Abstract
Cisplatin and its derivatives are the main metallodrugs used in cancer therapy. However, low selectivity, toxicity and drug resistance are associated with their use. The zinc(II) (ZnII) thiosemicarbazone complexes [Zn(atc-Et)2] (1) and [Zn(atc-Ph)2] (2) (atc-R: monovalent anion of 2-acetylpyridine N4-R-thiosemicarbazone) were synthesized and fully characterized in the solid state and in solution via elemental analysis, Fourier transform infrared (FTIR), ultraviolet-visible (UV-Vis) and proton nuclear magnetic resonance (1H NMR) spectroscopy, conductometry and single-crystal X-ray diffraction. The cytotoxicity of these complexes was evaluated in the HepG2, HeLa, MDA-MB-231, K-562, DU 145 and MRC-5 cancer cell lines. The strongest antiproliferative results were observed in MDA-MB-231 and HepG2 cells, in which these complexes displayed significant selective toxicity (3.1 and 3.6, respectively) compared with their effects on normal MRC-5 cells. In vivo studies were performed using an alternative model (Artemia salina L.) to assure the safety of these complexes, and the results were confirmed using a conventional model (BALB/c mice). Finally, tests of oral bioavailability showed maximum plasma concentrations of 3029.50 µg/L and 1191.95 µg/L for complexes 1 and 2, respectively. According to all obtained results, both compounds could be considered as prospective antiproliferative agents that warrant further research.
Keywords: zinc(II) complexes, cancer, antiproliferative activity, Artemia salina L., acute toxicity, oral bioavailability
1. Introduction
Cancer consists of a complex group of diseases in which uncontrolled cell growth invades tissues and organs and potentially spreads to other regions of the body (metastasis). Mutated cells divide rapidly and tend to behave aggressively, causing the formation of tumors or malignancies [1]. Today, cancer is responsible for one out of every eight deaths worldwide—more than human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), tuberculosis and malaria combined. Therefore, cancer is clearly a public health problem. The incidence of cancer is growing at an alarming rate, and simply due to the growth and aging of the world population, approximately 21.7 million new cases and 13.0 million deaths are expected by 2030 [2]. The modes of cancer treatment include surgery, chemotherapy, radiotherapy, transplantation, targeted therapy, immune therapy, and photodynamic therapy. Chemotherapy is an important cancer treatment option. However, its main drawback is non-selective toxicity, as chemotherapeutic agents act on both tumor cells (the targets) and normal cells [3].
The development of cisplatin represents one of the most significant advances in the treatment of various cancers. Since its approval in 1978 by the U.S. Food and Drug Administration (FDA), it has been widely used to treat a variety of solid and hematological tumors. However, there are problems associated with its use related to its low therapeutic index and high potential toxicity to the kidneys (nephrotoxicity) and the gastrointestinal tract [4,5]. Due to the frequent use of cisplatin alone or in combination with other chemotherapy drugs, drug resistance is observed in numerous types of cancer, such as ovarian cancer, lung cancer, pancreatic cancer and nasopharyngeal carcinoma [6,7,8,9]. Thus, several drug resistance mechanisms, including drug efflux, inhibition of apoptosis, and increased DNA repair, have been studied and described [10].
The study of inorganic chemistry in recent years has been demonstrated to be effective in the development of new therapeutic agents. Drugs based on metals are increasing in importance in therapies for cancer and other diseases [11,12]. It is known that certain metals such as zinc (Zn), a trace element in the human body, participate in various reactions in biological systems, in addition to their presence in metalloenzymes fundamental for our body [13]. Studies have noted that the activity of bio-metals, such as Zn, is modified after the formation of coordination compounds, and the thermodynamic and kinetic properties of these metals can also be modified to regulate their biological activities. These properties include permeability, lipophilicity and formation constants, which can be critical for transition metals in reaching target sites [14]. Previously studied Zn complexes showed antiproliferative activity on different tumor cell lines that was greater than that of their free ligands [15].
Within this context, our group has studied the effect of metal coordination on bioactivity. In the present study, we synthesized and characterized new ZnII complexes, [Zn(atc-Et)2] (1) and [Zn(atc-Ph)2] (2), and evaluated their in vitro antiproliferative activity, their toxicological safety using an alternative model (Artemia salina L.) as well as a BALB/c mouse model and, finally, their in vivo oral bioavailability using a rapid screening method.
2. Results and Discussion
2.1. Chemistry
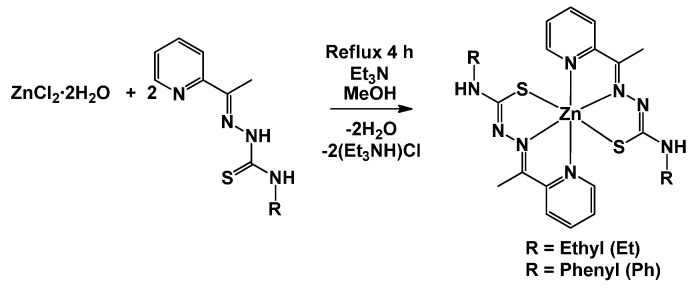
Reacting Hatc-Et or Hatc-Ph with ZnCl2·2H2O in the presence of Et3N under reflux in MeOH produced pure yellow precipitates of the Zn complexes at reasonable yields (Scheme 1). Complex 1 was very soluble in CH2Cl2 but was less soluble in methanol or ethanol. Alternatively, complex 2 was reasonably soluble only in dimethyl sulfoxide (DMSO). Elemental analyses and molar conductivity measurements produced results in accordance with the formation of neutral complexes of the type [Zn(atc-R)2].
Scheme 1.
Synthesis of the complexes.
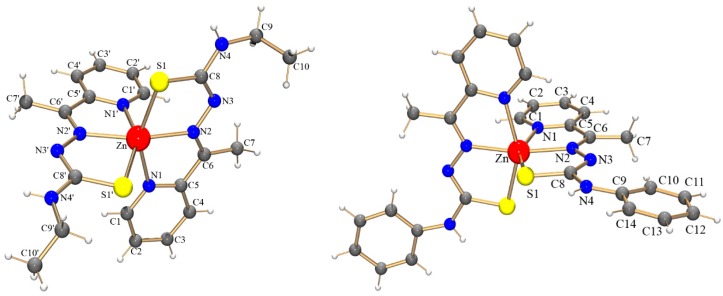
The Fourier transform infrared (FTIR) and proton nuclear magnetic resonance (1H NMR) spectra of these complexes were consistent with the N,N,S-coordination of the atc-R1− ligands to the ZnII metal center. Single-crystal X-ray diffraction was performed to determine the structures of the complexes (Figure 1). Table 1 summarizes selected bond lengths and angles. The thiosemicarbazone ligands coordinate to ZnII via their pyridine and azomethine nitrogen atoms as well as their sulfur atom, forming two four-membered chelated rings with a metal center. In both complexes, the tridentate ligands are nearly planar and perpendicular to each other. The coordination spheres of both complexes are best described as possessing a distorted octahedral geometry.
