Abstract
The circadian biological clock is essentially based on the light/dark cycle. Some people working with shift schedules cannot adjust their sleep/wake cycle to the light/dark cycle, and this may result in alterations of the circadian biological clock. This study explored the circadian biological clock of shift and daytime nurses using non-invasive methods. Peripheral skin temperature, cortisol and melatonin levels in saliva, and Per2 expression in pubic hair follicle cells were investigated for 24 h after a day off. Significant differences were observed in peripheral skin temperature and cortisol levels between shift and daytime nurses. No differences in melatonin levels were obtained. Per2 maximum values were significantly different between the two groups. Shift nurses exhibited lower circadian variations compared to daytime nurses, and this may indicate an adjustment of the circadian biological clock to continuous shift schedules. Non-invasive procedures, such as peripheral skin temperature measurement, determination of cortisol and melatonin in saliva, and analysis of clock genes in hair follicle cells, may be effective approaches to extensively study the circadian clock in shift workers.
Keywords: skin temperature, circadian rhythm, cortisol, melatonin, PER2 gene, circadian clocks, light dark cycle, circadian dysregulation, occupational health, shift work
1. Introduction
Many biological processes have a circadian rhythm. It is estimated that about 2% to 10% of the whole genome shows a circadian pattern of expression [1,2,3,4,5]. This physiological rhythmicity of the circadian biological clock is entrained by external stimuli such as light and temperature [6,7,8,9]. The need for continuous production in the industry sector, and continuous patient’s care in the health sector, suggest that many workers cannot adjust their sleep/wake cycle to the light/dark cycle [10,11,12]. This may result in alterations of their biological rhythms which, over time, can cause the development of diseases [13,14,15]. The circadian biological clock can be studied using parameters, such as body temperature, cortisol and melatonin secretion, and clock gene expression [16].
Changes in body temperature are the final effects of several physiological processes that result in the production of heat and successive dispersion through the skin. Thermal homeostasis of core temperature is regulated by the preoptic anterior hypothalamus; the circadian pattern of core temperature peaks in the afternoon and falls in the early hours of the morning during sleep [17,18]. The distal skin temperature rhythm has a pivotal role in the regulation of the core temperature rhythm and sleepiness, since heat loss from the extremities drives the core temperature circadian rhythm [19,20,21]. The peripheral temperature rises in the evening and nighttime causing heat dispersion, facilitating sleep and the decrease of core body temperature. In contrast, the peripheral temperature decreases in the daytime raising the core temperature [19,22,23,24,25]. In a subject living on a conventional day-oriented schedule, cortisol levels reach their minimal values early in the night and reach their maximal values around the regular time of awakening [26,27,28]. Cortisol in blood is bound up with serum protein and this affinity is temperature-dependent [29,30]. Melatonin secretion starts in the evening, levels peak in the middle of the night, and slowly decline thereafter to reach their lowest levels at the end of the morning [31]. Melatonin secretion is inhibited by exposure to light at short wavelengths, a deregulation in its secretion was noted in many disease processes, including cancer [32,33]. Shift workers experience light exposure and sleep deprivation during night shifts, which results in reduced levels of melatonin [15,34].
Clock genes are expressed in both the hypothalamic suprachiasmatic nucleus and in other mammalian tissues where they regulate tissue-specific gene expression [35]. Clock gene expression is self-maintained by two transcriptional activators, namely CLOCK (circadian locomoter output cycles kaput) and BMAL1 (brain and muscle ARNT-like protein 1), which activate the expression of clock genes Period (Per) and Cryptochrome (Cry). PER and CRY repress the CLOCK-BMAL1 dimer resulting in negative feedback on the transcription of Per and Cry, thus closing the cycle in approximately 24 h [36,37,38,39]. Among clock genes, Per2 expression is influenced by temperature and plays a role in the adaptation to cold [8,40,41].
In this study, we investigated peripheral skin temperature, cortisol and melatonin levels, and Per2 expression in shift and daytime nurses using non-invasive methods.
2. Results
No significant differences in demographic characteristics, Epworth scores, and Chronotype (MEQ score) were observed between shift-working (SW) and daytime (DT) nurses (Table 1).
Table 1.
Demographic characteristics, Epworth Sleepiness Scale scores, and Chronotype (MEQ score) of shift-working (SW) and daytime (DT) nurses.
| Parameters | SW Nurses (n = 23) | DT Nurses (n = 25) | p-Value |
|---|---|---|---|
| Age (years) mean ± SD | 38.8 ± 3.9 | 39.2 ± 3.2 | 0.699 |
| Job seniority (years) mean ± SD | 13.6 ± 3.4 | 12.8 ± 4.4 | 0.487 |
| Shift-work seniority (years) mean ± SD | 13.6 ± 3.4 | - | - |
| Night-shift work (nights per month) mean ± SD | 6.0 ± 1.0 | - | - |
| Body Mass Index (kg/m2) mean ± SD | 24.2 ± 4.7 | 25.0 ± 4.3 | 0.541 |
| Smokers (%) | 39.1 | 44.0 | 0.732 |
| Alcohol drinkers (%) | 34.8 | 40.0 | 0.709 |
| Epworth Sleepiness Scale (score) mean ± SD | 6.0 ± 3.7 | 5.9 ± 3.1 | 0.919 |
| Chronotype a (MEQ score) mean ± SD | 54.3 ± 7.8 | 57.2 ± 9.6 | 0.259 |
a An higher score is indicative of morningness preference.
Wrist skin temperature showed a circadian rhythm in both SW and DT nurses (ANOVA repeated measures and Cosinor analysis, p < 0.05). Wrist skin temperatures of SW nurses had a lower mesor, and a lower amplitude and higher minimum compared to DT nurses; there was no difference between maximum values (Table 2).
Table 2.
