conclusion) for people consuming some of the analyzed 28-tea samples.
Conclusion
All samples of tea showed the presence of 5 to 12 PAHs out of 18 PAHs (US EPA), and these are shown in Table 3. Benzo(a)pyrene classified as probable human carcinogen (2A) was found in all samples except the green tea sample #27. Fluorene, pyrene, chrysene, Indeno[l,2,3-cd] pyrene and Dibenz[a,h]anthracene were the major PAHs in the tea samples analyzed in this study. Green teas were the lowest in their content of the indicated PAHs and fine teas were the highest. It was also observed that decaffeinated teas were also low in their TEQ values and their contents of the PAHs. PAHs contents show that the PAH contamination depends on the drying process of tea leaves and special procedures during the manufacturing of different types of tea. Therefore, it is recommend that consumers should consider drinking decaffeinated tea.
References
- 1.Fiedler H, Cheung CK, Wong MH. PCDD/PCDF, chlorinated pesticides and PAH in Chinese teas. Chemosphere. 2002;46:1429–1433. doi: 10.1016/s0045-6535(01)00264-8. [DOI] [PubMed] [Google Scholar]
- 2.Pincemaille J, Schummer C, Heinen E, Moris G. Determination of polycyclic aromatic hydrocarbons in smoked and non-smoked black teas and tea infusions. Food Chem. 2014;145:807–813. doi: 10.1016/j.foodchem.2013.08.121. [DOI] [PubMed] [Google Scholar]
- 3.Sevastyanova O, Binkova B, Topinka J, Sram RJ, Kalina I, Popov T. In vitro genotoxicity of PAH mixtures and organic extract from urban air particles: Part II: Human cell lines. Mutat Res-Fund Mol M. 2007;620:123–134. doi: 10.1016/j.mrfmmm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Guidelines for Drinking Water Quality. II. World Health Organization; Geneva: 1984. [Google Scholar]
- 5.Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997;8:444–472. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- 6.Pereira-Netto A, Barreto R, Moreira J, Arbilla G. Preliminary comparison of PAHs in total suspended particulate samples taken at Niteroi and Rio de Janeiro Cities, Brazil, Bull. Environ. Contam. Toxicol. 2001;66:36–43. doi: 10.1007/s0012800202. [DOI] [PubMed] [Google Scholar]
- 7.Fiedler H, Cheung C, Wong M. PCDD/PCDF, chlorinated pesticides and PAH in Chinese teas. Chemosphere. 2002;46:1429–1433. doi: 10.1016/s0045-6535(01)00264-8. [DOI] [PubMed] [Google Scholar]
- 8.Schlemitz S, Pfannhauser W. Supercritical fluid extraction of mononitrated polycyclic aromatic hydrocarbons from tea-correlation with the PAH concentration. Z Lebensm. Unters For. 1997;205:305–310. [Google Scholar]
- 9.Ziegenhals K, Jira W, Speer K. Polycyclic aromatic hydrocarbons (PAHs) in various types of tea. Eur. Food Res. Technol. 2008;228:83–91. [Google Scholar]
- 10.Dabrova L, Pulkrabova J, Kalachova K, Tomaniova M, Kocourek V, Hajslova J. Rapid determination of polycyclic aromatic hydrocarbons (PAHs) in tea using two-dimensional gas chromatography coupled with time of flight mass spectrometry. Talanta. 2012;100:207–216. doi: 10.1016/j.talanta.2012.07.081. [DOI] [PubMed] [Google Scholar]
- 11.Ishizaki A, Saito K, Hanioka N, Narimatsu S, Kataoka H. Determination of polycyclic aromatic hydrocarbons in food samples by automated on-line intube solid-phase microextraction coupled with high-performance liquid chromatography-fluorescence detection. J. Chromatogr. A. 2010;1217:5555–5563. doi: 10.1016/j.chroma.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 12.US EPA . Code of federal regulation, title 40, part 60, subparts D, Da, Db, Dc. Environmental Protection Agency; Washington, DC.: 1997. p. 44. [Google Scholar]
- 13.Singh S, Vashishth A, Vishal A. PAHs in some brands of tea. Environ. Monit. Assess. 2011;177:35–38. doi: 10.1007/s10661-010-1615-0. [DOI] [PubMed] [Google Scholar]
- 14.Grover I, Singh S, Bonamali P. Priority PAHs in orthodox black tea during manufacturing process. Environ. Monit. Assess. 2013;185:6291–6294. doi: 10.1007/s10661-012-3025-y. [DOI] [PubMed] [Google Scholar]
- 15.Kayali-Sayadi MN, Rubio-Barroso S, Polo-Dıez LM. Rapid PAH Determination in Urban Particulate Air Samples by HPLC with Fluorometric Detection and Programmed Excitation and Emission Wavelength Pairs. J. Chromatogr. Sci. 1995;33(4):181–185. [Google Scholar]
- 16.Kayali-Sayadi MN, Rubio-Barroso S, Polo-Dıez LM. Determination of PAHs in Particulate Air by Micellar Liquid Chromatography. J. Liq. Chromatogr. 1994;17(17):3623–3640. [Google Scholar]
- 17.Kayali-Sayadi MN, Rubio-Barroso S, Beceiro-Roldan C, Polo-Dıez LM. Rapid Determination of PAHs in Drinking Water Samples Using Solid-Phase Extraction and HPLC with Programmed Fluorescence Detection. J. Liq. Chromatogr. Relat. Technol. 1996;19(19):3135–3146. [Google Scholar]
- 18.Koester CJ, Clement RE. Analysis of Drinking Water for Trace Organics. Crit. Rev. Anal. Chem. 1993;24(4):263–316. [Google Scholar]
- 19.Eisert R, Levsen K. Solid-phase microextraction coupled to gas chromatography: A new method for the analysis of organics in water. J. Chromatogr. 1996;733(1–2):143–157. [Google Scholar]
- 20.Camargo MCR, Toledo MCF. Polycyclic aromatic hydro-carbons in Brazilian vegetables and fruits. Food Control. 2003;14(1):49–53. [Google Scholar]
- 21.Nisbet C, LaGoy P. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharm. 1992;16:290–300. doi: 10.1016/0273-2300(92)90009-x. [DOI] [PubMed] [Google Scholar]