Figure 1.
Molecular structure determined for [Zn(atc-Et)2] (1) (left) and [Zn(atc-Ph)2] (2) (right) with atom labels.
Table 1.
Selected bond lengths (Å) and angles (°) for [Zn(atc-Et)2] (1) and [Zn(atc-Ph)2] (2).
| 1 | 2 | |
|---|---|---|
| Bond Lengths | ||
| Zn(1)-N(2)/Zn(1)-N(2’) | 2.1586(18)/2.1527(17) | 2.1549(17) |
| Zn(1)-N(1)/Zn(1)-N(1’) | 2.203(2)/2.2482(18) | 2.2139(18) |
| Zn(1)-S(1)/Zn(1)-S(1’) | 2.4539(10)/2.4464(9) | 2.4452(6) |
| S(1)-C(8)/S(1’)-C(8’) | 1.739(2)/1.719(2) | 1.729(2) |
| N(3)-C(8)/N(3’)-C(8’) | 1.321(3)/1.338(3) | 1.323(3) |
| Angles | ||
| N(2’)-Zn(1)-N(2) | 161.22(5) | 169.60(9) |
| N(2’)-Zn(1)-N(1) | 104.79(7) | 98.88(6) |
| N(2)-Zn(1)-N(1) | 73.95(7) | 73.62(7) |
| N(2’)-Zn(1)-N(1’) | 73.58(7) | 73.62(7) |
| N(2)-Zn(1)-N(1’) | 87.64(7) | 98.88(6) |
| N(1)-Zn(1)-N(1’) | 87.97(6) | 90.55(9) |
| N(2’)-Zn(1)-S(1’) | 79.18(6) | 79.08(5) |
| N(1)-Zn(1)-S(1) | 151.47(5) | 152.54(5) |
| N(1’)-Zn(1)-S(1’) | 150.67(5) | 152.54(5) |
| C(8)-S(1)-Zn(1) | 96.13(8) | 95.93(7) |
| C(1)-N(1)-C(5) | 118.95(19) | 118.58(19) |
The Zn-N bond lengths are similar but slightly shorter when involving the azomethine nitrogen atom. The Zn-S bonds for complexes 1 and 2, with lengths of approximately 2.45 Å, are very similar to those observed in similar complexes reported in the literature [16]. The bond lengths involving the donor atoms and the ZnII metal center in the complex [Zn(atc-Et)2] are slightly shorter than those in the analogous MnII complex [Mn(atc-Et)2] [17]. This result is in agreement with the smaller ionic radius of ZnII than that of MnII. In the CoIII cationic complex [Co(atc-Et)2]+, the bond lengths surrounding the metal center remain shorter than those in similar complexes containing MnII and ZnII, as expected [18].
2.2. Cytotoxic Activity toward MRC-5, HepG2, HeLa, MDA-MB-231, K-562 and DU 145 Cell Lines
The search for new molecules that exhibit selectivity for a tumor cell but not a normal cell is a major challenge in anticancer drug research [19]. Therefore, we are seeking new complexes with selectivity indices of greater than three, indicating that they are cytotoxic to tumor cells at a concentration at least three-fold lower than the concentration in which they are cytotoxic to normal cells [20]. Concentration inhibiting cellular proliferation by 50% (IC50) values are presented in Table 2. For comparison purposes, the cytotoxicity of cisplatin as a reference antitumor drug was also evaluated under the same conditions.
Table 2.
Cytotoxicity (IC50) of ZnCl2·6H2O, free ligands, complexes 1 and 2 and cisplatin against tumor cell lines and normal (MRC-5) cells.
| Complexes | IC50 (µM) ± SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MRC-5 1 | HepG2 2 | SI * | HeLa 2 | SI * | MDA-MB-231 2 | SI * | K-562 2 | SI * | DU 145 2 | SI * | |
| ZnCl2·6H2O | 239.9 ± 16.8 | 698.4 ± 10.9 | 0.3 | 864.5 ± 14.0 | 0.3 | 475.4 ± 64.3 | 0.5 | 1088.7 ± 73.8 | 0.2 | 382.9 ± 7.8 | 0.6 |
| Hatc-Et | 9.0 ± 0.0 | 552.8 ± 45.0 | 0.0 | 746.0 ± 23.2 | 0.0 | 903.5 ± 65.3 | 0.0 | 420.3 ± 3.7 | 0.0 | 531.5 ± 12.6 | 0.0 |
| Hatc-Ph | 7.4 ± 0.0 | 36.3 ± 1.5 | 0.2 | 25.0 ± 0.2 | 0.3 | 24.3 ± 5.0 | 0.3 | 11.1 ± 0.6 | 0.7 | 15.1 ± 2.1 | 0.5 |
| [Zn(atc-Et)2] (1) | 12.1 ± 4.0 | 7.1 ± 3.7 | 1.7 | 102.0 ± 17.6 | 0.1 | 3.9 ± 0.0 | 3.1 | 52.0 ± 8.6 | 0.2 | 9.4 ± 3.4 | 1.3 |
| [Zn(atc-Ph)2] (2) | 12.1 ± 3.0 | 3.3 ± 0.0 | 3.6 | 325.0 ± 58.4 | 0.0 | 5.5 ± 3.1 | 2.2 | 14.1 ± 3.8 | 1.0 | 34.1 ± 7.0 | 0.3 |
| Cisplatin | 46.2 ± 4.9 | 60.3 ± 15.1 | 0.8 | 85.0 ± 10.1 | 0.5 | 143.3 ± 3.3 | 0.3 | 75.4 ± 6.0 | 0.6 | 56.5 ± 6.8 | 0.8 |
* The SI of each complex was calculated as the ratio of the IC50 for MRC-5 cells 1/IC50 for tumor 2 cells. Substances with an SI > 3 are considered to be promising. IC50: concentration inhibiting cellular proliferation by 50%; SD: standard deviation; SI: selectivity index.
The free ligand Hatc-Et and the precursor salt ZnCl2·6H2O was cytotoxic to normal MRC-5 cells and showed no effects on all tumor cells tested; therefore, these compounds were considered to be inactive on tumor cells. Alternatively, Hatc-Ph displayed activity on the tumor cell lines as well as cytotoxicity to MRC-5 cells, resulting in low selectivity. In two cases, the coordination of the ligand to Zn led to the formation of complexes with higher antiproliferative activities. Complex 1 showed selective cytotoxicity to the MDA-MB-231 cell line with a selectivity index (SI) of 3.1, and complex 2 showed an SI of 3.6 in the HepG2 cell line. Both complexes were demonstrated to be more active and selective than cisplatin in all cell lines tested.
Importantly, DMSO (solvent control) alone showed no cytotoxic activity toward the MRC-5, HepG2, HeLa, MDA-MB-231, K-562 and DU 145 cell lines.
There is no report available in the literature regarding the SI of the antitumor activities of ZnII complexes, and this lack of data prevented us from comparing the results obtained in this study with previous findings.