Results of Cosinor analysis of wrist skin temperature of shift-working (SW) and daytime (DT) nurses.
| Wrist Skin Temperature | SW Nurses (n = 23) | DT Nurses (n = 25) | p-Value |
|---|---|---|---|
| Mesor (°C) mean ± SD | 34.47 ± 0.42 | 33.96 ± 0.62 | <0.001 |
| Maximum (°C) mean ± SD | 36.20 ± 0.45 | 36.31 ± 0.46 | 0.407 |
| Minimum (°C) mean ± SD | 32.85 ± 0.92 | 31.59 ± 0.99 | <0.001 |
| Amplitude (°C) mean ± SD | 0.95 ± 0.44 | 1.23 ± 0.38 | 0.022 |
| Acrophase (h) mean ± SD | 5:05 ± 7:43 | 4:05 ± 5:50 | 0.617 |
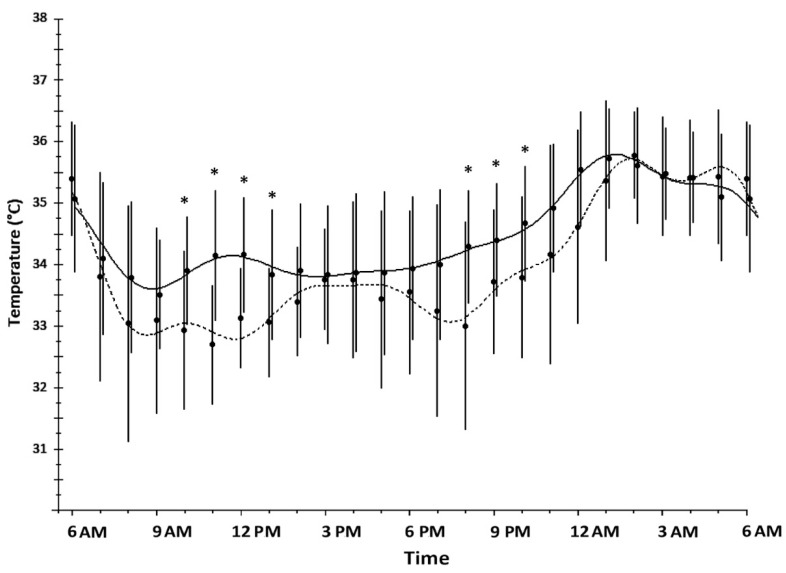
Wrist skin temperatures of SW nurses were significantly higher in the morning hours (from 10:00 AM to 1:00 PM) and in the evening (from 8:00 PM to 10:00 PM) (Figure 1).
Figure 1.
Profiles of wrist skin temperature of shift-working (solid line) and daytime (dashed line) nurses collected for a 24 h period. Data are expressed as the hourly mean ± SD. Statistical significance is indicated by * p < 0.05.
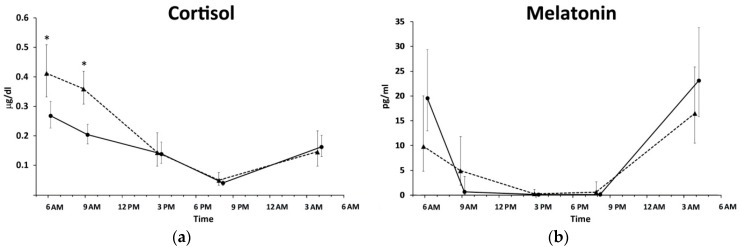
Cortisol levels were also significantly different in the 24 h period of both groups (ANOVA repeated measures, p < 0.05) with maximum values at 6:00 AM. Significant differences were found between SW and DT nurses in cortisol levels (ANOVA repeated measures, p < 0.05); cortisol levels were significantly lower in SW nurses than those of DT nurses at 6:00 AM and 8:00 AM (Figure 2).
Figure 2.
Profiles of cortisol (a) and melatonin (b) levels in saliva samples of shift-working (solid line) and daytime (dashed line) nurses collected for a 24 h period. Data are expressed as the geometric mean ±95% confidence interval. Statistical significance is indicated by * p < 0.05.
Melatonin levels showed significant differences in the 24 h period of both DT and SW nurses (ANOVA repeated measures, p < 0.05); no significant differences were found between the two groups (Figure 2). Maximum levels were noted at 4:00 AM in both groups but no significant difference was found between the two groups.
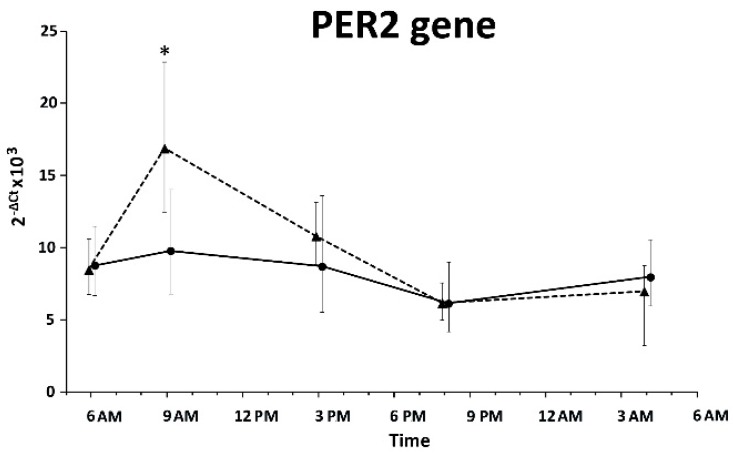
Samples collected from 20 SW and 22 DT nurses were tested for Per2 expression in pubic hair follicle cells. Sufficient amounts of mRNA were not obtained from six subjects. The expression of Per2 was significantly different in the 24 h period of both SW and DT nurses, the 24 h variations were less significant in SW nurses (ANOVA repeated measures, p < 0.05) (Figure 3). The expression of Per2 was statistically no different between the two groups except in the maximum levels at 8:00 AM which were significantly lower in SW nurses.
Figure 3.
Profiles of Per2 expression in pubic hair follicle cells of shift-working (solid line) and daytime (dashed line) nurses collected for a 24 h period. Data are expressed as the geometric mean ± 95% confidence interval. Statistical significance is indicated by * p < 0.05.
