Abstract
Arthrospira platensis biomass was used in order to obtain functional lipophilic compounds through green extraction technologies such as supercritical carbon dioxide fluid extraction (SFE) and microwave-assisted extraction (MAE). The temperature (T) factor was evaluated for MAE, while for SFE, pressure (P), temperature (T), and co-solvent (ethanol) (CS) were evaluated. The maximum extraction yield of the obtained oleoresin was (4.07% ± 0.14%) and (4.27% ± 0.10%) for SFE and MAE, respectively. Extracts were characterized by gas chromatography mass spectrometry (GC-MS) and gas chromatography flame ionization detector (GC-FID). The maximum contents of functional lipophilic compounds in the SFE and MAE extracts were: for carotenoids 283 ± 0.10 μg/g and 629 ± 0.13 μg/g, respectively; for tocopherols 5.01 ± 0.05 μg/g and 2.46 ± 0.09 μg/g, respectively; and for fatty acids 34.76 ± 0.08 mg/g and 15.88 ± 0.06 mg/g, respectively. In conclusion, the SFE process at P 450 bar, T 60 °C and CS 53.33% of CO2 produced the highest yield of tocopherols, carotenoids and fatty acids. The MAE process at 400 W and 50 °C gives the best extracts in terms of tocopherols and carotenoids. For yield and fatty acids, the MAE process at 400 W and 70 °C produced the highest values. Both SFE and MAE showed to be suitable green extraction technologies for obtaining functional lipophilic compounds from Arthrospira platensis.
Keywords: supercritical fluid extraction, microwave assisted extraction, Arthrospira platensis, cyanobacteria, functional lipophilic compounds, β-carotene, α-tocopherol, γ-linolenic acid
1. Introduction
Microalgae and cyanobacteria cultivation has attracted the interest of industrial companies and the scientific community as a natural and sustainable biomass source for bioactive compounds [1,2]. Furthermore, they are capable of fixating CO2 from the atmosphere, they do not need to use agricultural lands to grow, they are fast growers and they have a high tolerance to varying environmental conditions [3,4]. Arthrospira platensis is a photosynthetic filamentous microorganism [5] considered a “super food” due to its pharmaceutical and nutraceutical properties [6], including antiproliferative, antitumor, antifungal, antibacterial, antimalarial, antiviral, and antimycotic characteristics [7,8]. Commercially available products of A. platensis contain approximately 65% protein, 20% f carbohydrate, 7% minerals, 5% lipids and 8% moisture [9]. Lipids are important cellular constituents that have multiple critical roles in cellular functions [10], besides their multiple applications as nutraceuticals, food additives [11], antimicrobials [12], and biofuels [13]. The main components of the algae lipid fraction are fatty acids (FA), phospholipids, tocopherols, waxes, sterols, hydrocarbons, ketones and pigments (carotenoids, chlorophylls) [14]. Traditional extraction techniques of oils from algae are based on the same technology of plant oil extraction (organic solvent extraction, Soxhlet). These techniques require high energy and are time-consuming, and they also polluting the environment with hazardous solvents. Therefore, sustainable technologies must be implemented, such as supercritical fluid extraction (SFE) and microwave-assisted extraction (MAE). Both use eco-friendly solvents, and require lower energy input while reducing extraction time [15]. In the case of SFE, one of its advantages is the use of carbon dioxide (CO2) as the main solvent for the extraction, since it has a low critical point (T 30.9 °C and P 73.9 bar). Also, it is cheap, environmentally friendly and generally recognized as safe (GRAS) by the Food and Drug Administration (FDA) [16]. Furthermore, CO2 has a high diffusivity with a tunable solvent strength [17]. On the other hand, MAE uses non-ionizing electromagnetic waves, enabling the extraction of bioactive compounds from complex biological matrices. It also requires less solvent volume and time for the extraction [18,19]. Additionally, MAE offers more effective and selective transfer heating (without heat transfers), allowing the reduction of thermal gradients, and can exploit the use of different types of solvents to increase the range of polarity of the extraction, thus increasing the variety and amount of metabolites extracted [20,21]. SFE with CO2 has been previously used for the extraction of functional lipophilic compounds from Arthrospira platensis such as carotenoids [22,23], fatty acids [24,25] and tocopherols [26,27]. However, all of these studies have been performed for individual compounds and did not consider the interaction between the lipophilic compounds and process parameters. On the other hand, for MAE technology, the available information about Arthrospira only covers the extraction of protein pigments (C-phycocyanin) [28], while for other microalgae/cyanobacteria, the information is scarce and, similarly, only focused on pigments [21,29] without evaluation of temperature in the process. To our knowledge, there are no reports on the extraction of functional lipophilic compounds from Arthrospira by MAE.
The data provided below demonstrate the advantages of using green extraction technologies, since they are environmentally friendly and faster than traditional solvent extraction. Therefore, the goal of this study was to explore the effect of the extraction factors on the content of the functional lipophilic compounds in the extracts obtained by SFE and MAE, in order to determine the most important extraction factors and provide a framework for future work. In the present study, the model was chosen, as it allows the analysis of a variety of factors with a minimum of experimental runs [30,31]. The factors considered for this study were pressure, temperature and co-solvent (ethanol) for SFE and temperature for MAE.
