Abstract
Background:
Pulmonary hypertension (PHT), if present, can be a significant cause of increased morbidity and mortality in children undergoing surgery for congenital heart diseases (CHD). Various techniques and drugs have been used perioperatively to alleviate the effects of PHT. Intravenous (IV) sildenafil is one of them and not many studies validate its clinical use.
Aims and Objectives:
To compare perioperative PaO2 – FiO2 ratio peak filling rate (PFR), systolic pulmonary artery pressure (PAP) – systolic aortic pressure (AoP) ratio, extubation time, and Intensive Care Unit (ICU) stay between two groups of children when one of them is administered IV sildenafil perioperatively during surgery for CHDs.
Materials and Methods:
Patients with ventricular septal defects and proven PHT, <14 years of age, all American Society of Anesthesiologists physical status III, undergoing cardiac surgery, were enrolled into two groups – Group S (IV sildenafil) and Group C (control) – over a period of 14 months, starting from October 2013. Independent t-test and Mann–Whitney U-test were used to compare the various parameters between two groups.
Results:
PFR was higher throughout, perioperatively, in Group S. PAP/AoP was 0.3 and 0.4 in Group S and Group C, respectively. In Group S, mean group extubation time was 7 ± 7.34 h, whereas in Group C it was 22.1 ± 10.6. Postoperative ICU stay in Group S and Group C were 42.3 ± 8.8 h and 64.4 ± 15.9 h, respectively.
Conclusion:
IV sildenafil, when used perioperatively, in children with CHD having PHT undergoing corrective surgery, improves not only PaO2 – FiO2 ratio and PAP – AoP ratio but also reduces extubation time and postoperative ICU stay.
Keywords: Congenital heart disease, Intravenous sildenafil, Perioperative management, Pulmonary hypertension
INTRODUCTION
The high pulmonary artery pressure (PAP) during the intrauterine period drastically drops immediately after birth. The descent continues until first 2–6 weeks of age when the pressure approaches the adult value and thereafter normally tends to stay static. From around 3rd month of life mean PAP of 25 mmHg or above, in a resting individual at sea level, is diagnosed as pulmonary hypertension (PHT). In children, PHT is predominantly either idiopathic or related to congenital heart disease (CHD).[1,2] CHD can alter the pulmonary blood flow (Qp), pulmonary vascular resistance (PVR), and PAP. Whereas, PAP is the product of PVR and Qp, PHT may develop as a result of an increase in either PVR or Qp or both. Pulmonary vascular bed is a low pressure system with high flow and any increase in resistance would generate PHT. While increased PAP is due to hypoxic lesions of the endothelium, which release proteolytic enzymes that alter the balance of metabolites of arachidonic acid, regulators of pulmonary vasomotor tone, an increase of PVR is due to a combination of vasoconstrictive processes and remodeling, with hypertrophy of the pulmonary artery.[3] In particular, PVR is important in the diagnosis and management of PHT with CHD than in any other form of PHT in children.[4]
Perioperative management of PHT, during surgery for CHD in children, is a formidable task for the surgical team. As PVR generally implies a reduction in number or diameter of pulmonary vessels, patients with increased PVR are more likely to suffer from PHT immediately following a cardiac operation, and a progressive increase in PVR even after correction of cardiac lesion, than those with PHT associated with a large left-to-right shunt. Ventilation strategies directed toward adequate oxygenation and avoiding acidosis, as well as hypercarbia, adequate analgesia and sedation, and optimizing hematocrit, are a few modalities that have been employed to manage this challenge. The pharmacological management includes nitric oxide inhalation, phosphodiesterase (PDE) Type 5 inhibitors (sildenafil), prostacyclin analogues (epoprostenol), endothelin receptor antagonists (bosentan), and inodilators (milrinone).[5] All these agents have been tried with varying degrees of success though nitric oxide remains the treatment of choice, despite its shortcomings. Among the remaining substitutes, sildenafil is a very promising drug. Effect of oral sildenafil has been studied, but not many studies validate the use of intravenous (IV) sildenafil. In our study, we have tried to assess whether IV sildenafil clinically ameliorates PHT during perioperative management of CHD in children.