2.3. Toxicity in Artemia salina L.
Complexes 1 and 2 showed 3.3- and 5.7-fold lower toxicity in the microcrustacean Artemia salina L., respectively, relative to cisplatin. The 50% lethal concentration (LC50) values, defined as the concentration of a substance causing lethality to 50% of the organisms exposed, are presented in Table 3.
Table 3.
LC50 of zinc complexes 1 and 2 and cisplatin in Artemia salina L.
| Complexes | LC50 (µM) ± SD |
|---|---|
| [Zn(atc-Et)2] (1) | 962.6 ± 19.2 |
| [Zn(atc-Ph)2] (2) | 1672.7 ± 15.0 |
| Cisplatin | 295.1 ± 35.1 |
There are a few reports in the literature using Artemia salina L. to determine the toxicity of novel substances, and most of these studies were conducted using plant extracts [21,22]. However, with the increasing need to use alternative models, new toxicity studies of organic and inorganic molecules using microcrustaceans as model organisms are emerging [23,24].
The ZnII complexes investigated in this study were less toxic to the microcrustacean than the perchlorate compound of bis(isoniazid) ZnII hexahydrate (LC50 = 268 µM), as reported in the literature by Freitas et al. (2011) [25].
Copper(II) (CuII) complexes with 2-pyridineformamide-derived thiosemicarbazones studied by Ferraz et al. (2009) showed LC50 values ranging from 1.2 to 7.2 µM against Artemia salina L.; those values smaller than the LC50 values observed for the present ZnII complexes [23]. A comparison between the data on cytotoxicity to cell lines and toxicity in Artemia salina L. in the present study indicated that complexes 1 and 2 showed selectivity toward the MDA-MB-231 and HepG2 cell lines, respectively, but that neither complex was toxic to the microcrustacean. DMSO, used as the solvent, was not toxic to Artemia salina L.
2.4. Acute Toxicity in Vivo
The samples were used at a dose of 1000 mg/kg due to the low solubility of the complexes in sunflower oil, which further hindered the performance of the gavage technique in the animals. The Globally Harmonized System of Classification and Labelling of Chemicals (GSH) classifies oral toxic agents into five categories, and the fourth category includes the substances that display median lethal dose (LD50) >300 mg/kg ≤2000 mg/kg. Therefore, the dose used in our experiments is within the range recommended by the Organization for Economic Cooperation Development (OECD) (2001) [26].
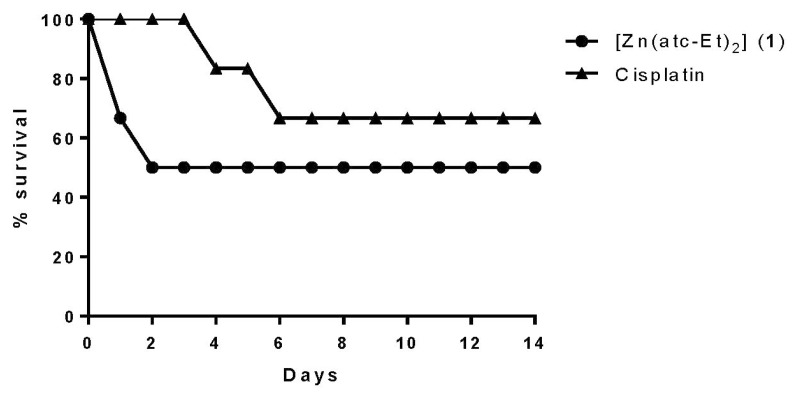
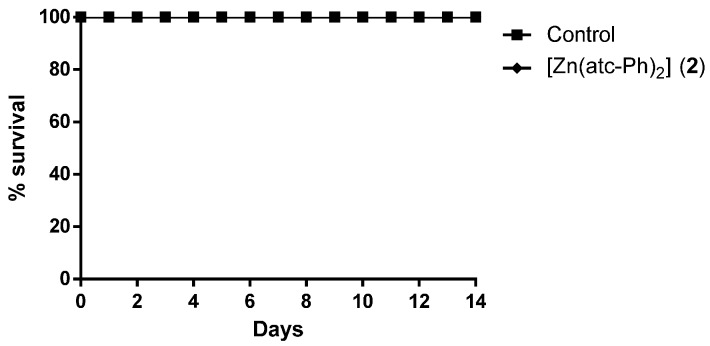
Complex 1 at a dose of 1000 mg/kg body weight caused mortality in all BALB/c mice during the experiment, so it was necessary to administer a dose of 500 mg/kg body weight via gavage to determine its LD50 (Figure 2). The solvent control and complex 2 at the concentration of 1000 mg/kg body weight showed no mortality, as shown in Figure 3. Cisplatin led to the death of two animals when administered at the dose of 500 mg/kg body weight. The effects of cisplatin at higher concentrations are reported in the literature [27].
Figure 2.
Percentage survival of BALB/c mice over 14 days of drug treatment at the concentration of 500 mg/kg body weight.
Figure 3.
Percent survival of BALB/c mice over 14 days of drug treatment at the concentration of 1000 mg/kg body weight.
The animals were monitored over 14 days of drug treatment, and behavioral parameters were evaluated (Hippocratic screening). However, no behavioral changes were observed.
According to the data obtained in this study concerning the toxicity of these complexes to Artemia salina L. and BALB/c mice, the results are in agreement. Specifically, complex 2 was less toxic than complex 1 in both the alternative and in vivo models.
Organ weight has long been accepted as a sensitive indicator of chemically induced changes to organs. In toxicological experiments, comparison of organ weights between treated and untreated groups of animals have conventionally been used to evaluate the toxic effect of a treatment [28,29].
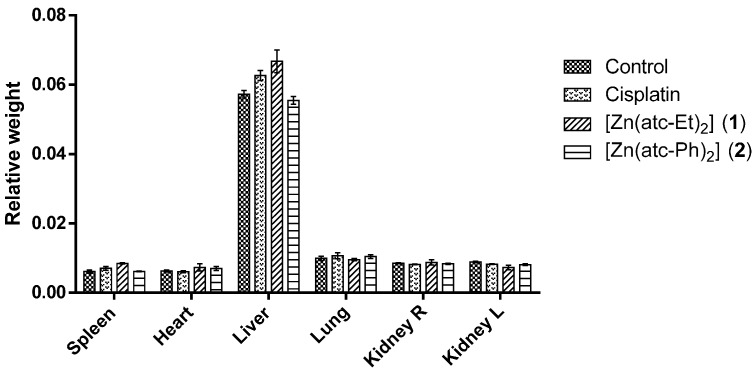
Macroscopic analysis of the organs showed no morphological changes and revealed no statistically significant difference in the mean ratio of organ weight to body weight among the animals treated with either of the ZnII complexes or cisplatin compared to the control animals (Figure 4).
Figure 4.
Relative weight (mean ratio of organ weight to body weight of BALB/c mice) and standard deviation (SD). R: right ; L: left.
