Abstract
Introduction:
Propofol has been suggested as a useful adjunct to cardiopulmonary bypass (CPB) because of its potential protective effect on the heart mediated by a decrease in ischemia-reperfusion injury and inflammation at clinically relevant concentrations. In view of these potentially protective properties, which modulate many of the deleterious mechanism of inflammation attributable to reperfusion injury and CPB, we sought to determine whether starting a low dose of propofol infusion at the beginning of CPB would decrease inflammation as measured by pro-inflammatory markers.
Materials and Methods:
We enrolled 24 patients undergoing elective coronary artery bypass graft (CABG). The study group received propofol at rate of 120 mcg/kg/min immediately after starting CPB and was maintained throughout the surgery and for the following 6 hours in the intensive care unit (ICU). The control group received propofol dose of 30-50 mcg/kg/min which was started at the time of chest closure with wires and continued for the next 6 hours in the ICU. Interleukins (IL) -6, -8 and -10 and tumor necrosis factor alpha (TNFalpha) were assayed.
Result:
The most significant difference was in the level of IL-6 which had a P value of less than 0.06. Starting a low dose propofol early during the CPB was not associated with significant hemodynamic instability in comparison with the control group.
Conclusion:
Our study shows that propofol may be suitable as an anti-inflammatory adjunct for patients undergoing CABG.
Keywords: Cardiopulmonary bypass, Coronary artery bypass graft, Interleukin, Propofol
INTRODUCTION
Cardiopulmonary bypass (CPB) is associated with the activation of a systemic inflammatory response. This is mainly caused by contact of blood with the nonendothelial surfaces of the CPB circuit leading to a massive activation of the extrinsic pathway of coagulation secondary to release and reinfusion of the tissue factor. In addition to the direct activation via the “contact system,” thrombin and activated platelets also stimulate the inflammatory response.[1,2] Cytokines such as interleukins (ILs)-6, -8, and -10, tumor necrosis factor (TNF) as well as complement factors C5a and C3d are released.[3]
Propofol has been suggested as a useful adjunct to CPB because of its potential protective effect on the heart mediated by a decrease in ischemia-reperfusion injury and inflammation at clinically relevant concentrations.[4] Reperfusion injury might manifest as a ventricular dysfunction known as myocardial stunning.[5] It can also produce myocardial necrosis which, if extensive, may result in cardiac failure.[6]
At the cellular level, propofol has been shown to inhibit lipid peroxidation induced by oxidation stress in rat liver mitochondria, microsomes, and brain synaptosomes.[7] It also increases basal endothelial nitric oxide release[8] and protects endothelial cells against the highly toxic-free radical peroxynitrite, another important molecule in the cellular toxicity of ischemia/reperfusion.[9] Mikawa et al. suggested that at clinically relevant concentrations, propofol can also suppress neutrophil chemotaxis, phagocytosis, and reactive oxygen species production.[10]
Corcoran et al. showed that the administration of propofol before aortic cross-clamp release in patients undergoing elective coronary artery bypass graft (CABG) surgery decreases myocardial lipid peroxidation, attenuates the inflammatory response to myocardial reperfusion, and limits the inflammatory cascade. They suggested giving a high dose of propofol around cross-clamp release time so that it reaches a steady state concentration of 6–8 ug/ml; followed by a lower infusion rate so as to maintain a steady state concentration of 2–4 ug/ml for 4 hours would decrease IL-6 production and also prevent an increase in IL-8 concentration, thus pointing to an attenuation of the inflammatory process in patients treated with propofol.[11]
Despite the benefits, negative effects of propofol on myocardial contractility and hemodynamic status have been revealed both in animal and human models.[12,13] The negative inotropic impact was found in nonischemic and acute ischemic myocardium in a dose-dependent manner, but only at a concentration higher than those typically used in clinical practice.[14,15] Another major concern is the propofol infusion syndrome (PRIS) which is believed to be triggered by a dose of propofol exceeding 4 mg/kg/h, time of infusion longer than 48 h, and implementation of concomitant infusions of catecholamines and steroids.[16,17]
MATERIALS AND METHODS
After obtaining approval by the Institutional Review Board (IRB no. 7163) and informed consent, we enrolled 24 American Society of Anesthesiologists (ASA) physical status IV patients undergoing elective CABG. One patient was subsequently removed from the study due to sample collection time error. Twenty-three patients were studied (study group = 12, control group = 11).