2. Results and Discussion
2.1. Effect of Conditions of Supercritical Fluid Extraction (SFE) and Microwave-Assisted Extraction (MAE) on Extraction Yield in Arthrospira platensis Extracts
SFE and MAE conditions were tested to cover a wide range of lipophilic compounds. Table 1 lists the different experiments performed (the first eight experiments correspond to SFE (n = 2) and the last two experiments correspond to MAE (n = 3)) along with the extraction yields (as % w/w) showed as the mean ± standard deviation. For SFE, the highest extraction yield (4.07% ± 0.14% w/w) was achieved with experiment BDF, at 450 bar, 60 °C and 53.33% of co-solvent. When working with CO2 at supercritical conditions, the yield increases with the addition of the co-solvent, meaning that the extracted compounds are of different polarity and, therefore, the process becomes less selective [32]. For yield extractions by SFE, the statistical analysis (ANOVA) showed that the temperature and co-solvent were significant factors (p < 0.05), where the highest temperature and highest amount of co-solvent gave the highest yield. In this response, pressure was not a significant factor (p > 0.05). For SFE, it has been reported that the main effect of a co-solvent is the solubility enhancement that results from an increase in solvent density and/or intermolecular interactions between the co-solvent and the solute [33]. According to our results, the highest yield was six times above the reported value for Arthrospira platensis extracts by SFE-CO2 [23], which can be attributed to the absence of co-solvent in the previous studies. In the case of MAE, the highest temperature (experiment H, 70 °C) produced the highest yield (4.27 ± 0.10) (p < 0.05). Therefore, this shows that the use of higher temperatures in SFE and MAE led to the highest yields in these extraction processes. Moreover, the highest yield in MAE can also be attributed to the solvents used, as they have been widely reported as effective for obtaining lipophilic compounds with different polarities from different microalgae/cyanobacteria sources [34,35].
Table 1.
Experimental matrix design for conditions for supercritical fluid extraction (SFE) and microwave-assisted extraction (MAE), and extraction yields of Arthrospira platensis extracts.
| Experiment * | P (bar) | T (°C) | CS (% of CO2) | Yield (% w/w) 1 |
|---|---|---|---|---|
| ADE | 150 | 60 | 26.70 | 3.09 ± 0.09 a,c,d |
| BDF | 450 | 60 | 53.33 | 4.07 ± 0.14 b |
| ACF | 150 | 45 | 53.33 | 3.05 ± 0.05 a,c,d |
| ADF | 150 | 60 | 53.33 | 3.13 ± 0.10 a,c.d |
| ACE | 150 | 45 | 26.70 | 1.21 ± 0.08 e |
| BCE | 450 | 45 | 26.70 | 1.40 ± 0.15 f |
| BDE | 450 | 60 | 26.70 | 1.96 ± 0.16 g |
| BCF | 450 | 45 | 53.33 | 1.72 ± 0.08 h |
| G | 1 | 50 | – | 2.03 ± 0.13 j |
| H | 1 | 70 | – | 4.27 ± 0.10 k |
* The extraction time for SFE and MAE was 50 and 15 min, respectively. Letters in the Experiment column are the acronym of the two levels of the tested variables: For SFE, Pressure (P) (150 (A), 450 (B)), Temperature (T) (45 (C), 60 (D)) and co-solvent (CS) (26.70 (E), 53.33 (F)). For MAE, Temperature (T) (50 (G), 70 (H)). 1 Values are represented as a mean ± standard deviation (n = 2 for SFE and n = 3 for MAE). a–h,j,k Different letters in the Yield column are significantly different (Least Significant Difference LSD test, p < 0.05).
2.2. Effect of Conditions of SFE and MAE on Carotenoids Content in Arthrospira platensis Extracts
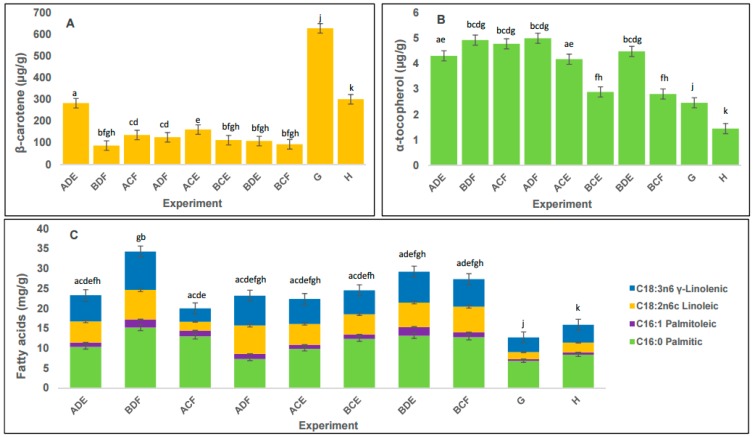
Figure 1A shows the values (means) of the concentration of carotenoids obtained for all experiments corresponding to SFE and MAE. For SFE, the highest content of carotenoids (283 ± 0.10 μg/g) was achieved (ADE) at 150 bar, 60 °C and 26.70% of co-solvent. Extraction with this pressure resulted in the highest efficiency in terms of carotenoid content; however, we observed that selectivity decreases at 450 bar (high pressure), because the experiment (BDF) that gave the highest yield uses different pressure and co-solvent levels compared with the experiment (ADE) which produced the highest content of carotenoids. This means that we extracted more compounds other than carotenoids, and these lipophilic compounds suffer a dilution effect. For the carotenoid content, the statistical analysis (ANOVA) showed that pressure was a significant factor (p < 0.05), while the temperature and co-solvent were not significant factors (p > 0.05). These results are similar to those obtained previously for Arthrospira species [22,36,37]. In the case of MAE, the highest content of carotenoids was obtained at experiment G, 50 °C (629 ± 0.13 μg/g) (p < 0.05). Furthermore, it was observed that a higher temperature (experiment H, 70 °C) produced the lowest content. These results agree with various reports, stating that the most sensitive carotenoids (violaxanthin) in MAE usually start degrading at temperatures higher than 60 °C [21]. Zhao et al. [29] reported excellent results with the solvents used in the MAE extraction of astaxanthin from Haematococcus pluvialis, where a high content of carotenoids was obtained. Also, our results agree with previous reports that suggest that efficient and fast extraction of carotenoids can be obtained using MAE without high pressure. Furthermore, the outstanding performance of MAE for carotenoids extraction could be attributed to the wall composition of A. platensis, since previous studies of pigment extractions report low efficiency of MAE with microalgae/cyanobacteria species that have a thick exopolysaccharide envelope [21]. Our results, in accordance with other reports for carotenoids extraction with SFE [38,39], show that the use of temperatures at about 60 °C led to the highest content of carotenoids from A. platensis. In the case of MAE, the highest content was observed at 50 °C.