2.5. Quantification of the Enzymatic Activity Levels of Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) in the Serum of BALB/c Mice
Quantification of enzymatic activity levels of AST and ALT is important in the diagnosis of heart and liver damage caused by heart attack, drug toxicity, or infection. AST is present in other tissues such as heart, skeletal muscle, kidney, brain, and pancreatic tissues. Therefore, AST is much more widely expressed than ALT, which is primarily expressed in the liver. Therefore, upon liver damage, both enzymes are released into serum/plasma. In contrast, if the lesion is purely hepatic, the serum ALT level increases slightly more than the serum AST level [30].
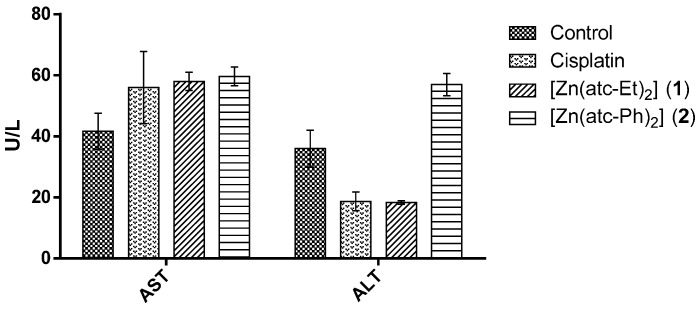
Regarding the enzymatic activity levels (of AST and ALT) in serum from mice, no significant difference was observed between the groups treated with either of the ZnII complexes or with cisplatin and the control group, as shown in Figure 5 and as demonstrated in another study [31]. This result confirms that no change in liver toxicity occurred in BALB/c mice.
Figure 5.
Quantification of the serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activity levels in BALB/c mice. U/L: units per liter.
As shown in Figure 5, the levels of ALT in the samples from mice treated with cisplatin or complex 1 were the half of the control levels. However, analysis of variance (ANOVA) and Dunnett’s test did not indicate that these differences were significant (p > 0.05).
2.6. Oral Bioavailability in Vivo
The in vivo test of oral bioavailability consists of a rapid screen that reveals whether an administered complex or the final product was absorbed into the bloodstream. The calibration curves used for quantitative evaluation of plasma samples showed an R2 correlation coefficient of 0.999, and the detection limit (DL) for Zn metal was 480 µg/L. For cisplatin (platinum (Pt) of atomic mass 195 u), the DL was 0.0003954 µg/L, and the quantification limit (QL) was 0.001318 µg/L.
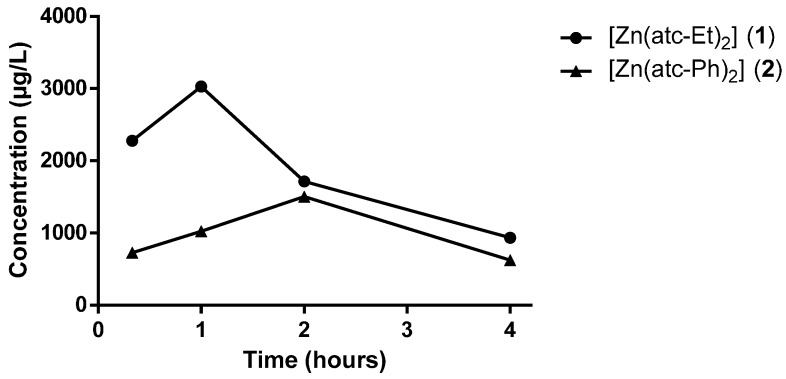
Figure 6 shows the time-concentration profiles for complexes 1 and 2 in plasma. The values presented in the figure were calculated relative to the control sample because the body itself contains a baseline concentration of Zn.
Figure 6.
Time-concentration profiles of ZnII complexes in plasma after a single administration via oral gavage at a dose of 300 mg/Kg body weight.
The plasma concentrations of complex 1 at 20 min, 1 h, 2 h and 4 h after administration were 2278.50 µg/L, 3029.50 µg/L, 1715.50 µg/L and 935.55 µg/L, respectively. The concentration of the complex at 20 min was the peak concentration observed (Cmax). For complex 2, the plasma concentrations at the same sampling time points were 727.83 µg/L, 1023.55 µg/L, 1191.95 µg/L and 624.70 µg/L, respectively. For this complex, Cmax in plasma was detected at 2 h, after which the plasma concentration of complex 2 decayed.
The time-concentration profiles of both complexes are in agreement with the findings of a previous study [32]. The differences in the profiles of ZnII complexes 1 and 2 observed in this study are probably due to their structural differences regarding the composition of their ligands.
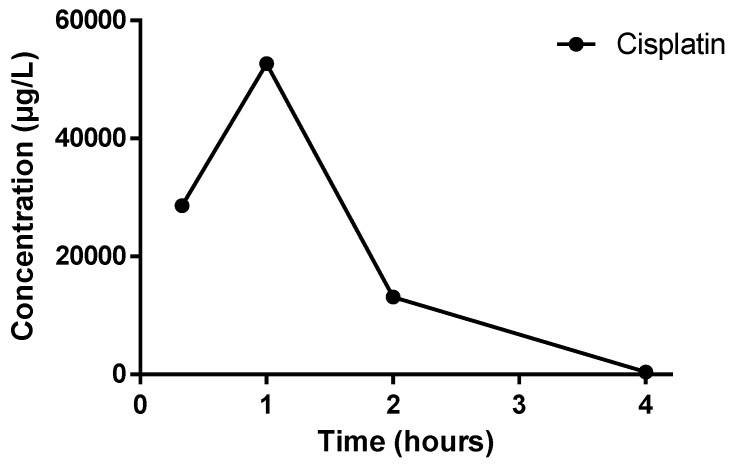
The platinum (Pt) concentration was quantified to determine the time of Cmax for the orally bioavailable drug cisplatin (Figure 7); Cmax of platinum ((Pt) of atomic mass 195 u)was determined to be 1 h after cisplatin administration.
Figure 7.
Time-concentration profile of cisplatin in plasma after a single administration via oral gavage at a dose of 300 mg/Kg body weight.
The complex profiles show that 1 and cisplatin display similar time points of Cmax occurrence (Tmax, time of maximum compound concentration), both of which were approximately 1 h. In contrast, 2 displayed a Tmax of 2 h.