3. Discussion
The circadian biological clock is primarily regulated by a light/dark cycle that is naturally based on sunlight. This cycle has caused humans to sleep during nighttime. The biological clock ensures both efficiency and energy saving in several physiologic processes of subjects living on a conventional day-oriented schedule. Shift work, involving night work, may desynchronize circadian rhythms causing persistent mismatching between the sleep/wake cycle and the light/dark cycle [15,42]. Workers in the study showed alterations in peripheral skin temperature. The peripheral skin temperature is found to have a higher mesor in SW nurses, the maximum is not different while the minimum is significantly greater, and consequently the amplitude is smaller. A higher wrist skin temperature was observed in SW nurses from 10:00 AM to 1:00 PM suggesting a minor ergotropic activation confirmed by the low levels of cortisol in the morning. We anticipated a higher diurnal sleepiness in SW, but the hypothesis was not confirmed by the Epworth score, which was similar between groups. A higher wrist skin temperature was observed also from 8:00 PM to 10:00 PM suggesting an anticipated propensity to sleep. SW nurses did not show the wrist skin temperature increase in the early afternoon present in DT; this phenomenon was observed in obese women, since alterations in peripheral temperature were found to be associated with metabolic syndrome [43,44]. No difference in Body Mass Index (BMI) was observed in our sample. However, it is not possible to exclude the possibility that wrist skin temperature rhythm alteration may be preliminary to a development of metabolic syndrome associated with shift-work [45,46].
Light exposure influences thermoregulation [9,47]. Nonphysiological light/dark cycles caused by night schedule may influence a thermophysiological response, as well as melatonin secretion [6,9]. Melatonin has thermoregulatory action both as a vascular modulator and as a molecule related to the amount and functionality of brown adipose tissue [48]. Alteration in melatonin secretion during the night shift may not persist after a day off [34,49]. The melatonin alteration is strongly influenced by the number of night shifts, specific work schedule, and light intensity exposure during night shifts [50,51,52,53,54]. In this study, the SW nurses did not show significant differences from DT nurses in melatonin levels. Since actual peaks may have been earlier than 4:00 AM, differences in the acrophase of melatonin may be present but not captured; results might also be affected by the night’s sleep of SW nurses the day before sampling. However, taking into consideration the low number (mean = 6.0) of participant’s night shifts per month in this study, results are in line with a previous study conducted where a decrease of melatonin was only found to be associated with ≥8 night shifts per month [53].
SW showed reduced cortisol levels in the morning. Typically, cortisol secretion increases at 6:00 AM and decreases at 9:00 PM [55]. Fluctuations in the cortisol profiles are lower during night shifts than during the day shifts [56,57]. Resumption of the regular cortisol secretion was observed in SW after one or more days off [56,58]. Our results support theories that 48 h of rest is not sufficient to SW to restore cortisol levels similar to DT nurses. The cortisol levels after awakening, and diurnal changes, can be used to indicate the adjustment required to meet environmental challenges [59]. Cortisol levels are the expression of the central circadian pacemaker and they represent a key molecule used to transfer circadian information from brain to peripheral tissue [60]. Glucocorthicoids stimulate clock gene expression [61,62], thus Per2 expression in hair follicle cells is probably modulated by cortisol levels. It could be interesting to study other clock gene expression levels in SW hair follicle cells, since clock genes as Per1 and Per3 were found to have robust circadian rhythms in beard follicle cells [63]. Few studies until now investigated clock genes in SW with different results [34,49,64,65,66]. Peripheral levels of clock genes expression fluctuate naturally in a circadian fashion, and they can be influenced by a number of internal and external cues [61,67,68]. Hair follicle cells permit the study of clock gene expression in multiple time samplings limiting the misinterpretation of data from a single time point.
A desynchronization of the sleep/wake cycle with the light/dark cycle is typical of SW. This may result in alterations of central and peripheral homeostatic mechanisms such as thermoregulation. The alterations observed in the SW nurses are not necessarily detrimental to health since they may be a physiologic adaptation to continuous circadian desynchronization. The necessity of achieving homeostasis is obtained with a lower investment in circadian variations during daytime. This energy saving strategy might lead, in the long term, to alterations related to metabolic syndrome.
Non-invasive procedures, such as peripheral skin temperature measurement, determinations of cortisol and melatonin in saliva, and the analysis of clock genes in hair follicle cells, may be effective approaches to extensively study the circadian biological clock in SW.
4. Experimental Section
4.1. Participants and Sampling
Participants were female nurses of the National Health Service hospital wards in Ancona (Italy). SW nurses were employed in a “clockwise rapidly rotating” type of shift-work. The schedule was as follows: Day 1: 7:00 AM–2:00 PM; Day 2: 2:00 PM–10:00 PM; Day 3: 10:00 PM–7:00 AM; 48 h of rest; resumption of the cycle. The work schedule of DT nurses was from 7:00 AM to 2:00 PM six days/week. The study was carried out in accordance with the Declaration of Helsinki’s ethical standards. As part of the standard occupational health surveillance, the study needed no formal approval by the local ethics committee. Nevertheless, the committee was consulted and it granted an informal authorization. Nurses were evaluated and selected based on the following criteria investigated during medical examination: fertile age (presence of a menstrual cycle), no current treatment with drugs, a negative history of psychiatric disorders, degenerative or cardiovascular diseases, insomnia, chronic viral infections, tumor or autoimmune diseases, no skin pathologies, no occupational exposure to ionizing radiation or involvement in antiblastic drug preparation, absence of artificial light (no light sources in the bedroom, no light from windows) when sleeping at home. Additional selection criteria were taken into account for SW and DT nurses. SW nurses had to be assigned to the current shift schedule involving at least 60 night-shifts/year without schedule breaks in the previous six6 months for at least two years. DT nurses had to possess a habitual sleep/wake schedule approximately between the hours of 11:00 PM and 6:00 AM without an episode of sleep deprivation for at least three weeks prior to the study. Among 65 nurses meeting the selection criteria, 23 SW nurses and 25 DT nurses agreed to participate in the study and gave their written informed consent. For both SW and DT nurses, biological samples were collected on the working day after a day off. In this working day, both groups had a morning-shift thus they were as comparable as possible preventing the acute alterations in SW nurses due to light at night exposure and sleep deprivation associated with the night-shift.