Using group sample sizes of 12 test patients and 11 control patients, this study has a statistical power of 0.80 (using a two-sided alpha level of 0.05) to detect an underlying effect size of 1.24. Using an approximate common standard deviation of 50 for the IL-6, IL-8, and IL-10 measures that effect size corresponds to an underlying mean difference of about 62 (e.g., a mean of 50 for test patients vs. 112 for control patients). Furthermore, using an approximate common standard deviation of 20 for the TNF-alpha measures that effect size corresponds to an underlying mean difference of about 25 (e.g., a mean of 10 for test patients vs. 35 for control patients). Therefore, this study was adequately powered to detect mean underlying differences of at least those magnitudes.
The inclusion criteria for the study were the patients with chronic stable angina and normal left ventricular function scheduled to undergo elective CABG. The exclusion criteria were impaired left ventricular function (ejection fraction <50%), clinically significant valvular dysfunction, myocardial infarction within the previous 6 weeks, diabetes mellitus, end-stage renal disease (ESRD), autoimmune disease, concurrent medication with anti-inflammatory effects (exception was made for aspirin 81 mg), immunosuppressive agents, and a history of allergic reaction to propofol.
In this randomized controlled, prospective investigation, patients were randomly assigned to either control arm (n = 11) or study arm (n = 12). Each of the surgeries was performed by a senior staff cardiothoracic surgeon and a physician assistant with no selection bias as to surgeon allocation. A blood sample was obtained from all participants in the preoperative holding area to establish a baseline inflammatory markers level (IL-6, -8, -10 and TNF-alpha).
After establishing standard ASA monitoring, anesthesia was induced with midazolam (0.05–0.1 mg/kg), sufentanil (1–1.5 mcg/kg or equivalent in fentanyl), etomidate (0.1–0.2 mg/kg), and inhalation of isoflurane at 1% with cisatracurium (0.15 mg/kg) to facilitate tracheal intubation. Intermitted positive-pressure ventilation was used to maintain normoxia and normocapnia. The ventilatory management for each patient was standardized to tidal volumes of 8 ml/kg to limit confounding factors.
Anesthesia was maintained with end tidal concentration of isoflurane of 1% in an oxygen air mix, cisatracurium infusion of 2 mcg/kg/min, and sufentanil 2–5 mcg/kg increments. Adequate dose of heparin was given to achieve activated clotting time of at least 400 s before cannulation and initiation of CPB.
The CPB circuit was primed with 1500 ml of plasmalyte. Patients received both anterograde and retrograde cardioplegia at the discretion of the operating surgeon. CPB was performed at mild hypothermia (bladder temperature of 35°C), using membrane oxygenator and nonpulsatile perfusion. Activated clotting time was kept above 400 s by giving intermittent 5,000 units of heparin increments. PaO2 was maintained at 150–250 mmHg and arterial pressure at 50–70 mmHg, with a low rate of 2–4 L/min/m2.
The study group received propofol at rate of 120 mcg/kg/min immediately after starting CPB and was maintained throughout the surgery and for the following 6 h in the Intensive Care Unit (ICU). The control group received propofol dose of 30–50 mcg/kg/min which was started at the time of chest closure with wires and continued for the next 6 h in the ICU.
During the peri-operative period, a total of 5 blood samples were obtained from each patient. First sample (T1) was obtained in preoperative holding area before the patient was transferred to the operating room. Second sample (T2) was obtained 20 min before aortic cross clamp placement. The remaining samples were obtained at 2 (T3), 6 (T4), and 24 h (T5) after aortic cross clamp removal. Samples were frozen at −70°C until all samples were collected and ready for analysis.
IL-6, -8 and -10, and TNF-alpha were assayed with a Bio-Plex 200 System (Bio-Rad, Hercules, CA, USA) using a custom 4-plex kit according to manufacturer's instructions. Analyte concentrations (pg/ml) were calculated from standard curves using Bio-Plex Manager 6.0 software (Bio-Rad, Hercules, CA, USA). Concentrations of each analyte below the lower limit of quantitation (LLOQ) (2.3, 1.9, 2.2, and 5.8 pg/ml for IL-6, -8 and -10, and TNF-alpha, respectively) were set to half the LLOQ.
Due to the distributional nonnormality of the numeric comparison variables, the Wilcoxon rank sum test (a nonparametric alternative to the two-sample t-test) has been used throughout. For the single categorical comparison variable [β-blocker usage; Table 1], the Fisher exact test has been used instead of the more standard Chi-square test due to sparse data (i.e., the presence of expected cell counts <5).
