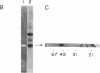
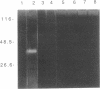
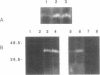
Abstract
The Bowman-Birk protease inhibitor (BBI) has been shown to be an effective suppressor of carcinogenesis in vivo and in vitro. To elucidate the mechanism(s) by which BBI suppresses carcinogenesis, we believe it will be necessary to identify and characterize the target enzymes that specifically interact with the BBI. We have shown previously that several cellular proteins in C3H/10T1/2 mouse embryo fibroblast cells specifically bind to a BBI affinity resin. In the current report, we demonstrate that one of these proteins has proteolytic activity as judged by its ability to degrade gelatin. The enzyme has a mass of 45 kDa and subcellular fractionation experiments demonstrate that this enzyme is located in the cytosol. Furthermore, the proteolytic activity was inhibited by diisopropylfluorophosphate but was not affected by EDTA, indicating that this enzyme is a serine protease. Higher levels of protease activity were found in logarithmic-phase C3H/10T1/2 cells compared with nondividing (confluent) cells, suggesting that this protease activity is growth regulated. Similar levels of this activity were present in nontransformed and in radiation-transformed C3H/10T1/2 cells. Treatment of nontransformed C3H/10T1/2 cells with phorbol 12-myristate 13-acetate increased the specific activity of this protease 5- to 10-fold. Our results suggest that this protease is a target enzyme of the BBI in these cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baturay N., Kennedy A. R. Pyrene acts as a cocarcinogen with the carcinogens benzo[a]pyrene, beta-propiolactone and radiation in the induction of malignant transformation in cultured mouse fibroblasts; soybean extract containing the Bowman-Birk inhibitor acts as an anticarcinogen. Cell Biol Toxicol. 1986 Mar;2(1):21–32. doi: 10.1007/BF00117704. [DOI] [PubMed] [Google Scholar]
- Billings P. C., Carew J. A., Keller-McGandy C. E., Goldberg A. L., Kennedy A. R. A serine protease activity in C3H/10T1/2 cells that is inhibited by anticarcinogenic protease inhibitors. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4801–4805. doi: 10.1073/pnas.84.14.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings P. C., Habres J. M., Kennedy A. R. Inhibition of radiation-induced transformation of C3H/10T1/2 cells by specific protease substrates. Carcinogenesis. 1990 Feb;11(2):329–332. doi: 10.1093/carcin/11.2.329. [DOI] [PubMed] [Google Scholar]
- Billings P. C., Jin T., Ohnishi N., Liao D. C., Habres J. M. The interaction of the potato-derived chymotrypsin inhibitor with C3H/10T1/2 cells. Carcinogenesis. 1991 Apr;12(4):653–657. doi: 10.1093/carcin/12.4.653. [DOI] [PubMed] [Google Scholar]
- Billings P. C., Morrow A. R., Ryan C. A., Kennedy A. R. Inhibition of radiation-induced transformation of C3H/10T1/2 cells by carboxypeptidase inhibitor 1 and inhibitor II from potatoes. Carcinogenesis. 1989 Apr;10(4):687–691. doi: 10.1093/carcin/10.4.687. [DOI] [PubMed] [Google Scholar]
- Billings P. C., Newberne P. M., Kennedy A. R. Protease inhibitor suppression of colon and anal gland carcinogenesis induced by dimethylhydrazine. Carcinogenesis. 1990 Jul;11(7):1083–1086. doi: 10.1093/carcin/11.7.1083. [DOI] [PubMed] [Google Scholar]
- Billings P. C., St Clair W., Owen A. J., Kennedy A. R. Potential intracellular target proteins of the anticarcinogenic Bowman Birk protease inhibitor identified by affinity chromatography. Cancer Res. 1988 Apr 1;48(7):1798–1802. [PubMed] [Google Scholar]
- Birk Y. Proteinase inhibitors from plant sources. Methods Enzymol. 1976;45:695–697. doi: 10.1016/s0076-6879(76)45059-0. [DOI] [PubMed] [Google Scholar]
- Birk Y. The Bowman-Birk inhibitor. Trypsin- and chymotrypsin-inhibitor from soybeans. Int J Pept Protein Res. 1985 Feb;25(2):113–131. doi: 10.1111/j.1399-3011.1985.tb02155.x. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Butler P. E. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carew J. A., Kennedy A. R. Identification of a proteolytic activity which responds to anticarcinogenic protease inhibitors in C3H-10T1/2 cells. Cancer Lett. 1990 Feb;49(2):153–163. doi: 10.1016/0304-3835(90)90152-n. [DOI] [PubMed] [Google Scholar]
- Correa P. Epidemiological correlations between diet and cancer frequency. Cancer Res. 1981 Sep;41(9 Pt 2):3685–3690. [PubMed] [Google Scholar]
- Doll R., Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981 Jun;66(6):1191–1308. [PubMed] [Google Scholar]