3. Experimental
3.1. Inorganic Complexes
2-Acetylpyridine, 4-ethyl-3-thiosemicarbazide, 4-phenyl-3-thiosemicarbazide, ZnCl2·2H2O and solvents were obtained commercially and used without further purification. The ligands Hatc-Et and Hatc-Ph were prepared as reported [17,18]. FTIR spectra between 400 and 4000 cm−1 were measured via the Potassium Bromide (KBr) pellet method using a Shimadzu IR Prestige-21 spectrophotometer (Shimadzu®, Tsukinowa, Otsu, Japan). Elemental analyses were performed using a Perkin-Elmer CHN 2400 elemental analyzer (Perkin Elmer®, Waltham, MA, USA). The conductivities of the complexes were measured in CH2Cl2 solution using an Orion Star Series conductometer (Thermo Scientific®, San Diego, CA, USA). Ultraviolet-visible (UV-Vis) spectra of solutions diluted in DMSO were measured using a Shimadzu UV-1800 spectrophotometer (Shimadzu®, Tsukinowa, Otsu, Japan). Proton nuclear magnetic resonance (1H NMR) spectra were acquired using an Agilent 400/54 Premium Shielded 9.4 T NMR spectrometer (Agilent®, Santa Clara, CA, USA), set to 399.8 MHz for 1H. The NMR spectra were internally referenced to the spectrum of Tetramethylsilane (TMS). Yellow crystals were produced via slow evaporation of the mother solution of the two ZnII complexes at room temperature. Data collection was performed at 296 K by applying Mo-Kα radiation (λ = 71.073 pm) using a Bruker Kappa APEX II Duo diffractometer (Bruker®, Karlsruhe, Germany). The multi-scan method was applied for absorption correction. The structures were solved with SHELXS97 software (Gottingen, Germany) [33] using direct methods, and all non-hydrogen atoms were refined with anisotropic displacement parameters in SHELXL97. The hydrogen atoms were calculated at idealized positions using the riding model option of SHELXL97 [33]. The asymmetric unit in the crystal structure of complex 2 contains only half of the entire molecule. Table 4 presents more detailed information about the structure determinations. The structural data were deposited at The Cambridge Crystallographic Data Centre (CCDC) as entries CCDC 1436883 (1) and CCDC1436884 (2), and these data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
Table 4.
Crystal data and structural refinement for [Zn(atc-Et)2] (1) and [Zn(atc-Ph)2] (2).
| 1 | 2 | |
|---|---|---|
| Empirical formula | C20H26N8S2Zn | C28H26N8S2Zn |
| Formula weight | 507.98 | 604.06 |
| Temperature (K) | 293(2) | 296(2) |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21 | C2/c |
| Unit cell dimensions | a = 8.8919(5) Å α = 90° b = 14.738(5) Å β = 103.917(5)° c = 9.248(5) Å γ = 90° |
a = 13.4421(10) Å α = 90° b = 18.9640(14) Å β = 95.5940(10)° c = 10.9390(8) Å γ = 90° |
| V (Å3) | 1,176.4(10) | 2775.2(4) |
| Z | 2 | 4 |
| Density (calculated) (Mg m−3) | 1.434 | 1.446 |
| Absorption coefficient (mm−1) | 1.246 | 1.069 |
| Crystal size/mm3 | 0.66 × 0.55 × 0.29 | 0.41 × 0.09 × 0.08 |
| Theta range for data collection | 2.36 to 25.12 | 1.86 to 25.07 |
| Index ranges | −10←h←10, −17←k←17, −11←l←9 | −16←h←16, −20←k←22, −12←l←13 |
| Reflections collected | 8001 | 8543 |
| Independent reflections | [R(int) = 0.0161] | [R(int) = 0.0227] |
| Abs. Corr. | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Final R indices [I > 2sigma(I)] | R1 = 0.0184, wR2 = 0.0484 | R1 = 0.0296, wR2 = 0.0668 |
| R indices (all data) | R1 = 0.0189, wR2 = 0.0485 | R1 = 0.0368, wR2 = 0.0699 |
| Goodness-of-fit on F2 | S = 1.047 | S = 1.037 |
V: unit cell volume; Z: number of molecules in unit cell; R1 = ; wR2 = where Fo and Fc are observed and calculated structure factors; I: reflection intensity; GoodF = S = , where n is the number of reflections and p is the total number of parameters refined.
3.2. Synthesis of the Complexes
The ZnII complexes were synthesized by adding one equivalent (0.25 mmol) of ZnCl2·2H2O to a solution containing two equivalents (0.50 mmol) of the ligand Hatc-R in 15 mL of MeOH supplemented with 3 drops of Et3N. The resulting solutions were stirred for 4 h under reflux. Subsequently, the yellow precipitate that formed was filtered off, washed with cold methanol and hexane and dried under vacuum. Via slow concentration of the mother solutions, yellow crystals suitable for X-ray diffraction were obtained for both complexes.
[Zn(atc-Et)2] (1): Color: Yellow. Yield: 0.078 g (61%). Analysis: found: C, 46.74%; H, 5.15%; and N, 23.05%; calculated for C20H26N8S2Zn (507.98 g/mol): C, 47.29%; H, 5.16%; and N, 22.06%. IR (νmax/cm−1): 3,216 ν (N−H), 1,594, 1,555 ν (C=N) + ν (C=C), 1,074 ν (N–N), and 781 ν (C−S). 1H NMR (DMSO, 399.8 MHz): δ 1.14 (t, J = 8 Hz, CH3CH2, 6H), 2.45 (s, CH3C=N, 6H), 3.43 (q, J = 8 Hz, CH2CH3, 4H), 7.44 (s, NHCH2, 2H), 7.58 (t, J = 6 Hz, Py, 2H), 7.83 (d, J = 8 Hz, Py, 2H), 8.09 (t, J = 8 Hz, Py, 2H), and 8.65 (d, J = 8 Hz, Py, 2H). UV-Vis peaks at 2.16 × 10−5 M in DMSO solution [λmax (ε, M−1·cm−1)]: 398.00 nm (3611) and 310.00 nm (3141). Molar conductivity (at 1 × 10−3 M in dichloromethane): 0.10 μS·cm−1.
[Zn(atc-Ph)2] (2): Color: Yellow. Yield: 0.077 g (50%). Analysis: found: C, 54.73%; H, 4.33%; and N, 18.23%; calculated for C28H26N8S2Zn (604.07 g/mol): C, 55.67%; H, 4.34%; and N, 18.55%. IR (νmax/cm−1): 3,255 ν (N−H), 1,596, 1,548 ν (C=N) + ν (C=C), 1,084 ν (N–N), and 781 ν (C−S). 1H NMR (DMSO, 399.8 MHz): δ 2.71 (s, CH3C=N, 6H), 6.94 (t, J = 8Hz, Ph, 2H), 7.28 (t, J = 8Hz, Ph, 4H), 7.39 (t, J = 8 Hz, Py, 2H), 7.87–7.96 (m, Py + Ph, 10H), and 9.29 (s, NHPh, 2H). UV-Vis peak at 2.15 × 10−5 M in DMSO solution [λmax (ε, M−1·cm−1)]: 396.50 nm (3,265). Molar conductivity (at 1 × 10−3 M in dichloromethane): 0.13 μS·cm−1.
3.3. Cell Culture
All adherent cell lines, including healthy lung fibroblast (MRC-5), hepatocellular carcinoma (HepG-2), cervix adenocarcinoma (HeLa), breast adenocarcinoma (MDA-MB-231), prostate carcinoma (DU 145) and suspended chronic myelogenous leukemia (K-562) cells, were cultured in a humidified atmosphere containing 5% CO2 at 37 °C. The cultures were maintained in Dulbecco's Modified Eagle Medium (DMEM) or Roswell Park Memorial Institute (RPMI) 1640 medium (Vitrocell®, Campinas, SP, Brazil) supplemented with 10% fetal bovine serum (FBS), 50 mg/L gentamicin sulfate, and 2 mg/L amphotericin B.