Participants filled in a questionnaire enquiring into their work schedule and other habits, such as smoking and alcohol consumption. The Epworth Sleepiness Scale was used to assess daytime sleepiness [69,70]. The chronotype was assessed by the “Morningness-Eveningness Questionnaire” (MEQ) [71], a 19-item questionnaire with a total score ranging from 16 to 86, extensively used in adults and workers [72,73,74]. All nurses had to participate in a tutorial about the procedure of sampling and successive conservation. Biological samples (saliva and pubic hair) were self-collected by nurses at 6:00 AM, 9:00 AM, 3:00 PM, 8:00 PM, and 4:00 AM of the successive day. Nurses conserved the biological samples at a temperature ranging from 2 to 8 °C, a thermal bag was used to transport samples. The temperature of conservation was continuously registered by a temperature data logger (Thermochron iBotton DS1922L, Maxim Integrated Products, Inc., Sunnyvale, CA, USA) fixed to the specimen containers. All samples were delivered to the laboratory the morning after the last sampling.
4.2. Peripheral Skin Temperature Measurement
Peripheral skin temperature was assessed continuously at the wrist on the nondominant hand as described by Sarabia et al. [75]. Temperature measurements were done by a Thermochron iButton DS1922L (Maxim Integrated Products, Inc., Sunnyvale, CA, USA). The use of iButtons for human skin temperature measurement has been noted by some authors [76,77,78]. The accuracy is −0.09 °C with a precision of 0.05 °C [77]. For the DS1922L, manufacturer’s specifications state this latest model functions throughout the full human thermo-physiological range. For this study, the iButton resolution was set at 0.0625 °C and the iButton real-time clock was synchronized with that of a laptop computer. The sensor was programmed to sample every 2 min for 24 h starting at 6:00 AM on the first working day after a day off. Wrist skin temperature was investigated the week before the sampling of the biological material in order to avoid artifacts of the wake-up for sampling at 4:00 AM. An iButton was attached to a cotton wrist band and the sensor surface was placed over the inside of the wrist on the radial artery. The data stored in the temperature sensor were transferred through an adapter (Thermochron iBotton DS9490B, Maxim Integrated Products, Inc., Sunnyvale, CA, USA) to a personal computer using OneWireViewer v. 3.15.50 (Maxim Integrated Products, Inc., Sunnyvale, CA, USA).
4.3. Cortisol and Melatonin Measurement
Saliva is an ideal matrix for multiple non-invasive sampling and allows the study of hormones such as cortisol and melatonin. Salivary cortisol levels are unaffected by salivary flow rate and are relatively resistant to degradation from enzymes or freeze-thaw cycles [79,80]. Studies reported that serum and salivary cortisol are strongly correlated, indicating that salivary cortisol levels reliably estimate serum cortisol levels [81,82]. Melatonin levels in plasma are paralleled by corresponding variations in saliva where the salivary concentration is about 30% of that found in plasma. Measurement of salivary melatonin is advantageous especially to avoid invasive venipuncture procedures [83,84]. Saliva samples were collected by unstimulated passive drool, passing the saliva directly into a polypropylene tube. Upon arrival at the laboratory, saliva samples were stored at −80 °C until required for assay. On the day of assay, the samples were thawed for approximately 4 h until room temperature (20.0–23.0 °C). Samples were then centrifuged at 3000 rpm for 15 min to remove mucins and other particulate matter which may interfere with analysis.
The free cortisol levels in saliva were determined in duplicate using a high-sensitivity salivary cortisol enzyme immunoassay kit (Salimetrics, LLC, State College, PA, USA) according to the manufacturer’s instructions. Samples from each subject were assayed in the same batch. The inter- and intra-assay variations were below 6.2% and 3.9%, respectively.
The melatonin levels were determined in duplicate using a salivary melatonin enzyme immunoassay kit (Salimetrics, LLC, State College, PA, USA) according to the manufacturer’s instructions. Samples from each subject were assayed in the same batch. The inter- and intra-assay variations were below 8.9% and 5.4%, respectively.
4.4. Circadian Expression of PER2 Clock Gene
The circadian expression of peripheral clock genes can be found in the blood and in other peripheral tissues [85,86,87]. Multiple blood sampling constitutes a major limit of studies on clock genes involving free-living subjects. This problem may be bypassed using hair follicle cells that constitute suitable biological samples for clock genes determination [63,88]. Total RNA quality and quantity in follicle cells were preliminarily tested in hairs collected from scalp, eyebrows, arms, pubic region, and legs of female volunteers. Hairs collected from the pubic region were selected for the study as they provided the best quality and quantity of total RNA. In each sampling, nurses harvested 15 pubic hairs. They were immediately submerged into tubes containing a RNA stabilization reagent (RNA Later, Sigma, Saint Louis, MO, USA).
When samples were delivered to the laboratory, RNA extraction was performed immediately. The isolation of total RNA was performed using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. Obtained RNA was concentrated by Eppendorf Concentrator 5301 (Eppendorf, Hamburg, Germany). RNA quality and quantification were evaluated with a Nanodrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). cDNA was synthesized according to the of High-Capacity cDNA Reverse Transcription Kit protocol (Applied Biosystems, Foster City, CA, USA). Gene expression was analyzed by real-time quantitative PCR using the TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, CA, USA). To control for variation in the amount of cDNA available for PCR in the different samples, the gene expression levels of the target sequences were normalized to the expression of an endogenous control, glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Primer and probe sets used for RT-PCR assays were as follows: Hs.PT.58.3464649 for Per2 and Hs.PT.39a.22214836 for Gapdh (Integrated DNA Technologies Inc., Coralville, IA, USA). The expression levels of Per2 was calculated applying the following equation: 2−∆Ct.