Figure 1.
Functional lipophilic compound concentration of SFE and MAE extracts of Arthrospira platensis. (A) β-Carotene (μg/g); (B) α-tocopherol (μg/g); (C) fatty acids (mg/g). Values are presented as a mean ± standard deviation (n = 2 for SFE and n = 3 for MAE). Different letters in the bars are significantly different (LSD test, p < 0.05).
2.3. Effect of Conditions of SFE and MAE on Tocopherols Content in Arthrospira platensis Extracts
Figure 1B shows the concentration (means) of tocopherols obtained by all experiments of SFE and MAE. According to the results from the mass spectrometer (Supplementary Materials Figure S1 and Table S1), the only element of tocopherols found in the samples was α-tocopherol. For SFE, the highest content of α-tocopherol was 5.01 ± 0.05 μg/g with experiment ADF (150 bar, 60 °C and 53.33% of co-solvent). Temperature was a significant factor according to the ANOVA analysis (p < 0.05). The highest content of tocopherols was lower than previously reported [27]; this may be due to the use of ethanol as a co-solvent in this study. Other studies have reported the use of non-polar solvents for tocopherol extractions [40]. Moreover, the main difference may be due to physiological differences between strains of Arthrospira used in previous reports and in this study [41]. For MAE, the highest content of tocopherols was with experiment G, 50 °C (2.46 ± 0.09 μg/g) (p < 0.05), where the higher temperature negatively affected the content of tocopherols. This can be attributed to a possible degradation by heat [42]. Comparing the content of α-tocopherol in the extracts obtained with MAE, it was lower than the content obtained by SFE. Therefore, in this special case, the polarity obtained with SFE was better for these metabolites.
2.4. Effect of Conditions of SFE and MAE on Fatty Acids Content in Arthrospira platensis Extracts
Fatty acids represent one of the major building blocks of lipids [43]. They can be used in different applications such as biofuels, food, etc.; therefore, it is important to determine the fatty acid content in the extracts obtained with SFE and MAE. Figure 1C shows the concentration (means) of fatty acids obtained for all experiments corresponding to SFE and MAE. For SFE, the highest content of fatty acids (34.76 ± 0.08 mg/g) was obtained with experiment BDF, with 450 bar, 60 °C, and 53.33% of co-solvent. Extraction with high pressure, high temperature and high co-solvent level gives the highest proficiency in terms of fatty acids extraction. Moreover, pressure, temperature and co-solvent were all significant factors (p < 0.05). The highest fatty acid content was found in treatments with 450 bar experiments (BDF, BCE, BDE and BCF); as a consequence, pressure was an important parameter in the process of extraction of fatty acids by SFE. These results agree with previous reports of extraction of fatty acids with SFE. Machmudah et al. [44] reported that an increase in the extraction pressure generates an increase in the density of carbon dioxide and, consequently, an increment in the solvating power for fatty acids. In the case of temperature, the results agree with those previously reported for SFE [45,46,47]. An increase in temperature at 450 bar resulted in a higher content of fatty acids, while an increase in temperature at 150 bar resulted in a lower fatty acid content. These results are explained by the “crossover point”: this parameter depends on analyte-supercritical fluid interactions, and appears when the pressure of a supercritical fluid is constant while the temperature increases. For example, if this pressure is below the “crossover point”, an increase in temperature leads to lower solvent strength of the fluid due to the decrease in fluid density. Above the “crossover point”, an increase in temperature can improve the extraction efficiency despite the decrease in fluid density, since the vapor pressure of the analyte is increased [46]. In the case of the co-solvent (ethanol), it acts as an important factor in SFE for the enhancement of the solubility of lipophilic compounds (fatty acids, β-carotene, squalene among others) [33]. For fatty acids, a significant increase in the solubility has been reported when ethanol is used as a co-solvent, due to the interactions of hydrogen bonds [33]. The composition of the fatty acids fraction in SFE extracts was: palmitic acid—45.10%, palmitoleic acid—4.91%, linoleic acid—21.27% and γ-linoleic acid—27.71%, agreeing partially with previous reports [25,48]. For example, for palmitic acid in Arthrospira, Sajilata et al. [25] reported a higher content (53.09%), while Andrich et al. [48] reported a lower content (39.10%). These differences may be due to the effect of growth conditions of cyanobacteria on the composition of their metabolites [49].