3.4. Cytotoxic Analysis (IC50) of Adherent Cell Lines
In these experiments, cells were collected in a solution of trypsin/ethylenediaminetetraacetic acid (EDTA) (Vitrocell®) and centrifuged (252× g for 5 min). The number of cells was counted using a Neubauer chamber (Celeromics, Valencia, Spain) after staining non-viable cells with 0.4% trypan blue solution (Sigma-Aldrich®) via the cell exclusion assay. Then, the cell concentration was adjusted to 7.5 × 104 cells/mL in DMEM for tumor cells and MRC-5 cells. Next, a 200 μL suspension was deposited into each well of a 96-well microplate to a cell density of 1.5 × 104 cells/well. The cells were incubated at 37 °C in an atmosphere of 5% CO2 for 24 h to allow the cells to attach to the plate [34]. The coordination compounds were solubilized in DMSO to an initial concentration of 10,000 µg/mL. Test solutions of the complexes were prepared to obtain concentrations from 500 to 1.95 μg/mL. The diluted solutions were added to the cells after changing the medium to remove any non-adherent cells, and the cultures were incubated for an additional 24 h. The cytotoxicity of the complexes was determined after incubating the cells in 30 μL of resazurin for approximately 2 h. The measurement was performed using a Synergy H1 microplate reader (BioTek®, Winooski, VT, USA) with excitation and emission filters at wavelengths of 530 and 590 nm, respectively. The assays were performed in three independent experiments.
As the reference and the control, 10,000 µg/mL cisplatin (Sigma-Aldrich®) and 5% DMSO, respectively, were used in all cytotoxicity assays.
3.5. Cytotoxic Analysis (IC50) of Suspended Cells
In these experiments, cells were centrifuged (252× g for 5 min), and the number of cells was counted using a Neubauer chamber after staining non-viable cells with 0.4% trypan blue solution (Sigma-Aldrich®) via the cell exclusion assay. Then, the cell concentration was adjusted to 2 × 105 cells/mL in RPMI 1640 medium. Next, 100 μL of the suspension was deposited into each well of a 96-well microplate to a final cell density of 2 × 106 cells/well [35]. The complexes were dissolved in DMSO to an initial concentration of 10,000 μg/mL. Test solutions of the complexes were prepared to obtain concentrations from 500 to 1.95 μg/mL. The diluted solutions were added to the cells, and the cultures were incubated for 24 h. The cytotoxicity of the complexes was determined after incubating the cells in 30 μL of resazurin for approximately 5 h. The measurement was performed using a Synergy H1 microplate reader (BioTek®, Winooski, VT, USA) with excitation and emission filters at wavelengths of 530 and 590 nm, respectively. The assays were performed in three independent experiments.
In all cytotoxicity assays, as the reference and the control, cisplatin (Sigma-Aldrich®) at a concentration of 10,000 µg/mL and 5% DMSO (Sigma-Aldrich®), respectively, were used.
3.6. SI Measurement
The SI of the complexes was calculated as the ratio of the IC50 for healthy MRC-5 cells to the IC50 for tumor cells. A greater IC50 value indicates that the compound is more active against tumor cells relative to normal host cells. Substances with SI values >3 are considered promising [20].
3.7. Artemia salina L. Toxicity Assay
To determine toxicity of the ZnII complexes to brine shrimp, 25 mg of egg (Natal, RN, Brazil) was incubated in a beaker (2000 mL) containing artificial salt water at a temperature ranging from 20 to 30 °C. The artificial salt water consisted of 23 g NaCl, 11 g MgCl2·6H2O, 4 g Na2SO4, 1.3 g CaCl2·H2O, and 0.7 g KCl in 1000 mL distilled water. The pH was adjusted to 9.0 using 5N NaOH to avoid risk of death to the Artemia larvae (nauplii) due to a low pH during incubation.
After 24 hours, 0.6 g of Saccharomyces cerevisiae was added to the beaker per liter of salt water to feed the nauplii; after the eggs were incubated for 48 h, the nauplii were extracted for the experiments.
A 120 µL suspension of 10-15 nauplii was added to each well of a 96-well microplate, including the control wells. The compounds were solubilized in DMSO at 15,000 µg/mL and serially diluted in a separate 96-well microplate. The tested concentrations ranged from 1500 to 5.85 µg/mL. After incubation for 24 h, the plates were examined under a Lupa binocular microscope (X3.0), and the numbers of live nauplii in each well were counted to determine the LC50 [21]. The assays were performed in three independent experiments.
In all toxicity assays, 15,000 µg/mL cisplatin (Sigma-Aldrich®) was used as the reference drug, and 2% DMSO (Sigma-Aldrich®) was used as a control at.
3.8. In Vivo Oral Bioavailability Tests
Female 4–8-week old BALB/c mice weighing 15–20 g were purchased from the central animal facilities of Campinas State University (UNICAMP, Campinas, SP, Brazil). The mice were maintained in polycarbonate cages at 23 ± 2 °C, 56% ± 2% humidity at a 12 h light/dark cycle under specific pathogen-free conditions (in a positive-pressure cabinet), and the animals were provided with food and water ad libitum. The in vivo oral bioavailability of the complexes was determined according to a previously suggested method [36]. The animals (n = 2 animals/complex) received a dose of 300 mg/Kg body weight via oral gavage. At 20 min, 1 h, 2 h, and 4 h after drug administration, blood was drawn from two animals via the retro-orbital veins, draining the submandibular vein and the face of the mice to the point at which the origins of the jugular vein join so that drops of blood exude from the point of penetration [37]. The plasma was separated and stored at −70 °C for quantification.
3.9. Specimen Preparation for Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
The metal ion (Zn) concentration was quantified via ICP-OES using a Thermo Scientific Model 6300 system (Thermo Scientific®, San Diego, CA, USA). Samples (50 µL) were diluted 1:100 with a solution containing 0.01% (v/v) Triton X-100 and 0.5% (v/v) nitric acid [38]. During the experiment, the recovery validation test was performed, and this test produced a result of 94%–104% recovery.
3.10. Specimen Preparation for Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
The concentration of the drug cisplatin was quantified via ICP-MS using a Thermo Scientific XSeries II system (Thermo Scientific®, San Diego, CA, USA). For digestion, 50 µL of plasma sample and 5950 µL of double-distilled 20% (v/v) nitric acid were used. The samples were digested in a microwave (Berghof speedwave D 72800, Goshen, NY, USA), reaching a temperature of 170 °C. During the experiment, the recovery validation test was performed, and this test produced a result of 95%–105% recovery.