4.5. Statistical Analysis
The normality distribution of variables was assessed by the Kolmogorov-Smirnov test. Natural logarithms of cortisol, melatonin, and Per2 expression values were used for analysis as transformed values more closely approximated a normal distribution. Continuous variables were expressed as mean and standard deviation, while log-transformed variables were expressed as a geometric mean along with a 95% confidence interval (95% CI). Repeated-measures of ANOVA were performed to analyze between- and within-groups a change of variables across time. The Mauchly test was performed to verify the sphericity assumption. When statistical differences were found by repeated measures of ANOVA, a multiple-comparison test, adjusted by the least significant difference, was applied to identify the differences between the two groups for each time point. Wrist skin temperature was further analyzed by Cosinor analysis, calculating its mesor, amplitude, acrophase, and maximum and minimum values. Student’s t-test was used to test differences of independent variables, and maximum and minimum values, of each variable between the two groups. Data was analyzed by Statistical Package Social Sciences (version 19) software (SPSS, Chicago, IL, USA) and Chronos-Fit 1.06 [89].
Acknowledgments
We thank Richard G. Stevens for revising the manuscript, Ernesta Pieragostini and Sayeed Md Abu for their kind aid in the laboratory and Megan Conner for her assistance to improve the English language of our manuscript.
Author Contributions
Massimo Bracci conceived and designed the study, analyzed the data and wrote the paper; Veronica Ciarapica performed the experiments, analyzed the data and contributed to the writing of the paper; Alfredo Copertaro conceived and designed the study and wrote the paper; Mariella Barbaresi contributed to samples collection; Nicola Manzella contributed to design of the study; Marco Tomasetti supervised the experiments; Simona Gaetani and Federica Monaco performed the experiments; Monica Amati and Matteo Valentino contributed reagents and materials; Venerando Rapisarda contributed to data analysis; Lory Santarelli supervised the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Akhtar R.A., Reddy A.B., Maywood E.S., Clayton J.D., King V.M., Smith A.G., Gant T.W., Hastings M.H., Kyriacou C.P. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr. Biol. 2002;12:540–550. doi: 10.1016/S0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- 2.Archer S.N., Laing E.E., Moller-Levet C.S., van der Veen D.R., Bucca G., Lazar A.S., Santhi N., Slak A., Kabiljo R., von Schantz M., et al. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc. Natl. Acad. Sci. USA. 2014;111:E682–E691. doi: 10.1073/pnas.1316335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller B.H., McDearmon E.L., Panda S., Hayes K.R., Zhang J., Andrews J.L., Antoch M.P., Walker J.R., Esser K.A., Hogenesch J.B., et al. Circadian and clock-controlled regulation of the mouse transcriptome and cell proliferation. Proc. Natl. Acad. Sci. USA. 2007;104:3342–3347. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panda S., Antoch M.P., Miller B.H., Su A.I., Schook A.B., Straume M., Schultz P.G., Kay S.A., Takahashi J.S., Hogenesch J.B. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/S0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 5.Ueda H.R., Chen W., Adachi A., Wakamatsu H., Hayashi S., Takasugi T., Nagano M., Nakahama K., Suzuki Y., Sugano S., et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- 6.Stevens R.G., Zhu Y. Electric light, particularly at night, disrupts human circadian rhythmicity: Is that a problem? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015;370 doi: 10.1098/rstb.2014.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright K.P., McHill A.W., Birks B.R., Griffin B.R., Rusterholz T., Chinoy E.D. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhr E.D., Yoo S.H., Takahashi J.S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Nicolas A., Madrid J.A., Rol M.A. Day-night contrast as source of health for the human circadian system. Chronobiol. Int. 2014;31:382–393. doi: 10.3109/07420528.2013.861845. [DOI] [PubMed] [Google Scholar]
- 10.Boudreau P., Dumont G.A., Boivin D.B. Circadian adaptation to night shift work influences sleep, performance, mood and the autonomic modulation of the heart. PLoS ONE. 2013;8:623. doi: 10.1371/journal.pone.0070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrov M.E., Clark C.B., Molzof H.E., Johnson R.L., Jr., Cropsey K.L., Gamble K.L. Sleep strategies of night-shift nurses on days off: Which ones are most adaptive? Front. Neurol. 2014;5 doi: 10.3389/fneur.2014.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa G., Anelli M.M., Castellini G., Fustinoni S., Neri L. Stress and sleep in nurses employed in “3 × 8” and “2 × 12” fast rotating shift schedules. Chronobiol. Int. 2014;31:1169–1178. doi: 10.3109/07420528.2014.957309. [DOI] [PubMed] [Google Scholar]
- 13.Arendt J. Shift work: Coping with the biological clock. Occup. Med. 2010;60:10–20. doi: 10.1093/occmed/kqp162. [DOI] [PubMed] [Google Scholar]
- 14.Munch M., Bromundt V. Light and chronobiology: Implications for health and disease. Dialogues Clin. Neurosci. 2012;14:448–453. doi: 10.31887/DCNS.2012.14.4/mmuench. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens R.G. Working against our endogenous circadian clock: Breast cancer and electric lighting in the modern world. Mutat. Res. 2009;680:106–108. doi: 10.1016/j.mrgentox.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Cermakian N., Boivin D.B. The regulation of central and peripheral circadian clocks in humans. Obes. Rev. 2009;10(Suppl. 2):25–36. doi: 10.1111/j.1467-789X.2009.00660.x. [DOI] [PubMed] [Google Scholar]