MATERIALS AND METHODS
The prospective randomized study was conducted at a tertiary level cardiac referral center. It was reviewed and approved by the Ethical Committee of the Institute. Written parental informed consent was taken from patients younger than 14 years, all with proven PHT, with American Society of Anesthesiologists (ASA) physical status III undergoing repair for ventricular septal defects (VSD), from October 2013 to December 2014. Exclusion criteria were, patients having PHT associated with congenital cardiac defects other than VSD, presence of any ongoing inotropic or ventilatory support prior to surgery, and presence of contraindications to central neuraxial blocks namely deranged coagulation, spinal deformity, etc.
A night prior to the surgery, the patients were randomized to one of the two groups, Group S (IV sildenafil) and Group C (control), by randomly generated chits with the specified group name sealed in an envelope, which was done by an investigator not participating in data collection. However, investigators collecting data could not be blinded to the method of allocation of groups. On the morning of the surgery, oral triclofos 50 mg/kg was administered in the ward, 45 min prior to shifting to the operation theater. In preoperative room, intranasal ketamine 7 mg/kg and nasal midazolam 0.4 mg/kg were given 15 min prior to induction. Once inside the operation theater, basic monitoring was instituted (electrocardiography [ECG], NIBP, and SpO2) and a peripheral IV access secured using a 22 G or 24 G cannula.
Each group was finally allotted 23 patients. In Group S, oral sildenafil was started prior to surgery in the dose 0.5 mg/kg, two doses administered, one at the night and one on the morning of surgery. This was followed by administration of IV sildenafil (0.4 mg/kg) after removal of aortic cross clamp and thereafter continuous infusion of IV sildenafil (total 1.6 mg/kg) over 24 h. Termination of infusion was overlapped with initiation of oral sildenafil (0.5 mg/kg). In Group C, no sildenafil was administered. Rest of the anesthetic technique used was same for both the groups. After inducing with 2 mg/kg IV ketamine, supplemented with 2 μg/kg IV fentanyl and IV rocuronium 1 mg/kg, endotracheal tube was chosen on the bases of weight and age of the baby, as well as experience of the anesthesiologist. Once intubated, every child was placed in left lateral position and epidural catheter inserted in T4–T6 space. Epidural catheter used was 23 G until 3–4 years age and 20 G for bigger children. Epidural solution used was 1 ml/kg bolus of 0.25% bupivacaine and 75 μg/kg morphine administered in two divided doses 30–45 min apart, followed by 0.125% bupivacaine at the rate 0.2 ml/kg/h, throughout intraoperative period and 0.1 ml/kg/h in the postoperative period thereafter in the Intensive Care Unit (ICU). Anesthesia was maintained with 50% FiO2 (air and oxygen mixture) and 0.2–1.5% isoflurane. IV methyl prednisolone 30 mg/kg was given to all patients. Injection dexmedetomidine 0.25 μg/kg/h was started after induction and continued throughout the perioperative period. Invasive blood pressure monitoring was established with radial artery cannulation. Right internal jugular vein was cannulated with 4.5–5.5 Fr triple lumen catheters. Right subclavian vein was cannulated with 22 G single lumen catheter placed in the right atrium that was intraoperatively surgically placed in left atrium across the interatrial septum to monitor the left atrial pressure (LAP).
Once the surgery was commenced, 300 Units/kg IV heparin was administered prior to placing the patient on cardiopulmonary bypass (CPB) and supplemented if indicated by activated clotting time. Dopamine 5 μg/kg/min and vasopressin 0.0003 units/kg/min were started in all patients and the dose titrated as per the demands of hemodynamic status at that time. Milrinone was started with the loading dose 100 μg/kg at the release of aortic cross clamp, and infusion of milrinone at the dose of 0.75 μg/kg/min was maintained thereafter. After surgery, the patients were assessed for fitness to be extubated on table with the following criteria:
Hemodynamic stability with minimal inotropic support
No ongoing inappropriate bleeding
Adequately rewarmed, CPB time <150 min
Adequate spontaneous respiratory effort after reversal with standard doses of neostigmine and glycopyrrolate
-
Any of the following present
- Gag reflex on suctioning
- Child opening eyes
- Appropriate limb movements.