3.11. In Vivo Acute Toxicity Assay
Female 16–20-week old BALB/c mice weighing 24–26 g were used after utilizing these animals for the bioavailability experiments. As described by Gruppo et al. (2006), the surviving animals were reused to reduce the number of animals required for research. The mice were maintained in polycarbonate cages at 23 ± 2 °C, 56% ± 2% humidity at a 12-h light/dark cycle under specific-pathogen-free conditions (in a positive-pressure cabinet), and the animals were provided with food and water ad libitum [36].
The safety profile of the complexes to BALB/c mice was evaluated via acute toxicity assays according to protocols established by the OECD using the classical method to measure the acute toxicity (LD50) of a single oral dose (administered via gavage) of the complexes [26].
Each complex was suspended in a sunflower oil vehicle, and the vehicle was used as a control (control group). The complexes were administered as a single dose at 1000 mg/kg body weight via gavage (n = 6 animals/complex). When treatment with any complex resulted in greater than 50% mortality, doses of 500 and 300 mg/kg body weight were administered. The animals were monitored for 14 days, and behavioral parameters (Hippocratic screening) were evaluated.
At the end of the experiment, the animals were weighed, and blood was collected via the submandibular vein. Subsequently, the animals were euthanized in CO2 chambers, and their organs (heart, lungs, right and left kidneys, spleen and liver) were surgically removed, weighed and macroscopically analyzed. Statistical analysis was performed using GraphPad Prism Version 1.5 software (La Jolla, CA, USA) via ANOVA and Dunnett’s test, and p < 0.05 was established as the significance level.
3.12. Quantification of the Serum AST and ALT Enzymatic Activity Levels in BALB/c Mice
To analyze whether there were any biochemical changes in the liver of the animals, we quantified the serum AST and ALT enzymatic activity levels in mice after administration of the complexes via gavage before sacrificing, as described above. A total of 500 µL of blood was collected via the submandibular vein. The blood samples were placed in collection tubes, centrifuged at 1097.6× g for 15 min to separate the serum, and stored at −70 °C. The samples were analyzed on the day of collection.
Enzymatic activity measurements were performed using biochemical diagnostic kits from Labtest S.A. (Labtest S.A., Lagoa Santa, MG, Brazil).
Statistical analysis was performed using GraphPad Prism Version 1.5 software via ANOVA and Dunnett’s test, and p < 0.05 was established as the significance level.
4. Conclusions
In conclusion, the results indicate that the ZnII complexes [Zn(atc-Et)2] and [Zn(atc-Ph)2] might be a promising source of new metal-based antitumor agents. Based on the investigated toxicity parameters, the complexes have been demonstrated to be safe to BALB/c mice when orally administered at the doses tested. In addition, we determined the times of Cmax of the ZnII complexes after administration via oral gavage: one hour for complex 1 and two hours for complex 2.
Acknowledgments
This work was financially supported by grant#2009/54011-8, grant#2013/09265-7 and grant#2013/14957-5, CNPQ-Glaxo (ref. Process: 406827/2012-5) and Programa de Apoio ao Desenvolvimento Científico (PADC) of School of Pharmaceutical Sciences/UNESP, Brazil.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/5/781/s1.
Author Contributions
Carolina G. de Oliveira synthesized and characterized the complexes and assisted in drafting the paper. Patricia B. da Silva performed the in vivo oral bioavailability and acute toxicity assays, quantified the Zn concentrations in plasma and assisted in drafting the paper. Carlos E. Eismann quantified the Zn concentrations in plasma and assisted in drafting the paper. Carlos A. Suárez quantified the Zn concentrations in plasma and assisted in drafting the paper. Amauri A. Menegário quantified the Zn concentrations in plasma and assisted in drafting the paper. Clarice Q. F. Leite performed the cytotoxicity assays and assisted in drafting the paper. Victor M. Deflon synthesized and characterized the complexes and assisted in drafting the paper. Fernando R. Pavan provided guidance to the primary author (Erica de O. Lopes), conceived of the project, and assisted in drafting the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization (WHO) [(accessed on 6 April 2015)]. Available online: http://www.who.int/mediacentre/factsheets/fs297/es/
- 2.American Cancer Society. [(accessed on 15 May 2015)]. Available online: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/
- 3.National Cancer Institute (NCI) [(accessed on 6 April 2015)]; Available online: www.cancer.gov/about-cancer/treatment/types.
- 4.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 5.Bielawski K., Czarnomysy R., Muszynska A., Bielawska A., Poplawska B. Cytotoxicity and induction of apoptosis of human breast cancer cells by novel platinum(II) complexes. Environ. Toxicol. Pharmacol. 2013;35:254–264. doi: 10.1016/j.etap.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z., Xie Z., Sun G., Yang P., Li J., Yang H., Xiao S., Liu Y., Qiu H., Qiu L., et al. Reversing drug resistance of cisplatin by hsp90 inhibitors in human ovarian cancer cells. Int. J. Clin. Exp. Med. 2015;8:687–701. [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H.M., Jiang Z.F., Ding P.S., Shao L.J., Lui R.Y. Hypoxia-induced autophagy mediates cisplatin resistance in lung cancer cells. Sci. Rep. 2015;5:1–15. doi: 10.1038/srep12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valenzuela M.M., Neidigh J.W., Wall N.R. Antimetabolite treatment for pancreatic cancer. Chemotherapy (Los Angel) 2014;3:1–16. doi: 10.4172/2167-7700.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z., Lui J., Li L., Nie D., Tao Q., Wu J., Fan J., Lin C., Zhao S., Ju D. Inhibition of autophagy potentiated the antitumor effect of nedaplatin in cisplatin-resistant nasopharyngeal carcinoma cells. PLoS ONE. 2015;10:1–16. doi: 10.1371/journal.pone.0135236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florea A.M., Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Do Nascimento F.B., Poelhsitz G.V., Pavan F.R., Sato D.N., Leite C.Q.F., Araújo H.S.S., Ellena J., Castellano E.E., Deflon V.M., Bastista A.A. Synthesis, characterization, X-ray structure and in vitro antimycobacterial and antitumoral activities of Ru(II) phosphine/diimine complexes containing the “SpymMe2” ligand, SpymMe2=4,6-dimethyl-2-mercaptopyrimidine. J. Inorg. Biochem. 2008;102:1783–1789. doi: 10.1016/j.jinorgbio.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Mondelli M.A., Graminha A.E., Côrrea R.S., Silva M.M., Carnizello A.P., Poelhsitz G.V., Ellena J., Deflon V.M., Caramori G.F., Torre M.H., et al. Ruthenium(II)/4,6-dimethyl-2-mercaptopyrimidine complexes: Synthesis, characterization, X-ray structures and in vitro cytotoxicity activities on cancer cell lines. Polyhedron. 2014;68:312–318. doi: 10.1016/j.poly.2013.11.001. [DOI] [Google Scholar]