- 17.Romanovsky A.A. Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 18.Tansey E.A., Johnson C.D. Recent advances in thermoregulation. Adv. Physiol. Educ. 2015;39:139–148. doi: 10.1152/advan.00126.2014. [DOI] [PubMed] [Google Scholar]
- 19.Krauchi K., Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am. J. Physiol. 1994;267:R819–R829. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- 20.Taylor N.A., Tipton M.J., Kenny G.P. Considerations for the measurement of core, skin and mean body temperatures. J. Therm. Biol. 2014;46:72–101. doi: 10.1016/j.jtherbio.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Krauchi K. The human sleep-wake cycle reconsidered from a thermoregulatory point of view. Physiol. Behav. 2007;90:236–245. doi: 10.1016/j.physbeh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Van Someren E.J. More than a marker: Interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol. Int. 2000;17:313–354. doi: 10.1081/CBI-100101050. [DOI] [PubMed] [Google Scholar]
- 23.Krauchi K., Gompper B., Hauenstein D., Flammer J., Pfluger M., Studerus E., Schotzau A., Orgul S. Diurnal blood pressure variations are associated with changes in distal-proximal skin temperature gradient. Chronobiol. Int. 2012;29:1273–1283. doi: 10.3109/07420528.2012.719961. [DOI] [PubMed] [Google Scholar]
- 24.Raymann R.J., Swaab D.F., van Someren E.J. Cutaneous warming promotes sleep onset. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1589–R1597. doi: 10.1152/ajpregu.00492.2004. [DOI] [PubMed] [Google Scholar]
- 25.Krauchi K., Wirz-Justice A. Circadian clues to sleep onset mechanisms. Neuropsychopharmacology. 2001;25:S92–S96. doi: 10.1016/S0893-133X(01)00315-3. [DOI] [PubMed] [Google Scholar]
- 26.Weibel L., Brandenberger G. Disturbances in hormonal profiles of night workers during their usual sleep and work times. J. Biol. Rhythms. 1998;13:202–208. doi: 10.1177/074873098129000048. [DOI] [PubMed] [Google Scholar]
- 27.Hucklebridge F., Hussain T., Evans P., Clow A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology. 2005;30:51–57. doi: 10.1016/j.psyneuen.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Knutsson U., Dahlgren J., Marcus C., Rosberg S., Bronnegard M., Stierna P., Albertsson-Wikland K. Circadian cortisol rhythms in healthy boys and girls: Relationship with age, growth, body composition, and pubertal development. J. Clin. Endocrinol. Metab. 1997;82:536–540. doi: 10.1210/jc.82.2.536. [DOI] [PubMed] [Google Scholar]
- 29.Cameron A., Henley D., Carrell R., Zhou A., Clarke A., Lightman S. Temperature-responsive release of cortisol from its binding globulin: A protein thermocouple. J. Clin. Endocrinol. Metab. 2010;95:4689–4695. doi: 10.1210/jc.2010-0942. [DOI] [PubMed] [Google Scholar]
- 30.Aardal E., Holm A.C. Cortisol in saliva—Reference ranges and relation to cortisol in serum. Eur. J. Clin. Chem. Clin. Biochem. 1995;33:927–932. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- 31.Burgess H.J., Fogg L.F. Individual differences in the amount and timing of salivary melatonin secretion. PLoS ONE. 2008;3:623. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill S.M., Belancio V.P., Dauchy R.T., Xiang S., Brimer S., Mao L., Hauch A., Lundberg P.W., Summers W., Yuan L., et al. Melatonin: An inhibitor of breast cancer. Endocr. Relat. Cancer. 2015;22:R183–R204. doi: 10.1530/ERC-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens R.G., Brainard G.C., Blask D.E., Lockley S.W., Motta M.E. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J. Clin. 2014;64:207–218. doi: 10.3322/caac.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bracci M., Copertaro A., Manzella N., Staffolani S., Strafella E., Nocchi L., Barbaresi M., Copertaro B., Rapisarda V., Valentino M., et al. Influence of night-shift and napping at work on urinary melatonin, 17-beta-estradiol and clock gene expression in pre-menopausal nurses. J. Biol. Regul. Homeost. Agents. 2013;27:267–274. [PubMed] [Google Scholar]
- 35.Brown S.A., Azzi A. Peripheral circadian oscillators in mammals. Handb. Exp. Pharmacol. 2013;217:45–66. doi: 10.1007/978-3-642-25950-0_3. [DOI] [PubMed] [Google Scholar]
- 36.Buhr E.D., Takahashi J.S. Molecular components of the mammalian circadian clock. Handb. Exp. Pharmacol. 2013;217:3–27. doi: 10.1007/978-3-642-25950-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowrey P.L., Takahashi J.S. Genetics of circadian rhythms in mammalian model organisms. Adv. Genet. 2011;74:175–230. doi: 10.1016/B978-0-12-387690-4.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi J.S. Molecular components of the circadian clock in mammals. Diabetes Obes. Metab. 2015;17(Suppl. 1):6–11. doi: 10.1111/dom.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chappuis S., Ripperger J.A., Schnell A., Rando G., Jud C., Wahli W., Albrecht U. Role of the circadian clock gene Per2 in adaptation to cold temperature. Mol. Metab. 2013;2:184–193. doi: 10.1016/j.molmet.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohnishi N., Tahara Y., Kuriki D., Haraguchi A., Shibata S. Warm water bath stimulates phase-shifts of the peripheral circadian clocks in PER2::Luciferase mouse. PLoS ONE. 2014;9:623. doi: 10.1371/journal.pone.0100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boivin D.B., Boudreau P. Impacts of shift work on sleep and circadian rhythms. Pathol. Biol. 2014;62:292–301. doi: 10.1016/j.patbio.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Corbalan-Tutau M.D., Gomez-Abellan P., Madrid J.A., Canteras M., Ordovas J.M., Garaulet M. Toward a chronobiological characterization of obesity and metabolic syndrome in clinical practice. Clin. Nutr. 2015;34:477–483. doi: 10.1016/j.clnu.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Corbalan-Tutau M.D., Madrid J.A., Ordovas J.M., Smith C.E., Nicolas F., Garaulet M. Differences in daily rhythms of wrist temperature between obese and normal-weight women: Associations with metabolic syndrome features. Chronobiol. Int. 2011;28:425–433. doi: 10.3109/07420528.2011.574766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Proper K.I., van de Langenberg D., Rodenburg W., Vermeulen R.C., van der Beek A.J., van Steeg H., van Kerkhof L.W. The relationship between shift work and metabolic risk factors: A systematic review of longitudinal studies. Am. J. Prev. Med. 2016;50 doi: 10.1016/j.amepre.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Copertaro A., Bracci M., Barbaresi M., Santarelli L. Assessment of cardiovascular risk in shift healthcare workers. Eur. J. Cardiovasc. Prev. Rehabil. 2008;15:224–229. doi: 10.1097/HJR.0b013e3282f364c0. [DOI] [PubMed] [Google Scholar]