Once extubated, nasal – continuous positive airway pressure was instituted in all the children. If the patient did not meet the extubation criteria he/she was shifted to cardiac surgical ICU and was mechanically ventilated with inotropic support (dopamine and milrinone) till the extubation criteria were met. During postoperative stay in ICU, rescue sedation of propofol 25–50 μg/kg/min IV was started as per requirement.
Routine monitoring as per the institutional protocol was done in the form of ECG, heart rate, IBP, end tidal CO2 (EtCO2) LAP, NIRS, core temperature, blood sugar, and urine output. Clinical parameters assessed to observe the effect of IV sildenafil were PaO2 – FiO2 ratio or peak filling rate (PFR) (arterial blood gas done at induction – T0, at initiation of CPB – T1, at termination of CPB – T2, at the end of surgery – T3, and thereafter 6 hourly for three more times, in the ICU – T4, T5 and T6), PAP (expressed as ratio of systolic PAP and systolic aortic pressure [AoP]) as assessed with echocardiography after shifting the patient to ICU postoperatively, extubation time, ICU stay, and deterioration of patient including pulmonary hypertensive (PH) crisis. Universal protocol for fasting status in pediatric age group was adhered to. Intraoperatively, IV fluids were administered as bolus containing Ringer's lactate and Dextrose[6] in the dose of 10 ml/kg in 30 min followed by maintenance rate at 1 ml/kg/h and postoperatively in the ICU at 2 ml/kg/h along with 5% albumin at 1 ml/kg/h for 12 h. Magnesium sulfate (100 mg/kg/day) and KCl (2 mEq/kg/day) were also added to maintenance fluid.
Data are expressed in mean and standard deviation. Independent t-test was used to test whether the groups are comparable, based on age, weight, PAP, and AoP of the patients and Chi-square test was used to test the homogeneity of gender distribution. Quantitative data with respect to postoperative extubation time and ICU stay were compared between the two groups using Mann–Whitney U-test. Independent t-test was used to compare the mean value of PFR at different time periods between groups and also the mean value of PAP/AoP between the two groups. P < 0.05 was considered as statistically significant.
RESULTS
Forty-six children were enrolled in the study and randomized to one of the two groups: Sildenafil (Group S) or control (Group C). There was no fall out seen due to death or any other reason and all 23 children in each group could be assessed till the end of the study [Figure 1]. The patient characteristics were similar between both the groups with respect to age, weight, gender, PAP (basal), and AoP (basal) [Table 1]. All the patients undergoing cardiac surgery were accepted under ASA physical status III.
Figure 1.
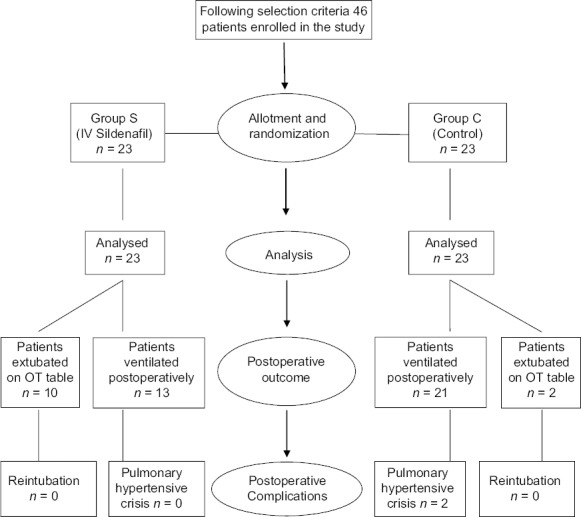
Flowchart outlining study design and perioperative outcome
Table 1.