Table 1.
Group comparison results for other study variables
| Variable | Test (n=12) | Control (n=11) | P |
|---|---|---|---|
| Beta blocker (%) | 7 (58.3) | 10 (90.9) | 0.155 (F) |
| Midazolam | 9.4±3.4 | 12.0±5.1 | 0.143 (W) |
| Etomidate | 4.1±5.2 | 6.8±3.9 | 0.277 (W) |
| Sufentanil | 325.0±406.5 | 730.0±460.8 | 0.056 (W) |
| Grafts | 3.5±0.8 | 3.6±1.3 | 0.842 (W) |
| Infusion T | 248.0±81.0 | 77.3±21.4 | <0.001 (W)* |
| Cross clamp | 87.3±29.4 | 94.0±34.2 | 0.498 (W) |
| CPB | 117.3±31.9 | 129.9±33.1 | 0.479 (W) |
| Crystalloid | 1283.3±327.1 | 1190.9±258.7 | 0.508 (W) |
| Colloids | 791.7±1351.9 | 454.5±187.7 | 0.818 (W) |
| Cell Saver | 2.9±3.6 | 1.5±2.0 | 0.270 (W) |
| Urine output | 637.9±319.2 | 623.2±345.4 | 0.368 (W) |
| Fentanyl | 912.5±1427.4 | 227.3±517.9 | 0.137 (W) |
*Statistically significant, P<0.05. Categorical data are given as frequency (percentage of group). Numeric data are given as mean±SD. F: Fisher exact test, W: Wilcoxon rank sum test, CPB: Cardiopulmonary bypass, SD: Standard deviation
As an additional evaluation of the IL-6, -8 and -10, and TNF-alpha data, repeated-measures analysis of variance (ANOVA) has been used. The repeated-measures modeling contains a group factor which represents the overall difference between the two groups when the 5 time points are combined together. That analysis has been performed on a logarithm transformed data, thereby conforming to the ANOVA requirement of distributional normality.
RESULTS
The groups were similar in terms of demographics, procedure performed, and doses of medication as shown in Table 1. All patients required short-term inotropic support upon separation from CPB. The change in markers was most profound at the 2 h mark. We believe that 2 h mark is representative of 2 h after cross clamp removal, which is consistent with our investigative findings that propofol plays a role in modulation of the inflammatory cascade, particularly around the time of reperfusion.
The group comparison results are summarized in Tables 2–5. The infusion T result indicated that the test group has significantly higher levels of that variable than the control group. A statistically significant difference between the two study groups has been detected for the infusion T variable as shown in Table 1.
Table 2.
Group comparison results for IL-6
| Variable | Test (n=12) | Control (n=11) | P |
|---|---|---|---|
| IL-6_1 | 10.1±24.8 | 11.6±12.7 | 0.275 (W) |
| IL-6_2 | 34.9±30.4 | 65.6±63.0 | 0.479 (W) |
| IL-6_3 | 229.1±150.7 | 867.9±1237.7 | 0.166 (W) |
| IL-6_4 | 558.2±670.2 | 1368.2±2305.6 | 0.117 (W) |
| IL-6_5 | 342.9±495.3 | 183.5±89.2 | 0.782 (W) |
Data are given as mean±SD. W: Wilcoxon rank sum test, SD: Standard deviation, IL-6: Interleukins-6
Table 5.
Group comparison results for TNF-alpha
| Variable | Test (n=12) | Control (n=11) | P |
|---|---|---|---|
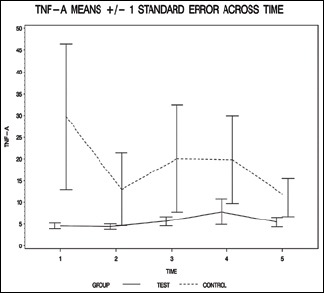
| TNF-alpha_1 | 4.6±2.2 | 29.7±55.6 | 0.335 (W) |
| TNF-alpha_2 | 4.4±2.1 | 13.0±27.7 | 1.000 (W) |
| TNF-alpha_3 | 5.7±3.6 | 20.1±40.8 | 0.204 (W) |
| TNF-alpha_4 | 7.8±10.1 | 19.8±33.6 | 0.319 (W) |
| TNF-alpha_5 | 5.5±3.8 | 11.1±14.5 | 0.765 (W) |
Data are given as mean±SD. W: Wilcoxon rank sum test, SD: Standard deviation, TNF-alpha: Tumor necrosis factor-alpha
Table 3.