- Hozumi M., Ogawa M., Sugimura T., Takeuchi T., Umezawa H. Inhibition of tumorigenesis in mouse skin by leupeptin, a protease inhibitor from Actinomycetes. Cancer Res. 1972 Aug;32(8):1725–1728. [PubMed] [Google Scholar]
- Kennedy A. R. The conditions for the modification of radiation transformation in vitro by a tumor promoter and protease inhibitors. Carcinogenesis. 1985 Oct;6(10):1441–1445. doi: 10.1093/carcin/6.10.1441. [DOI] [PubMed] [Google Scholar]
- Kuroki T., Drevon C. Inhibition of chemical transformation in C3H/10T1/2 cells by protease inhibitors. Cancer Res. 1979 Jul;39(7 Pt 1):2755–2761. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long S. D., Quigley J. P., Troll W., Kennedy A. R. Protease inhibitor antipain suppresses 12-O-tetradecanoyl-phorbol-13-acetate induction of plasminogen activator in transformable mouse embryo fibroblasts. Carcinogenesis. 1981;2(9):933–936. doi: 10.1093/carcin/2.9.933. [DOI] [PubMed] [Google Scholar]
- Masuzawa M., Hamazaki H., Nishioka K., Nishiyama S., Ryan T. J. A fibroblast-derived urokinase-inhibitor differing from protease nexin. Biochem Biophys Res Commun. 1987 Dec 31;149(3):866–873. doi: 10.1016/0006-291x(87)90488-8. [DOI] [PubMed] [Google Scholar]
- Messadi D. V., Billings P., Shklar G., Kennedy A. R. Inhibition of oral carcinogenesis by a protease inhibitor. J Natl Cancer Inst. 1986 Mar;76(3):447–452. [PubMed] [Google Scholar]
- Mills P. K., Beeson W. L., Abbey D. E., Fraser G. E., Phillips R. L. Dietary habits and past medical history as related to fatal pancreas cancer risk among Adventists. Cancer. 1988 Jun 15;61(12):2578–2585. doi: 10.1002/1097-0142(19880615)61:12<2578::aid-cncr2820611232>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Phillips R. L. Role of life-style and dietary habits in risk of cancer among seventh-day adventists. Cancer Res. 1975 Nov;35(11 Pt 2):3513–3522. [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- St Clair W. H., Billings P. C., Carew J. A., Keller-McGandy C., Newberne P., Kennedy A. R. Suppression of dimethylhydrazine-induced carcinogenesis in mice by dietary addition of the Bowman-Birk protease inhibitor. Cancer Res. 1990 Feb 1;50(3):580–586. [PubMed] [Google Scholar]
- St Clair W. H. Suppression of 3-methylcholanthrene-induced cellular transformation by timed administration of the Bowman-Birk protease inhibitor. Carcinogenesis. 1991 May;12(5):935–937. doi: 10.1093/carcin/12.5.935. [DOI] [PubMed] [Google Scholar]
- Terzaghi M., Little J. B. X-radiation-induced transformation in a C3H mouse embryo-derived cell line. Cancer Res. 1976 Apr;36(4):1367–1374. [PubMed] [Google Scholar]
- Troll W., Klassen A., Janoff A. Tumorigenesis in mouse skin: inhibition by synthetic inhibitors of proteases. Science. 1970 Sep 18;169(3951):1211–1213. doi: 10.1126/science.169.3951.1211. [DOI] [PubMed] [Google Scholar]
- Umezawa H. Structures and activities of protease inhibitors of microbial origin. Methods Enzymol. 1976;45:678–695. doi: 10.1016/s0076-6879(76)45058-9. [DOI] [PubMed] [Google Scholar]
- Weed H. G., McGandy R. B., Kennedy A. R. Protection against dimethylhydrazine-induced adenomatous tumors of the mouse colon by the dietary addition of an extract of soybeans containing the Bowman-Birk protease inhibitor. Carcinogenesis. 1985 Aug;6(8):1239–1241. doi: 10.1093/carcin/6.8.1239. [DOI] [PubMed] [Google Scholar]
- Wigler M., Weinstein I. B. Tumour promotor induces plasminogen activator. Nature. 1976 Jan 22;259(5540):232–233. doi: 10.1038/259232a0. [DOI] [PubMed] [Google Scholar]
- Witschi H., Kennedy A. R. Modulation of lung tumor development in mice with the soybean-derived Bowman-Birk protease inhibitor. Carcinogenesis. 1989 Dec;10(12):2275–2277. doi: 10.1093/carcin/10.12.2275. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Yavelow J., Caggana M., Beck K. A. Proteases occurring in the cell membrane: a possible cell receptor for the Bowman-Birk type of protease inhibitors. Cancer Res. 1987 Mar 15;47(6):1598–1601. [PubMed] [Google Scholar]
- Yavelow J., Collins M., Birk Y., Troll W., Kennedy A. R. Nanomolar concentrations of Bowman-Birk soybean protease inhibitor suppress x-ray-induced transformation in vitro. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5395–5399. doi: 10.1073/pnas.82.16.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavelow J., Scott C. B., Mayer T. C. Fluorescent visualization of binding and internalization of the anticarcinogenic Bowman-Birk type protease inhibitors in transformed fibroblasts. Cancer Res. 1987 Mar 15;47(6):1602–1607. [PubMed] [Google Scholar]