Patient characteristics in the two groups
| Group S | Group C | P | |
|---|---|---|---|
| Age (mean±SD) | 3.2±3 | 3.5±3.4 | 0.734 |
| Male (n (%)) | 10 (43.5) | 15 (65.2) | 0.139 |
| Weight (mean±SD) | 12.6±7.1 | 12.8±7.6 | 0.927 |
| PAP (basal) (mean±SD) | 44.2±7.2 | 46.2±8.4 | 0.653 |
| AoP (basal) (mean±SD) | 82.2±11.6 | 85.4±11.4 | 0.275 |
SD: Standard deviation, AoP: Aortic pressure, PAP: Pulmonary artery pressure
The study clearly displayed the superiority of Group S with respect to fast track extubation, postoperative ICU stay, PFR, as well as PAP/AoP. In Group S, 10 out of 23 children were extubated on table in the operating room (OR), whereas in Group C two children were extubated in the OR. The mean time period for postoperative extubation in Group S was 7 ± 7.24 h, whereas that for Group C was 22.1 ± 10.6 h [Figure 2]. The postoperative ICU stay in Group S was 42.3 ± 8.8 h and Group C was 64.4 ± 15.9 h [Figure 3]. PFR was significantly lower in Group C compared to Group S, throughout perioperative period [Figure 4]. Postoperative systolic PAP/AoP was 0.3 and 0.4 for Group S and Group C, respectively [Figure 5].
Figure 2.
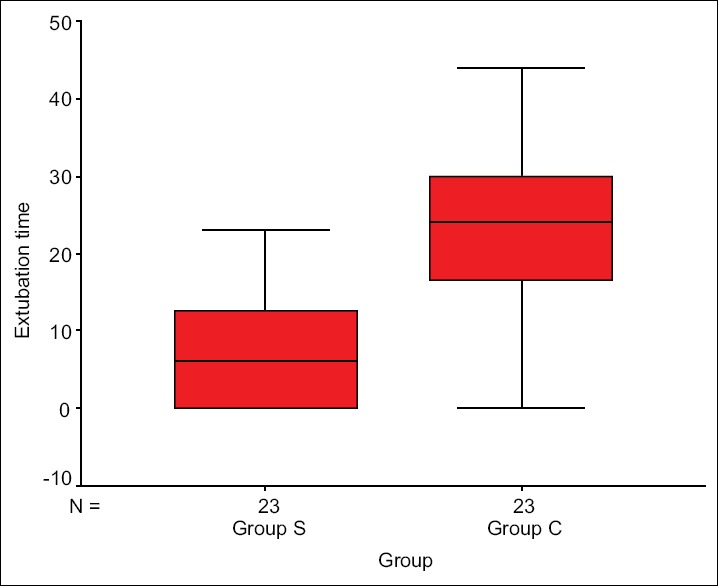
Box plot showing extubation time distribution in two groups
Figure 3.
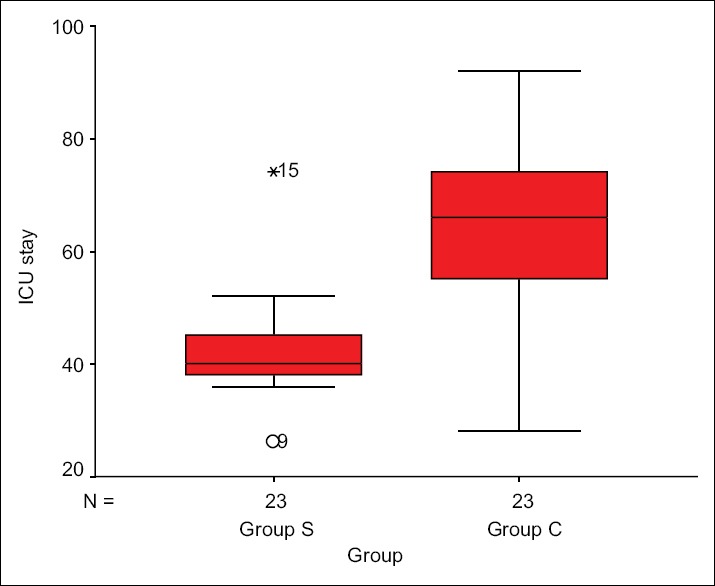
Box plot showing intensive care unit stay time distribution in two groups
Figure 4.