For MAE the highest content of fatty acids (15.88 ± 0.06 mg/g) (p < 0.05) was obtained with experiment H, 70 °C. This result is similar to those obtained by extraction yield at the same temperature. In the case of MAE, temperature is an important factor, since higher temperature produces the highest yield of compounds, although with the limitation of the thermal stability of the compounds present in the extract. According to the obtained results, the content of tocopherols and carotenoids decreased with increasing temperature. The composition of fatty acids in MAE extracts was found to be palmitic acid—52.64%, palmitoleic acid—3.72%, linoleic acid—15.47% and γ-linoleic acid—28.15%. Moreover, the content of γ-linolenic acid from MAE extracts was similar to the content of γ-linolenic acid from SFE extracts. However, there were differences in the content of palmitic acid, where MAE extracts (52.64%) were higher than SFE extracts (45.10%). In respect to linoleic acid, SFE extracts had higher content (21.27%) than MAE extracts (15.47%). This was due to differences in the affinity of the solvents used in the extraction process. This information is important since MAE offers higher purity extracts than other extraction technologies [28], so this can be exploited according to the different applications of fatty acids. For instance, palmitic acid can be used for biofuels production [50,51], while γ-linolenic acid has shown positive effects in treating inflammatory conditions [52,53,54]. Therefore, these findings provide information for the production of functional lipophilic compounds such as palmitic and γ-linolenic acid from a natural and sustainable source.
3. Materials and Methods
3.1. Samples and Chemicals
Cyanobacteria samples (Arthrospira platensis) were obtained from Tecnologia Ambiental Biomex S.A. de C.V. [55] (Guadalajara, Mexico). Arthrospira was grown in open raceway ponds with modified Jourdan medium [56] during 45 days. The geographical location of the ponds was 20°14′0″ N, 103°35′0″ W. The biomass was harvested with a mesh, air dried to 20% moisture in order to maintain the stability of lipophilic compounds and stored under dry and dark conditions until the extracts were prepared (2 weeks). Methanol, ethyl acetate and light petroleum 60–80 °C boiling point were purchased from Tedia (Monterrey, Mexico). Fatty acids methyl esters, β-carotene, undecanoic acid, sulfuric acid and α-tocopherol were obtained from Sigma-Aldrich (Toluca, Mexico). Carbon dioxide (Industrial grade) was acquired from Praxair S.A. (Guadalajara, Mexico) and ethanol from Reactivos Guadalajara (Guadalajara, Mexico).
3.2. Supercritical Fluid Extraction (SFE)
All extractions were carried out in a pilot-scale plant for supercritical fluid extraction (Biomex, Mexico) with a 100 mL extraction vessel (Waters Thar SFC SFE 100 Equipment). All extractions were carried out using a flow of 15 g/min of CO2 for 50 min and ethanol/water 96% (v/v) was used as co-solvent. An automated back pressure regulator (ABPR) controlled the extraction pressure, carbon dioxide pump was purchased from Thar Instruments (Pittsburgh, PA, USA) and the co-solvent pump from Waters (Milford, CT, USA).
For each experiment, the extraction vessel was filled with 20 g of cyanobacteria powder (milled with mortar and pestle). The solvent/feed ratio (g/g) was 37.5 in order to obtain the highest possible yield for SFE [57]. Pressure and temperature in the separator were the same in all experiments (1 bar and 25 °C). The effects of three factors, pressure (P), temperature (T) and co-solvent (CS) on the extraction of functional lipophilic compounds were examined using a (150 (A), 450 (B)); T (°C) (45 (C), 60 (D)); CS (as % of CO2) (26.7 (E), 53.33 (F)). All extracts were concentrated in a vacuum rotary evaporator IKA Works (Wilmington, NC, USA) and kept under N2 at −20 °C in the dark. The selected response variables were extraction yield, concentration of carotenoids, concentration of tocopherols and concentration of fatty acids. The statistical method used for the data analysis was ANOVA, using the statistical package Minitab version 16 (State College, PA, USA). All extractions were performed in duplicate.
3.3. Microwave-Assisted Extraction (MAE)
All extractions were carried out in a microwave-assisted extraction equipment MARS 5® (CEM Corporation, Matthews, USA) with a 100 mL extraction vessel GreenChem™. The extraction vessel was made of PFA Teflon™. All extractions were done using methanol/ethyl acetate/light petroleum (1:1:1 v/v) at 400 W power, 1 bar, and 15 min extraction time. For each experiment, the extraction vessel was filled with a 0.06 ratio of biomass/solvent (w/v). The effect of temperature was tested at two experimental levels T (°C) (50 (G), 70 (H)). All extracts were centrifuged at 4500 rpm, 4 °C for 7 min; the supernatants were transferred to evaporation flasks for concentration in a vacuum rotary evaporator (IKA Works, Wilmington, NC, USA) and kept under N2 at −20 °C in the dark; the residual pellets were discarded. The selected response variables were extraction yield, concentration of carotenoids, concentration of tocopherols and concentration of fatty acids. The statistical method used for the data analysis was ANOVA, using the statistical package Minitab version 16 (State College, USA). All Extractions were performed in triplicate.