Group comparison results for IL-8
| Variable | Test (n=12) | Control (n=11) | P |
|---|---|---|---|
| IL-8_1 | 44.0±48.9 | 55.3±74.6 | 0.926 (W) |
| IL-8_2 | 22.4±11.1 | 37.5±26.9 | 0.148 (W) |
| IL-8_3 | 79.2±65.9 | 98.2±77.9 | 0.460 (W) |
| IL-8_4 | 109.5±90.9 | 119.4±91.7 | 0.460 (W) |
| IL-8_5 | 55.7±36.0 | 56.7±23.4 | 0.538 (W) |
Data are given as mean±SD. W: Wilcoxon rank sum test, SD: Standard deviation, IL-8: Interleukins-8
Table 4.
Group comparison results for IL-10
| Variable | Test (n=12) | Control (n=11) | P |
|---|---|---|---|
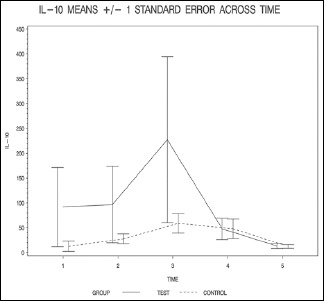
| IL-10_1 | 91.7±275.9 | 12.8±34.9 | 0.368 (W) |
| IL-10_2 | 96.7±267.0 | 27.6±33.0 | 0.758 (W) |
| IL-10_3 | 227.4±579.0 | 58.9±65.1 | 0.689 (W) |
| IL-10_4 | 47.8±74.4 | 48.0±64.9 | 0.829 (W) |
| IL-10_5 | 13.3±16.6 | 12.5±12.3 | 1.000 (W) |
Data are given as mean±SD. W: Wilcoxon rank sum test, SD: Standard deviation, IL-10: Interleukins-10
The resulting group factor P values are all >0.05, indicating that none of the overall group differences are statistically significant (P = 0.099 for IL-6, P = 0.348 for IL-8, P = 0.641 for IL-10, and P = 0.273 for TNF-alpha).
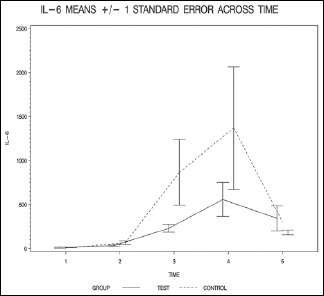
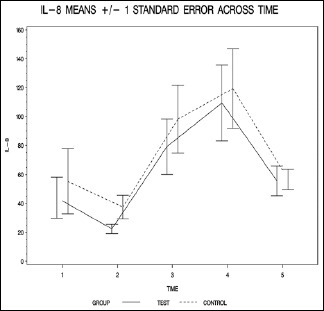
The finding of this study suggests that propofol administration, starting from the beginning of CPB and continuing it for 6 h after cross clamp removal, may have beneficial effects on the inflammatory process that develops during the procedure. Propofol administration prevented the rise of IL-6, -8, and TNF-alpha pointing to a possible attenuation of the inflammatory process. On the other hand, there was a slight increase in the level of IL-10. The most significant difference was in the level of IL-6 which had a P value of <0.06 while the rest of markers had a P value that was higher than 0.1.
DISCUSSION
Fransen et al. reported that the acute inflammatory response in CABG patients, which has historically been ascribed to CPB, may actually be initiated by the surgical procedure itself, which includes, but is not limited to skin incision and median sternotomy.[18] Brasil et al. reported a significantly higher release of TNF-alpha in the CPB group when compared with off-pump surgery.[19] However, recent data from Tarnok et al. suggest that similar increased serum levels of inflammatory mediators and increased consumption of complement and adhesion molecules occur during cardiovascular surgery with or without CPB in children. They concluded that these changes are the combined effect of anesthesia, surgical trauma, and endothelial lesions.[20]
Reactive oxygen species free radical generation is a critical early event in myocardial reperfusion injury. They are produced in the myocardium and endothelium during reperfusion.[21,22] Reactive oxygen species are responsible for many of the adverse effects of reperfusion, including cellular calcium dysregulation, endothelial and neutrophil activation, and proinflammatory cytokine release.