- 13.Malone R. Bioinorganic Chemistry: A Short Course. 2nd ed. John Wiley & Sons; New Jersey, NJ, USA: 2002. pp. 1–368. [Google Scholar]
- 14.Das A.K. Metallotherapy: A Textbook on Medicinal Aspects of Bioinorganic Chemistry. CBS Publishers and Distributors; New Dehli, India: 1990. [Google Scholar]
- 15.Li M.X., Zhang L.Z., Yang M., Niu J.Y., Zhou J. Synthesis, crystal structures, in vitro biological evaluation of zinc(II) and bismuth(III) complexes of 2-acetylpyrazine N(4)-phenylthiosemicarbazone. Bioorg. Med. Chem. Lett. 2012;22:2418–2423. doi: 10.1016/j.bmcl.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Li M.X., Chen C.L., Zhang D., Niu J.Y., Ji B.S. Mn(II), Co(II) and Zn(II) complexes with heterocyclic substituted thiosemicarbazones: Synthesis, characterization, X-ray crystal structures and antitumor comparison. Eur. J. Med. Chem. 2010;45:3169–3177. doi: 10.1016/j.ejmech.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira C.G., Maia P.I.S., Souza P.C., Pavan F.R., Leite C.Q.F., Vianna R.B., Batista A.A., Nascimento O.R., Deflon V.M. Manganese(II) complexes with thiosemicarbazones as potential anti-Mycobacterium tuberculosis agents. J. Inorg. Biochem. 2013;132:21–29. doi: 10.1016/j.jinorgbio.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira C.G., Maia P.I.S., Miyata M., Pavan F.R., Leite C.Q.F., Almeida E.T., Deflon V.M. Cobalt(III) complexes with thiosemicarbazones as potential anti-Mycobacterium tuberculosis agents. J. Braz. Chem. Soc. 2014;25:1848–1856. [Google Scholar]
- 19.Stefani C., Jansson P.J., Gutierrez E., Bernhardt P.V., Richardson D.R., Kalinowski D.S. Alkyl substituted 2'-benzoylpyridine thiosemicarbazone chelators with potent and selective anti-neoplastic activity: Novel ligands that limit methemoglobin formation. J. Med. Chem. 2013;56:357–370. doi: 10.1021/jm301691s. [DOI] [PubMed] [Google Scholar]
- 20.Chelopo M.P., Pawar S.A., Sokhela M.K., Govender T., Kruger H.G., Maguire G.E.M. Anticancer activity of ruthenium(II) arene complexes bearing 1,2,3,4-tetrahydroisoquinoline amino alcohol ligands. Eur. J. Med. Chem. 2013;66:407–414. doi: 10.1016/j.ejmech.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 21.Parra A.L., Yhebra R.S., Sardiñas I.G., Buela L.I. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001;8:395–400. doi: 10.1078/0944-7113-00044. [DOI] [PubMed] [Google Scholar]
- 22.Meyer B.N., Ferrigni N.R., Putnam J.E., Jacobsen L.B., Nichols D.E., McLaughlin J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
- 23.Ferraz K.O., Wardell S.M.S.V., Wardell J.L., Louro S.R.W., Beraldo H. Copper(II) complexes with 2-pyridineformamide-derived thiosemicarbazones: Spectral studies and toxicity against Artemia salina. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009;73:140–145. doi: 10.1016/j.saa.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Krstic N.M., Matuc I.Z., Juranic Z.D., Novakovic I.T., Sladic D.M. Steroid dimers-in vitro cytotoxic and antimicrobial activities. J. Steroid Biochem. Mol. Biol. 2014;143:365–375. doi: 10.1016/j.jsbmb.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Freitas M.C.R., Antonio J.M.S., Ziolli R.L., Yoshida M.I., Rey N.A., Diniz R. Synthesis and structural characterization of a zinc(II) complex of the mycobactericidal drug isoniazid—Toxicity against Artemia salina. Polyhedron. 2011;30:1922–1926. doi: 10.1016/j.poly.2011.04.027. [DOI] [Google Scholar]
- 26.OECD, 2011. Guideline 423: Acute Oral Toxicity - Acute Toxic Class Method. [(accessed on 18 November 2014)]. Paris: Head of Publications Service. Available online: http://www.oecd.org/publications.
- 27.Muscella A., Vetrugno C., Migoni D., Biagioni F., Fanizzi F.P., Fornai F., De Pascali S.A., Marsigliante S. Antitumor activity of [Pt(O,O′-acac)(gamma-acac)(DMS)] in mouse xenograft model of breast cancer. Cell. Death Dis. 2014;5:1014. doi: 10.1038/cddis.2013.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piao Y., Liu Y., Xiel X. Change trends of organ weight background data in sprague dawley rats at diferente ages. J. Toxicol. Pathol. 2013;26:29–34. doi: 10.1293/tox.26.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ping K.Y., Darah I., Chen Y., Sreeramanan S., Sasidharan S. Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. BioMed Res. Int. 2013;2013:1–14. doi: 10.1155/2013/628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang X.-J., Choi Y.-K., Im H.-S., Yarimaga O., Yoon E., Kim H.-S. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors. 2006;6:756–782. doi: 10.3390/s6070756. [DOI] [Google Scholar]
- 31.Araujo R.C.P., Neves F.A.R., Formagio A.S.N., Kasseya C.A.L., Stefanello M.E.A., Souza V.V., Pavan F.R., Croda J. Evaluation of the anti-mycobacterium tuberculosis activity and in vivo acute toxicity of Annona sylvatic. BMC Complement. Altern. Med. 2014;14:781. doi: 10.1186/1472-6882-14-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S., Zhang Y., Peng N., Zhang H., Yao J., Li Z., Liu L. Pharmacokinetics and biodistribution of zinc-enriched yeast in rats. Sci. World J. 2014;2014:1–4. doi: 10.1155/2014/217142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.G.M. Sheldrick, SHELXL97 . Program. for the Refinement of Crystal Structures. University of Göttingen; Göttingen, Germany: 1997. [Google Scholar]
- 34.O’brien J., Wilson I., Orton T., Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 35.Akhtar M.N., Zareen S., Yeap S.K., Ho W.Y., Lo K.M., Hasan A., Alitheen B. Total synthesis, cytotoxic effects of damnacanthal, nordamnacanthal and related anthraquinone analogues. Molecules. 2013;18:10042–10055. doi: 10.3390/molecules180810042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gruppo V., Johnson C.M., Marietta K.S., Scherman H., Zink E.E., Crick D.C., Adams L.B., Orme I.M., Lenaerts J.A. Rapid microbiologic and pharmacologic evaluation of experimental compounds against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2006;50:1245–1250. doi: 10.1128/AAC.50.4.1245-1250.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golde W.T., Gollobin P., Rodriguez L.L. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab. Anim. 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 38.Batista B.L., Rodrigues J.L., Nunes J.A., Souza V.C.O., Barbosa F.J. Exploiting dynamic reaction cell inductively coupled plasma mass spectrometry (DRC-ICP-MS) for sequential determination of trace elements in blood using a dilute-and-shoot procedure. Anal. Chim. Acta. 2009;639:13–18. doi: 10.1016/j.aca.2009.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.