3.4. Carotenoids Analysis
All extracts were made up to a final volume of 15 and 5 mL with ethyl acetate for SFE and MAE extracts, respectively. Total carotenoid content was estimated with UV-Vis spectrophotometer Hach DR5000 (Loveland, CO, USA) at absorbance maxima of 450 nm for β-carotene. Carotenoid content was calculated using an of 2100 and was quantified as equivalents of β-carotene in mg/g.
3.5. Tocopherols Analysis
Tocopherols were analyzed with an Agilent 6890N GC (Agilent Technologies, Santa Clara, CA, USA) equipped with a HP-5MS capillary column (30 m, 0.25 mm i.d., 0.25 μm film thickness) and a mass spectrometer 5973 N as a detector. The carrier gas was helium at a flow rate of 0.8 mL/min. The column temperature was kept initially at 190 °C for 1 min, then gradually increased to 300 °C at 15 °C/min, and finally, maintained at 300 °C for 10 min. For GC-MS detection, an electron ionization system was used with 70 eV of energy. An aliquot of the extracts (1 µL) was injected automatically with 20:1 in split mode. Injector and detector temperatures were set at 270 and 230 °C, respectively.
3.6. Fatty Acids Analysis
Fatty acids were determined through derivatization to fatty acid methyl esters (FAMEs). To prepare FAMEs of the extracts, samples (2 mL) were mixed with 400 μL of internal standard (undecanoic acid 1000 mg/L in hexane/acetone 80:20 v/v) and 2 mL of methanol/sulfuric acid (93:7 v/v). These mixtures were maintained for 60 min at 80 °C. Then, the samples were allowed to cool down to room temperature. After addition of 5 mL of hexane, the samples were mixed in a vortex for 1 min and two phases were formed. The organic phase was transferred to a volumetric flask (10 mL), and the re-extraction of the polar phase was carried out three to four more times until a volume of 10 mL was obtained.
The FAMEs were analyzed with an Agilent 6890N GC (Agilent Technologies, Santa Clara, CA, USA), equipped with a SP2380 capillary column (30 m, 0.25 mm i.d., 0.20 μm film thickness) and flame ionization 19244–80560 as a detector. Nitrogen gas was used as carrier at a flow rate of 0.8 mL/min. The column temperature was kept initially at 50 °C for 2 min, then gradually increased to 240 °C at 4 °C/min, and finally, maintained at 240 °C for 1 min. An aliquot of the extracts (1 μL) was injected automatically with 20:1 in split mode. Injector and detector temperatures were set at 260 and 280 °C, respectively.
4. Conclusions
Arthrospira platensis showed a significant amount of carotenoids, tocopherols and fatty acids compared to other cyanobacteria species. These extracts represent a sustainable and safe source of functional lipophilic compounds. In general, SFE extractions with ethanol produced higher amounts of these compounds as compared with MAE extractions. This represents an important advantage because ethanol is an economical and environmentally friendly solvent. However, for carotenoids, MAE was more effective than SFE. The SFE extraction (BDF) with 450 bar, 60 °C and 53.33% of co-solvent gives the best extracts in terms of yield, tocopherols, carotenoids and fatty acids. For MAE, the extraction at 400 W and 50 °C (G) gives the best extracts in terms of tocopherols and carotenoids. For yield and fatty acids, the best extracts were prepared at 400 W and 70 °C (H). SFE and MAE proved to be suitable green extraction procedures for functional lipophilic compounds from A. platensis.
Acknowledgments
This work was financially supported by Tecnologia Ambiental Biomex S.A. de C.V. (PEI CONACYT project 221518, 2015), the strategic research group Emerging Technologies and Molecular Nutrition. Food, Pharmaceutical and Bioproducts Development and Environmental Bioprocess Group from Tecnologico de Monterrey, Campus Monterrey. The authors acknowledge the support provided by CONACYT (Mexico) in the form of PhD studies fellowship No. 325470 for Diego Armando Esquivel Hernandez. The authors are grateful to Rodrigo Ferraez-Villarreal, Zaira Falcon-Valdes and Esteban Lopez-Tavera for technical assistance with the experimental setup and our special thanks to QFB Carmen Salinas Salazar and IBT Angelica Mendoza Macias for critically reading this manuscript. The authors gratefully acknowledge the Water Center for Latin America and the Caribbean and Environmental Quality Center for their support with the laboratory facilities.
Abbreviations
| SFE | Supercritical fluid extraction |
| MAE | Microwave assisted extraction |
| P | Pressure |
| T | Temperature |
| CS | Co-solvent |
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/5/658/s1.