- 47.Te Kulve M., Schellen L., Schlangen L.J., van Marken Lichtenbelt W.D. The influence of light on thermal responses. Acta Physiol. 2016;216:163–185. doi: 10.1111/apha.12552. [DOI] [PubMed] [Google Scholar]
- 48.Seron-Ferre M., Reynolds H., Mendez N.A., Mondaca M., Valenzuela F., Ebensperger R., Valenzuela G.J., Herrera E.A., Llanos A.J., Torres-Farfan C. Impact of maternal melatonin suppression on amount and functionality of brown adipose tissue (BAT) in the newborn sheep. Front. Endocrinol. 2014;5 doi: 10.3389/fendo.2014.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bracci M., Manzella N., Copertaro A., Staffolani S., Strafella E., Barbaresi M., Copertaro B., Rapisarda V., Valentino M., Santarelli L. Rotating-shift nurses after a day off: Peripheral clock gene expression, urinary melatonin, and serum 17-beta-estradiol levels. Scand. J. Work Environ. Health. 2014;40:295–304. doi: 10.5271/sjweh.3414. [DOI] [PubMed] [Google Scholar]
- 50.Davis S., Mirick D.K., Chen C., Stanczyk F.Z. Night shift work and hormone levels in women. Cancer Epidemiol. Biomark. Prev. 2012;21:609–618. doi: 10.1158/1055-9965.EPI-11-1128. [DOI] [PubMed] [Google Scholar]
- 51.Grundy A., Tranmer J., Richardson H., Graham C.H., Aronson K.J. The influence of light at night exposure on melatonin levels among canadian rotating shift nurses. Cancer Epidemiol. Biomark. Prev. 2011;20:2404–2412. doi: 10.1158/1055-9965.EPI-11-0427. [DOI] [PubMed] [Google Scholar]
- 52.Marie Hansen A., Helene Garde A., Hansen J. Diurnal urinary 6-sulfatoxymelatonin levels among healthy danish nurses during work and leisure time. Chronobiol. Int. 2006;23:1203–1215. doi: 10.1080/07420520601100955. [DOI] [PubMed] [Google Scholar]
- 53.Peplonska B., Bukowska A., Gromadzinska J., Sobala W., Reszka E., Lie J.A., Kjuus H., Wasowicz W. Night shift work characteristics and 6-sulfatoxymelatonin (MT6s) in rotating night shift nurses and midwives. Occup. Environ. Med. 2012;69:339–346. doi: 10.1136/oemed-2011-100273. [DOI] [PubMed] [Google Scholar]
- 54.Schernhammer E.S., Rosner B., Willett W.C., Laden F., Colditz G.A., Hankinson S.E. Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer Epidemiol. Biomark. Prev. 2004;13:936–943. [PubMed] [Google Scholar]
- 55.Knauth P., Rutenfranz J. Experimental shift work studies of permanent night, and rapidly rotating, shift systems. I. Circadian rhythm of body temperature and re-entrainment at shift change. Int. Arch. Occup. Environ. Health. 1976;37:125–137. doi: 10.1007/BF00378059. [DOI] [PubMed] [Google Scholar]
- 56.Niu S.F., Chung M.H., Chu H., Tsai J.C., Lin C.C., Liao Y.M., Ou K.L., O’Brien A.P., Chou K.R. Differences in cortisol profiles and circadian adjustment time between nurses working night shifts and regular day shifts: A prospective longitudinal study. Int. J. Nurs. Stud. 2015;52:1193–1201. doi: 10.1016/j.ijnurstu.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Ulhoa M.A., Marqueze E.C., Burgos L.G., Moreno C.R. Shift work and endocrine disorders. Int. J. Endocrinol. 2015;2015 doi: 10.1155/2015/826249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merkus S.L., Holte K.A., Huysmans M.A., Hansen A.M., van de Ven P.M., van Mechelen W., van der Beek A.J. Neuroendocrine recovery after 2-week 12-h day and night shifts: An 11-day follow-up. Int. Arch. Occup. Environ. Health. 2015;88:247–257. doi: 10.1007/s00420-014-0954-5. [DOI] [PubMed] [Google Scholar]
- 59.Adam E.K., Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Kalsbeek A., van der Spek R., Lei J., Endert E., Buijs R.M., Fliers E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol. Cell. Endocrinol. 2012;349:20–29. doi: 10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 61.Balsalobre A., Brown S.A., Marcacci L., Tronche F., Kellendonk C., Reichardt H.M., Schutz G., Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 62.Cuesta M., Cermakian N., Boivin D.B. Glucocorticoids entrain molecular clock components in human peripheral cells. FASEB J. 2015;29:1360–1370. doi: 10.1096/fj.14-265686. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe M., Hida A., Kitamura S., Enomoto M., Ohsawa Y., Katayose Y., Nozaki K., Moriguchi Y., Aritake S., Higuchi S., et al. Rhythmic expression of circadian clock genes in human leukocytes and beard hair follicle cells. Biochem. Biophys. Res. Commun. 2012;425:902–907. doi: 10.1016/j.bbrc.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 64.James F.O., Cermakian N., Boivin D.B. Circadian rhythms of melatonin, cortisol, and clock gene expression during simulated night shift work. Sleep. 2007;30:1427–1436. doi: 10.1093/sleep/30.11.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reszka E., Peplonska B., Wieczorek E., Sobala W., Bukowska A., Gromadzinska J., Lie J.A., Kjuus H., Wasowicz W. Circadian gene expression in peripheral blood leukocytes of rotating night shift nurses. Scand. J. Work Environ. Health. 2013;39:187–194. doi: 10.5271/sjweh.3303. [DOI] [PubMed] [Google Scholar]