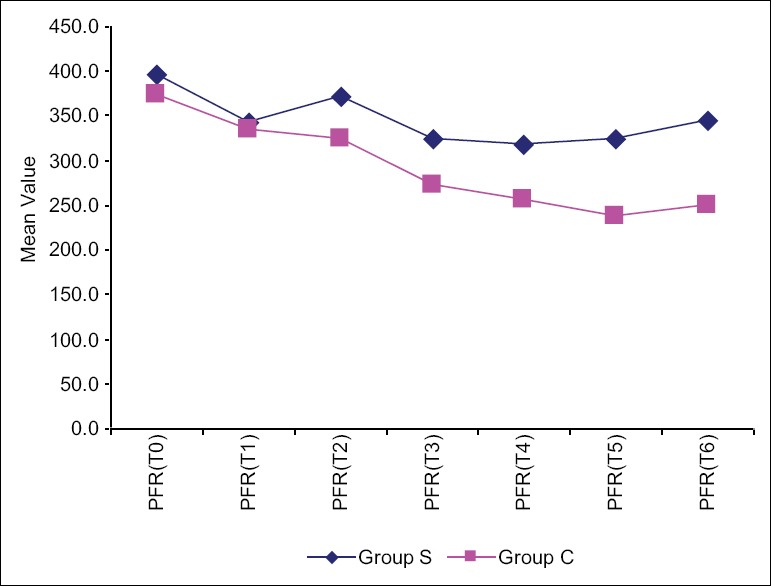
Comparison of the two groups with respect to PaO2 – FiO2 ratio (peak filling rate) at different time points
Figure 5.
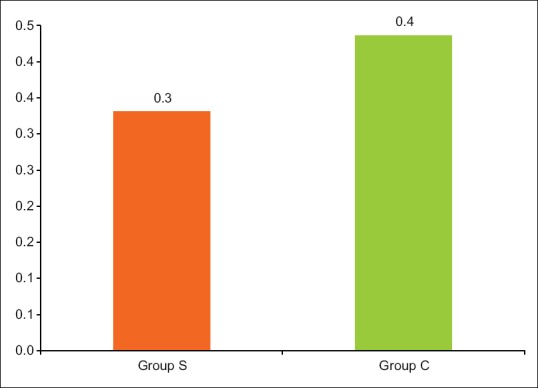
Comparison of the two groups with respect to systolic pulmonary artery pressure/aortic pressure
DISCUSSION
PHT associated with CHD has been classified into four types:[4] Type 1 – Eisenmenger Syndrome, Type 2 – left to right shunts (operable and inoperable), Type 3 – PHT with co-incidental CHD (small atrial septal defects and VSDs), and Type 4 – postoperative PHT. Presently the gold standard management for PHT associated with surgery for CHD is inhaled nitric oxide (iNO), which has its inherent shortcomings. Discontinuation of iNO can cause fatal rebound PHT.[7,8] Also, PH crisis, if it occurs is not completely responsive to it.[9] Administration itself mandates the use of special equipment which may not be present at all centers. To get over these shortcomings, various other pulmonary vasodilators have been used e.g. sildenafil, epoprostenol, bosentan, and milrinone. Whereas, all of them have been used with varying degrees of success; sildenafil has shown great promise. Sildenafil has been used either in combination with iNO[10,11] or as a single drug.[12,13,14,15,16] It is a selective and potent inhibitor of PDE Type 5 which specifically degrades cyclic-guanosine monophosphate (c-GMP) and is found in high concentration in pulmonary arteries. Normally, endothelium – derived nitric oxide stimulates intracellular soluble guanylate cyclase resulting in increased levels of c-GMP, which then acts to mediate smooth muscle relaxation. Sildenafil inhibits the degradation by PDE 5 and prolongs the action of c-GMP.[17,18]
Most of the studies pertaining to the therapeutic potential of sildenafil with respect to PHT during pediatric cardiac surgeries have been conducted using oral sildenafil. Only a few studies substantiate the use of IV sildenafil.[19,20,21] Of these, the one conducted by Fraisse Alain et al. was a double-blinded, placebo-controlled, dose-ranging, and parallel-group trial. Unfortunately, the trial was heavily underpowered with a final analysis of only 17 patients. Nevertheless, the study concluded that IV sildenafil reduces PAP and shortened time to extubation and ICU stay, in children with postoperative PHT. In another study by Stocker et al., the combined effect of iNO and IV sildenafil was studied in 15 infants. The authors concluded that IV sildenafil augmented the pulmonary vasodilator effects of iNO in infants early after cardiac surgery. However, they found that sildenafil produced systemic hypotension and impaired oxygenation, which was not improved by iNO. In a study by Schulze-Neicke et al., the effects of iNO (20 ppm) were compared before and after the stepwise infusion of sildenafil in 24 children and it was concluded that IV sildenafil is as effective as iNO as a pulmonary vasodilator in children with CHD.