Author Contributions
Roberto Parra-Saldívar, José Rodríguez-Rodríguez and Sara P. Cuéllar-Bermúdez conceived the original idea and designed the experiments; Diego A. Esquivel-Hernández and Víctor H. López performed the experiments; Diego A. Esquivel-Hernández, José Rodríguez-Rodríguez and Magdalena Rostro-Alanis analyzed the data; Gibrán S. Alemán-Nava contributed reagents/materials/analysis tools; and Diego A. Esquivel-Hernández wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cardozo K.H., Guaratini T., Barros M.P., Falcão V.R., Tonon A.P., Lopes N.P., Campos S., Torres M.A., Souza A.O., Colepicolo P. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C. 2007;146:60–78. doi: 10.1016/j.cbpc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Cuellar-Bermudez S.P., Aguilar-Hernandez I., Cardenas-Chavez D.L., Ornelas-Soto N., Romero-Ogawa M.A., Parra-Saldivar R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015;8:190–209. doi: 10.1111/1751-7915.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naik S., Goud V.V., Rout P.K., Dalai A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010;14:578–597. doi: 10.1016/j.rser.2009.10.003. [DOI] [Google Scholar]
- 4.Cuellar-Bermudez S.P., Garcia-Perez J.S., Rittmann B.E., Parra-Saldivar R. Photosynthetic bioenergy utilizing CO2: An approach on flue gases utilization for third generation biofuels. J. Clean. Prod. 2015;98:53–65. doi: 10.1016/j.jclepro.2014.03.034. [DOI] [Google Scholar]
- 5.Paliwal C., Ghosh T., George B., Pancha I., Maurya R., Chokshi K., Ghosh A., Mishra S. Microalgal carotenoids: Potential nutraceutical compounds with chemotaxonomic importance. Algal Res. 2016;15:24–31. doi: 10.1016/j.algal.2016.01.017. [DOI] [Google Scholar]
- 6.Nuhu A.A. Spirulina (Arthrospira): An important source of nutritional and medicinal compounds. J. Mar. Biol. 2013;2013 doi: 10.1155/2013/325636. [DOI] [Google Scholar]
- 7.Kumari D.J., Babitha B., Jaffar S., Prasad M.G., Ibrahim M., Khan M.S.A. Potential Health Benefits of Spirulina platensis. Int. J. Adv. Pharm. Sci. 2011;2:417–422. [Google Scholar]
- 8.Dixit R.B., Suseela M. Cyanobacteria: Potential candidates for drug discovery. Antonie Van Leeuwenhoek. 2013;103:947–961. doi: 10.1007/s10482-013-9898-0. [DOI] [PubMed] [Google Scholar]
- 9.Campanella L., Crescentini G., Avino P. Chemical composition and nutritional evaluation of some natural and commercial food products based on Spirulina. Analusis. 1999;27:533–540. doi: 10.1051/analusis:1999130. [DOI] [Google Scholar]
- 10.Tocher D.R., Glencross B.D. Lipids and Fatty Acids. In: Cheng-Sheng L., Chhorn L., Delbert-M G.I., Webster C.D., editors. Dietary Nutrients, Additives and Fish Health. John Wiley & Sons; Hoboken, NJ, USA: 2015. p. 47. [Google Scholar]
- 11.Osborn H., Akoh C. Structured Lipids-Novel Fats with Medical, Nutraceutical, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2002;1:110–120. doi: 10.1111/j.1541-4337.2002.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 12.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Hoekman S.K., Broch A., Robbins C., Ceniceros E., Natarajan M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012;16:143–169. doi: 10.1016/j.rser.2011.07.143. [DOI] [Google Scholar]
- 14.Halim R., Gladman B., Danquah M.K., Webley P.A. Oil extraction from microalgae for biodiesel production. Bioresour. Technol. 2011;102:178–185. doi: 10.1016/j.biortech.2010.06.136. [DOI] [PubMed] [Google Scholar]
- 15.Chemat F., Vian M.A., Cravotto G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012;13:8615–8627. doi: 10.3390/ijms13078615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez J.L. Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds. CRC Press; Boca Raton, FL, USA: 2007. [Google Scholar]
- 17.Herrero M., Mendiola J.A., Cifuentes A., Ibáñez E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A. 2010;1217:2495–2511. doi: 10.1016/j.chroma.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Kerem Z., German-Shashoua H., Yarden O. Microwave-assisted extraction of bioactive saponins from chickpea (Cicer arietinum L.) J. Sci. Food Agric. 2005;85:406–412. doi: 10.1002/jsfa.1989. [DOI] [Google Scholar]
- 19.Routray W., Orsat V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess Technol. 2012;5:409–424. doi: 10.1007/s11947-011-0573-z. [DOI] [Google Scholar]
- 20.Chemat F., Lucchesi M., Smadja J., Favretto L., Colnaghi G., Visinoni F. Microwave accelerated steam distillation of essential oil from lavender: A rapid, clean and environmentally friendly approach. Anal. Chim. Acta. 2006;555:157–160. doi: 10.1016/j.aca.2005.08.071. [DOI] [Google Scholar]