- 66.Zhu Y., Stevens R.G., Hoffman A.E., Tjonneland A., Vogel U.B., Zheng T., Hansen J. Epigenetic impact of long-term shiftwork: Pilot evidence from circadian genes and whole-genome methylation analysis. Chronobiol. Int. 2011;28:852–861. doi: 10.3109/07420528.2011.618896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ackermann K., Plomp R., Lao O., Middleton B., Revell V.L., Skene D.J., Kayser M. Effect of sleep deprivation on rhythms of clock gene expression and melatonin in humans. Chronobiol. Int. 2013;30:901–909. doi: 10.3109/07420528.2013.784773. [DOI] [PubMed] [Google Scholar]
- 68.Stevens R.G. Circadian disruption and breast cancer: From melatonin to clock genes. Epidemiology. 2005;16:254–258. doi: 10.1097/01.ede.0000152525.21924.54. [DOI] [PubMed] [Google Scholar]
- 69.Johns M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 70.Vignatelli L., Plazzi G., Barbato A., Ferini-Strambi L., Manni R., Pompei F., D’Alessandro R. Italian version of the Epworth sleepiness scale: External validity. Neurol. Sci. 2003;23:295–300. doi: 10.1007/s100720300004. [DOI] [PubMed] [Google Scholar]
- 71.Horne J.A., Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 72.Bajaj A., Rosner B., Lockley S.W., Schernhammer E.S. Validation of a light questionnaire with real-life photopic illuminance measurements: The harvard light exposure assessment questionnaire. Cancer Epidemiol. Biomark. Prev. 2011;20:1341–1349. doi: 10.1158/1055-9965.EPI-11-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung M.H., Chang F.M., Yang C.C., Kuo T.B., Hsu N. Sleep quality and morningness-eveningness of shift nurses. J. Clin. Nurs. 2009;18:279–284. doi: 10.1111/j.1365-2702.2007.02160.x. [DOI] [PubMed] [Google Scholar]
- 74.Yazdi Z., Sadeghniiat-Haghighi K., Javadi A.R., Rikhtegar G. Sleep quality and insomnia in nurses with different circadian chronotypes: Morningness and eveningness orientation. Work. 2014;47:561–567. doi: 10.3233/WOR-131664. [DOI] [PubMed] [Google Scholar]
- 75.Sarabia J.A., Rol M.A., Mendiola P., Madrid J.A. Circadian rhythm of wrist temperature in normal-living subjects a candidate of new index of the circadian system. Physiol. Behav. 2008;95:570–580. doi: 10.1016/j.physbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Smith A.D., Crabtree D.R., Bilzon J.L., Walsh N.P. The validity of wireless ibuttons and thermistors for human skin temperature measurement. Physiol. Meas. 2010;31:95–114. doi: 10.1088/0967-3334/31/1/007. [DOI] [PubMed] [Google Scholar]
- 77.Van Marken Lichtenbelt W.D., Daanen H.A., Wouters L., Fronczek R., Raymann R.J., Severens N.M., van Someren E.J. Evaluation of wireless determination of skin temperature using ibuttons. Physiol. Behav. 2006;88:489–497. doi: 10.1016/j.physbeh.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 78.Hasselberg M.J., McMahon J., Parker K. The validity, reliability, and utility of the ibutton(r) for measurement of body temperature circadian rhythms in sleep/wake research. Sleep Med. 2013;14:5–11. doi: 10.1016/j.sleep.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 79.Garde A.H., Hansen A.M. Long-term stability of salivary cortisol. Scand. J. Clin. Lab. Investig. 2005;65:433–436. doi: 10.1080/00365510510025773. [DOI] [PubMed] [Google Scholar]
- 80.Vining R.F., McGinley R.A. The measurement of hormones in saliva: Possibilities and pitfalls. J. Steroid Biochem. 1987;27:81–94. doi: 10.1016/0022-4731(87)90297-4. [DOI] [PubMed] [Google Scholar]
- 81.Daniel M., Moore D.S., Decker S., Belton L., DeVellis B., Doolen A., Campbell M.K. Associations among education, cortisol rhythm, and BMI in blue-collar women. Obesity. 2006;14:327–335. doi: 10.1038/oby.2006.42. [DOI] [PubMed] [Google Scholar]
- 82.Dorn L.D., Kolko D.J., Susman E.J., Huang B., Stein H., Music E., Bukstein O.G. Salivary gonadal and adrenal hormone differences in boys and girls with and without disruptive behavior disorders: Contextual variants. Biol. Psychol. 2009;81:31–39. doi: 10.1016/j.biopsycho.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Almeida E.A., Di Mascio P., Harumi T., Spence D.W., Moscovitch A., Hardeland R., Cardinali D.P., Brown G.M., Pandi-Perumal S.R. Measurement of melatonin in body fluids: Standards, protocols and procedures. Childs Nerv. Syst. 2011;27:879–891. doi: 10.1007/s00381-010-1278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nowak R., McMillen I.C., Redman J., Short R.V. The correlation between serum and salivary melatonin concentrations and urinary 6-hydroxymelatonin sulphate excretion rates: Two non-invasive techniques for monitoring human circadian rhythmicity. Clin. Endocrinol. 1987;27:445–452. doi: 10.1111/j.1365-2265.1987.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 85.Fukuya H., Emoto N., Nonaka H., Yagita K., Okamura H., Yokoyama M. Circadian expression of clock genes in human peripheral leukocytes. Biochem. Biophys. Res. Commun. 2007;354:924–928. doi: 10.1016/j.bbrc.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 86.Bjarnason G.A., Jordan R.C., Wood P.A., Li Q., Lincoln D.W., Sothern R.B., Hrushesky W.J., Ben-David Y. Circadian expression of clock genes in human oral mucosa and skin: Association with specific cell-cycle phases. Am. J. Pathol. 2001;158:1793–1801. doi: 10.1016/S0002-9440(10)64135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boivin D.B., James F.O., Wu A., Cho-Park P.F., Xiong H., Sun Z.S. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood. 2003;102:4143–4145. doi: 10.1182/blood-2003-03-0779. [DOI] [PubMed] [Google Scholar]
- 88.Akashi M., Soma H., Yamamoto T., Tsugitomi A., Yamashita S., Yamamoto T., Nishida E., Yasuda A., Liao J.K., Node K. Noninvasive method for assessing the human circadian clock using hair follicle cells. Proc. Natl. Acad. Sci. USA. 2010;107:15643–15648. doi: 10.1073/pnas.1003878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zuther P., Gorbey S., Lemmer B. Chronos-Fit 1.06. [(accessed on 14 July 2015)]. Available online: http://www.ma.uni-heidelberg.de/inst/phar/lehre/chrono.html.