In our study, we compared the clinical impact of perioperative administration of IV sildenafil in one group (Group S) to the absence of the drug in the other (Group C). Rest of the management was exactly similar in both the groups. All the patients were known to have PHT preoperatively. The common perioperative protocol was aimed to reduce PHT in both the groups. Adequate sedation and anesthesia were maintained right from preoperative period in the form of nasal midazolam and ketamine, intraoperatively with the use of IV dexmedetomidine and inhalational anesthetics and postoperatively, by continuation of dexmedetomidine and propofol if required. Adequate perioperative analgesia was ensured using epidural infusion of local anesthetic. Muscle relaxation too was appropriately provided whenever the patient was ventilated. Ventilation strategy, whether intraoperatively or postoperatively in the ICU, was aimed to deliver adequate oxygenation, and avoid acidosis and hypercarbia. It was particularly ensured that any kind of stimulus to the child, e.g., suctioning would be avoided in lighter plane of anesthesia. Another important aspect was IV fluid management. Correct fluid management is the basis for recovery of acute PHT. In patients with PHT, right ventricular (RV) dysfunction/failure can reduce left ventricular (LV) filling and lead to cardiogenic shock. In addition to decreased RV contractility and cardiac output, RV dilatation can further limit LV filling via ventricular interdependence, which causes shifting of the interventricular septum toward the LV cavity. The challenge in fluid management in these patients is to find the optimal RV preload necessary to avoid the detrimental effects of ventricular interdependence on LV function.[22] Pharmacological intervention to manage PHT, in both the groups, was in the form of milrinone infusion.[23,24] Magnesium sulphate was also administered perioperatively, though only observational studies have shown its role in pulmonary vasodilation.[25]
Despite the general management on the lines of PHT, two children developed PH crisis, postoperatively in the ICU. Fundamental management of PH crisis after cardiac surgery includes two aspects (1) Appropriate assessment and treatment of RV failure and (2) acute interventions to compensate for extreme acidosis and tissue hypoxia. PH crisis often requires an aggressive combination of therapies for RV failure, and we should carefully manage inotropes and vasopressors (e.g., dobutamine and norepinephrine), appropriate fluid balance, and maintenance of sinus rhythm and atrioventricular synchrony. The general treatment of acidosis and hypoxia includes oxygenation, alkalinization, hypocapnia, and muscle relaxation.[26] Current clinical studies support the efficacy of gentle ventilation (smaller tidal volumes and limited inspiratory plateau pressure), instead of moderate hyperventilation, for the management of perioperative PHT in pediatric patients.[27] Both the children recovered with this approach though their ICU stay was prolonged.
In our study, better clinical outcome was clearly apparent in sildenafil group. Extubation time was less compared to control group and in fact 10 children could be extubated in the operation room. ICU stay, also, in the former group was truncated. PaO2 – FiO2 ratio was better as well in this group and so was the systolic PAP – systolic AoP ratio. As with the study by Stocker et al., systemic hypotension was not seen in our study with the use of IV sildenafil. This could be because of dopamine infusion (5 μg/kg/min) that is a part of anesthetic protocol followed at our institute. A limitation of our study was that we did not include patients with PHT associated with non-VSD congenital cardiac defects (e.g. TAPVC, truncus arteriosus, and aortopulmonary window). We thought that different basic congenital cardiac pathologies could have impacted the study outcome with respect to the parameters that were assessed and the results would have been confounded. However, a larger study may be conducted in future to include other congenital cardiac defects as well, and interrelation among different lesions and parameters may be analyzed with respect to IV sildenafil.
CONCLUSIONS
IV sildenafil, when used perioperatively, in children with CHD having PHT and undergoing corrective surgery, improves PaO2 – FiO2 ratio and PAP – AoP ratio. It also reduces extubation time and postoperative ICU stay thus reducing the complications related to prolonged postoperative ventilation and ICU stay.