- 21.Pasquet V., Chérouvrier J.-R., Farhat F., Thiéry V., Piot J.-M., Bérard J.-B., Kaas R., Serive B., Patrice T., Cadoret J.-P. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011;46:59–67. doi: 10.1016/j.procbio.2010.07.009. [DOI] [Google Scholar]
- 22.Mendiola J.A., Marín F.R., Hernandez S., Arredondo B.O., Señoráns F.J., Ibañez E., Reglero G. Characterization via liquid chromatography coupled to diode array detector and tandem mass spectrometry of supercritical fluid antioxidant extracts of Spirulina platensis microalga. J. Sep. Sci. 2005;28:1031–1038. doi: 10.1002/jssc.200500035. [DOI] [PubMed] [Google Scholar]
- 23.Mendiola J., Jaime L., Santoyo S., Reglero G., Cifuentes A., Ibanez E., Senorans F. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chem. 2007;102:1357–1367. doi: 10.1016/j.foodchem.2006.06.068. [DOI] [Google Scholar]
- 24.Mouahid A., Crampon C., Toudji S.-A.A., Badens E. Supercritical CO2 extraction of neutral lipids from microalgae: Experiments and modelling. J. Supercrit. Fluid. 2013;77:7–16. doi: 10.1016/j.supflu.2013.01.024. [DOI] [Google Scholar]
- 25.Sajilata M., Singhal R.S., Kamat M.Y. Supercritical CO2 extraction of γ-linolenic acid (GLA) from Spirulina platensis ARM 740 using response surface methodology. J. Food Eng. 2008;84:321–326. doi: 10.1016/j.jfoodeng.2007.05.028. [DOI] [Google Scholar]
- 26.Gómez-Coronado D.J., Ibanez E., Rupérez F.J., Barbas C. Tocopherol measurement in edible products of vegetable origin. J. Chromatogr. A. 2004;1054:227–233. doi: 10.1016/j.chroma.2004.08.072. [DOI] [PubMed] [Google Scholar]
- 27.Mendiola J.A., García-Martínez D., Rupérez F.J., Martín-Álvarez P.J., Reglero G., Cifuentes A., Barbas C., Ibanez E., Señoráns F.J. Enrichment of vitamin E from Spirulina platensis microalga by SFE. J. Supercrit. Fluid. 2008;43:484–489. doi: 10.1016/j.supflu.2007.07.021. [DOI] [Google Scholar]
- 28.Vali Aftari R., Rezaei K., Mortazavi A., Bandani A.R. The Optimized Concentration and Purity of Spirulina platensis C-Phycocyanin: A Comparative Study on Microwave-Assisted and Ultrasound-Assisted Extraction Methods. J. Food Process. Preserv. 2015;39:3080–3091. doi: 10.1111/jfpp.12573. [DOI] [Google Scholar]
- 29.Zhao L., Chen G., Zhao G., Hu X. Optimization of microwave-assisted extraction of astaxanthin from Haematococcus pluvialis by response surface methodology and antioxidant activities of the extracts. Sep. Sci. Technol. 2009;44:243–262. doi: 10.1080/01496390802282321. [DOI] [Google Scholar]
- 30.Montgomery D.C. Design and Analysis of Experiments. John Wiley & Sons; Hoboken, NJ, USA: 2008. pp. 203–224. [Google Scholar]
- 31.Sharif K., Rahman M., Azmir J., Mohamed A., Jahurul M., Sahena F., Zaidul I. Experimental design of supercritical fluid extraction—A review. J. Food Eng. 2014;124:105–116. doi: 10.1016/j.jfoodeng.2013.10.003. [DOI] [Google Scholar]
- 32.Lang Q., Wai C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta. 2001;53:771–782. doi: 10.1016/S0039-9140(00)00557-9. [DOI] [PubMed] [Google Scholar]
- 33.Güçlü-Üstündağ Ö., Temelli F. Solubility behavior of ternary systems of lipids, cosolvents and supercritical carbon dioxide and processing aspects. J. Supercrit. Fluid. 2005;36:1–15. doi: 10.1016/j.supflu.2005.03.002. [DOI] [Google Scholar]
- 34.Cheng C.-H., Du T.-B., Pi H.-C., Jang S.-M., Lin Y.-H., Lee H.-T. Comparative study of lipid extraction from microalgae by organic solvent and supercritical CO2. Bioresour. Technol. 2011;102:10151–10153. doi: 10.1016/j.biortech.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 35.Lee J.-Y., Yoo C., Jun S.-Y., Ahn C.-Y., Oh H.-M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010;101:S75–S77. doi: 10.1016/j.biortech.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 36.Canela A.P.R., Rosa P.T., Marques M.O., Meireles M.A.A. Supercritical fluid extraction of fatty acids and carotenoids from the microalgae Spirulina maxima. Ind. Eng. Chem. Res. 2002;41:3012–3018. doi: 10.1021/ie010469i. [DOI] [Google Scholar]
- 37.Careri M., Furlattini L., Mangia A., Musci M., Anklam E., Theobald A., von Holst C. Supercritical fluid extraction for liquid chromatographic determination of carotenoids in Spirulina Pacifica algae: A chemometric approach. J. Chromatogr. A. 2001;912:61–71. doi: 10.1016/S0021-9673(01)00545-3. [DOI] [PubMed] [Google Scholar]