Financial support and sponsorship
Military Hospital (Cardiothoracic Center), Armed Forces Medical College, Pune.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
All the technical support staff at OT and surgical ICU at Military Hospital (Cardiothoracic Center), Pune namely perfusionists, operating room assistants and all the associated nursing staff, also the technicians at Radiology Department, Hospital Pathology Lab and Transfusion Medicine Department
Maj Gen V K Sinha, Commandant, Military Hospital (Cardiothoracic Center), Pune.
REFERENCES
- 1.Barst RJ, McGoon MD, Elliott CG, Foreman AJ, Miller DP, Ivy DD. Survival in childhood pulmonary arterial hypertension: Insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation. 2012;125:113–22. doi: 10.1161/CIRCULATIONAHA.111.026591. [DOI] [PubMed] [Google Scholar]
- 2.Berger RM, Beghetti M, Humpl T, Raskob GE, Ivy DD, Jing ZC, et al. Clinical features of paediatric pulmonary hypertension: A registry study. Lancet. 2012;379:537–46. doi: 10.1016/S0140-6736(11)61621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falcone N. Pulmonary hypertension in pediatric heart surgery. Rev Esp Anestesiol Reanim. 2001;48:462–4. [PubMed] [Google Scholar]
- 4.Ivy DD, Abman SH, Barst RJ, Berger RM, Bonnet D, Fleming TR, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D117–26. doi: 10.1016/j.jacc.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 5.Oishi P, Datar SA, Fineman JR. Advances in the management of pediatric pulmonary hypertension. Respir Care. 2011;56:1314–39. doi: 10.4187/respcare.01297. [DOI] [PubMed] [Google Scholar]
- 6.Murat I, Dubois MC. Perioperative fluid therapy in pediatrics. Paediatr Anaesth. 2008;18:363–70. doi: 10.1111/j.1460-9592.2008.02505.x. [DOI] [PubMed] [Google Scholar]
- 7.Atz AM, Wessel DL. Sildenafil ameliorates effects of inhaled nitric oxide withdrawal. Anesthesiology. 1999;91:307–10. doi: 10.1097/00000542-199907000-00041. [DOI] [PubMed] [Google Scholar]
- 8.Namachivayam P, Theilen U, Butt WW, Cooper SM, Penny DJ, Shekerdemian LS. Sildenafil prevents rebound pulmonary hypertension after withdrawal of nitric oxide in children. Am J Respir Crit Care Med. 2006;174:1042–7. doi: 10.1164/rccm.200605-694OC. [DOI] [PubMed] [Google Scholar]
- 9.Atz AM, Lefler AK, Fairbrother DL, Uber WE, Bradley SM. Sildenafil augments the effect of inhaled nitric oxide for postoperative pulmonary hypertensive crises. J Thorac Cardiovasc Surg. 2002;124:628–9. doi: 10.1067/mtc.2002.125265. [DOI] [PubMed] [Google Scholar]
- 10.Laquay N, Lévy ML, Vaccaroni L, Mauriat P, Carli P. Use of oral sildenafil (Viagra) in pulmonary hypertension after cardiac pediatric surgery. Ann Fr Anesth Reanim. 2003;22:140–3. doi: 10.1016/s0750-7658(02)00863-8. [DOI] [PubMed] [Google Scholar]
- 11.Trachte AL, Lobato EB, Urdaneta F, Hess PJ, Klodell CT, Martin TD, et al. Oral sildenafil reduces pulmonary hypertension after cardiac surgery. Ann Thorac Surg. 2005;79:194–7. doi: 10.1016/j.athoracsur.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 12.El Midany AA, Mostafa EA, Azab S, Hassan GA. Perioperative sildenafil therapy for pulmonary hypertension in infants undergoing congenital cardiac defect closure. Interact Cardiovasc Thorac Surg. 2013;17:963–8. doi: 10.1093/icvts/ivt353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palma G, Giordano R, Russolillo V, Cioffi S, Palumbo S, Mucerino M, et al. Sildenafil therapy for pulmonary hypertension before and after pediatric congenital heart surgery. Tex Heart Inst J. 2011;38:238–42. [PMC free article] [PubMed] [Google Scholar]
- 14.Peiravian F, Amirghofran AA, Borzouee M, Ajami GH, Sabri MR, Kolaee S. Oral sildenafil to control pulmonary hypertension after congenital heart surgery. Asian Cardiovasc Thorac Ann. 2007;15:113–7. doi: 10.1177/021849230701500207. [DOI] [PubMed] [Google Scholar]
- 15.Barst RJ, Layton GR, Konourina I, Richardson H, Beghetti M, Ivy DD. STARTS – 2: Long-term survival with oral sildenafil monotherapy in treatment – Naïve patients with pediatric pulmonary arterial hypertension. Eur Heart J. 2012;33(Suppl 1):979. doi: 10.1161/CIRCULATIONAHA.113.005698. [DOI] [PubMed] [Google Scholar]
- 16.Barst RJ, Ivy DD, Gaitan G, Szatmari A, Rudzinski A, Garcia AE, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012;125:324–34. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 17.Barnett CF, Machado RF. Sildenafil in the treatment of pulmonary hypertension. Vasc Health Risk Manag. 2006;2:411–22. doi: 10.2147/vhrm.2006.2.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reffelmann T, Kloner RA. Therapeutic potential of phosphodiesterase 5 inhibition for cardiovascular disease. Circulation. 2003;108:239–44. doi: 10.1161/01.CIR.0000081166.87607.E2. [DOI] [PubMed] [Google Scholar]
- 19.Fraisse A, Butrous G, Taylor MB, Oakes M, Dilleen M, Wessel DL. Intravenous sildenafil for postoperative pulmonary hypertension in children with congenital heart disease. Intensive Care Med. 2011;37:502–9. doi: 10.1007/s00134-010-2065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stocker C, Penny DJ, Brizard CP, Cochrane AD, Soto R, Shekerdemian LS. Intravenous sildenafil and inhaled nitric oxide: A randomised trial in infants after cardiac surgery. Intensive Care Med. 2003;29:1996–2003. doi: 10.1007/s00134-003-2016-4. [DOI] [PubMed] [Google Scholar]
- 21.Schulze-Neick I, Hartenstein P, Li J, Stiller B, Nagdyman N, Hübler M, et al. Intravenous sildenafil is a potent pulmonary vasodilator in children with congenital heart disease. Circulation. 2003;108(Suppl 1):II167–73. doi: 10.1161/01.cir.0000087384.76615.60. [DOI] [PubMed] [Google Scholar]
- 22.Hui-li G. The management of acute pulmonary arterial hypertension. Cardiovasc Ther. 2011;29:153–75. doi: 10.1111/j.1755-5922.2009.00095.x. [DOI] [PubMed] [Google Scholar]
- 23.Majure DT, Greco T, Greco M, Ponschab M, Biondi-Zoccai G, Zangrillo A, et al. Meta-analysis of randomized trials of effect of milrinone on mortality in cardiac surgery: An update. J Cardiothorac Vasc Anesth. 2013;27:220–9. doi: 10.1053/j.jvca.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Tempe DK. Perioperative management of pulmonary hypertension. Ann Card Anaesth. 2010;13:89–91. doi: 10.4103/0971-9784.62926. [DOI] [PubMed] [Google Scholar]
- 25.Rao S, Bartle D, Patole S. Current and future therapeutic options for persistent pulmonary hypertension in the newborn. Expert Rev Cardiovasc Ther. 2010;8:845–62. doi: 10.1586/erc.09.186. [DOI] [PubMed] [Google Scholar]
- 26.Brunner N, de Jesus Perez VA, Richter A, Haddad F, Denault A, Rojas V, et al. Perioperative pharmacological management of pulmonary hypertensive crisis during congenital heart surgery. Pulm Circ. 2014;4:10–24. doi: 10.1086/674885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umenai T, Shime N, Hashimoto S. Hyperventilation versus standard ventilation for infants in postoperative care for congenital heart defects with pulmonary hypertension. J Anesth. 2009;23:80–6. doi: 10.1007/s00540-008-0682-7. [DOI] [PubMed] [Google Scholar]