- 38.Shi J., Yi C., Xue S.J., Jiang Y., Ma Y., Li D. Effects of modifiers on the profile of lycopene extracted from tomato skins by supercritical CO2. J. Food Eng. 2009;93:431–436. doi: 10.1016/j.jfoodeng.2009.02.008. [DOI] [Google Scholar]
- 39.Kassama L.S., Shi J., Mittal G.S. Optimization of supercritical fluid extraction of lycopene from tomato skin with central composite rotatable design model. Sep. Purif. Technol. 2008;60:278–284. doi: 10.1016/j.seppur.2007.09.005. [DOI] [Google Scholar]
- 40.Sánchez-Machado D., López-Hernández J., Paseiro-Losada P. High-performance liquid chromatographic determination of α-tocopherol in macroalgae. J. Chromatogr. A. 2002;976:277–284. doi: 10.1016/S0021-9673(02)00934-2. [DOI] [PubMed] [Google Scholar]
- 41.Tomaselli L., Boldrini G., Margheri M. Physiological behaviour of Arthrospira (Spirulina) maxima during acclimation to changes in irradiance. J. Appl. Phycol. 1997;9:37–43. doi: 10.1023/A:1007956210329. [DOI] [Google Scholar]
- 42.Sabliov C.M., Fronczek C., Astete C., Khachaturyan M., Khachatryan L., Leonardi C. Effects of temperature and UV light on degradation of α-tocopherol in free and dissolved form. J. Am. Oil Chem. Soc. 2009;86:895–902. doi: 10.1007/s11746-009-1411-6. [DOI] [Google Scholar]
- 43.Christie W., Han X. Lipid Analysis: Isolation, Separation, Identification and Lipidomic Analysis. 4th ed. Oily Press; Bridgwater, UK: 2010. [Google Scholar]
- 44.Machmudah S., Kawahito Y., Sasaki M., Goto M. Supercritical CO2 extraction of rosehip seed oil: Fatty acids composition and process optimization. J. Supercrit. Fluid. 2007;41:421–428. doi: 10.1016/j.supflu.2006.12.011. [DOI] [Google Scholar]
- 45.Cheung P.C. Temperature and pressure effects on supercritical carbon dioxide extraction of N-3 fatty acids from red seaweed. Food Chem. 1999;65:399–403. doi: 10.1016/S0308-8146(98)00210-6. [DOI] [Google Scholar]
- 46.Turner C., King J.W., Mathiasson L. Supercritical fluid extraction and chromatography for fat-soluble vitamin analysis. J. Chromatogr. A. 2001;936:215–237. doi: 10.1016/S0021-9673(01)01082-2. [DOI] [PubMed] [Google Scholar]
- 47.Crampon C., Boutin O., Badens E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011;50:8941–8953. doi: 10.1021/ie102297d. [DOI] [Google Scholar]
- 48.Andrich G., Zinnai A., Nesti U., Venturi F. Supercritical fluid extraction of oil from microalga Spirulina (Arthrospira) platensis. Acta Alimentaria. 2006;35:195–203. doi: 10.1556/AAlim.35.2006.2.6. [DOI] [Google Scholar]
- 49.Cuellar-Bermudez S.P., Romero-Ogawa M.A., Vannela R., Lai Y.S., Rittmann B.E., Parra-Saldivar R. Effects of light intensity and carbon dioxide on lipids and fatty acids produced by Synechocystis sp. PCC6803 during continuous flow. Algal. Res. 2015;12:10–16. doi: 10.1016/j.algal.2015.07.018. [DOI] [Google Scholar]
- 50.Carmo A.C., de Souza L.K., da Costa C.E., Longo E., Zamian J.R., da Rocha Filho G.N. Production of biodiesel by esterification of palmitic acid over mesoporous aluminosilicate Al-MCM-41. Fuel. 2009;88:461–468. doi: 10.1016/j.fuel.2008.10.007. [DOI] [Google Scholar]
- 51.Giro-Paloma J., Konuklu Y., Fernández A. Preparation and exhaustive characterization of paraffin or palmitic acid microcapsules as novel phase change material. Solar Energy. 2015;112:300–309. doi: 10.1016/j.solener.2014.12.008. [DOI] [Google Scholar]
- 52.Horrobin D. The role of essential fatty acids and prostaglandins in the premenstrual syndrome. J. Reprod. Med. 1983;28:465–468. [PubMed] [Google Scholar]
- 53.Biessels G.J., Smale S., Duis S.E., Kamal A., Gispen W.H. The effect of γ-linolenic acid–α-lipoic acid on functional deficits in the peripheral and central nervous system of streptozotocin-diabetic rats. J. Neurol. Sci. 2001;182:99–106. doi: 10.1016/S0022-510X(00)00456-1. [DOI] [PubMed] [Google Scholar]
- 54.Bordoni A., Biagi P., Masi M., Ricci G., Fanelli C., Patrizi A., Ceccolini E. Evening primrose oil (Efamol) in the treatment of children with atopic eczema. Drugs Exp. Clin. Res. 1987;14:291–297. [PubMed] [Google Scholar]
- 55.Biomex. [(accessed on 25 April 2016)]. Available online: https://biomexalgae.com.
- 56.Jourdan J. Cultivate Your Spirulina; Artisan Culture Manual for the Production of Spirulina. 3rd ed. Antenna Technologies; Gèneve, Switzerland: 1996. pp. 14–17. [Google Scholar]
- 57.Valderrama J.O., Perrut M., Majewski W. Extraction of astaxantine and phycocyanine from microalgae with supercritical carbon dioxide. J. Chem. Eng. Data. 2003;48:827–830. doi: 10.1021/je020128r. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.