Propofol's anti-inflammatory effects have attributed to its ability to modulate macrophage functions via the suppression of migration, phagocytosis, and oxidative ability.[23] Data from Chang et al. revealed that therapeutic concentrations of propofol can protect macrophages from nitric oxide-induced insults to the cell.[24]
The anti-inflammatory properties of propofol can be exerted using a dose as low as 10 μg/ml in blood.[25] However, abusive treatment with propofol can cause severe complications including PRIS in patients with critical illnesses.[26,27] Clinical manifestations and pathological observations showed a variety of cellular injury in PRIS patients, including lipemic plasma, fatty liver enlargement, metabolic acidosis, rhabdomyolysis, and myoglobinuria. Murphy et al. showed that treatment with a high dosage of propofol (25 μg/ml) resulted in macrophage apoptosis.[28]
Propofol's antioxidant properties have been attributed to its phenolic, Vitamin E–like structure.[29] This limits tissue oxidative stress effects,[30] which may be accounted for by its reaction with toxic peroxynitrite molecules to form a less reactive phenoxyl molecule or by its ability to bind to reactive hydroxyl radicals more avidly than the spin-trap 5,5-dimethyl pyrroline-N-oxide, detoxifying these radicals, and preventing free radical-mediated lipid peroxidation.[31]
Our study showed that despite lower inflammatory markers in the test group, P value remained about the 0.05 mark which might suggest that starting propofol early during CPB may not be as protective as starting a higher dose infusion during the period of cross clamp removal. It is well-known that the pattern of peak release of cytokines is around this period.[32]
IL-10 levels might have been influenced by factors other than the inflammation process. As it is mainly cleared by the kidney, its plasma half-life is markedly increased in ESRD leading to elevated plasma level. Furthermore, uremic monocytes produce higher amount of IL-10 compared to healthy individual.[33]
Our rationale for this study was to utilize the theoretical antioxidant effects of propofol prior to reperfusion. On reperfusion, activated neutrophils are flushed from pulmonary and cardiac capillaries into systemic circulation. Accordingly, the reperfusion period has been shown to entail an increase in circulating concentrations of free radicals. Previous investigations have shown that the peak concentration of free radicals in the peripheral circulation system appears at the reperfusion period.[34]
We acknowledge some limitations in our pilot study. The sample size was only 24 patients, which might have affected the final result of the study. Also, the propofol concentration in blood was not measured for the patients, and given the dilutional effect of bypass and decreased perfusion to both liver and kidney; propofol level in blood may have had a fluctuated course, instead of being at the desired steady state. Also, the use of inhalational agents might have played a role in potentiating the anti-inflammatory effect.
Our study shows that despite using a relatively higher dose of propofol (120 mcg/kg/min) throughout CPB, there was no significant hemodynamic difference between the study and the control group. This was evident by stable vital signs during the propofol infusion with no need to adjust the inotropes and pressors provided to the patient. Also, there was no statistical difference in the amount crystalloid, colloids, blood products given to the patients in both groups as shown in Table 1.
CONCLUSION
Propofol may be suitable as an anti-inflammatory adjunct for patients undergoing CABG. Starting a low dose propofol early during the CPB was not associated with significant hemodynamic instability in comparison with the control group. However, larger sample sizes are needed to further elucidate if its anti-inflammatory effects during CPB are statistically significant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Despotis GJ, Avidan MS, Hogue CW., Jr Mechanisms and attenuation of hemostatic activation during extracorporeal circulation. Ann Thorac Surg. 2001;72:S1821–31. doi: 10.1016/s0003-4975(01)03211-8. [DOI] [PubMed] [Google Scholar]
- 2.Mojcik CF, Levy JH. Aprotinin and the systemic inflammatory response after cardiopulmonary bypass. Ann Thorac Surg. 2001;71:745–54. doi: 10.1016/s0003-4975(00)02218-9. [DOI] [PubMed] [Google Scholar]
- 3.Diegeler A, Doll N, Rauch T, Haberer D, Walther T, Falk V, et al. Humoral immune response during coronary artery bypass grafting: A comparison of limited approach, “off-pump” technique, and conventional cardiopulmonary bypass. Circulation. 2000;102(19 Suppl 3):III95–100. doi: 10.1161/01.cir.102.suppl_3.iii-95. [DOI] [PubMed] [Google Scholar]
- 4.Lim KH, Hale strap AP, Angelini GD, Suleiman MS. Propofol is cardioprotective in a clinically relevant model of normothermic blood cardioplegic arrest and cardiopulmonary bypass. Exp Biol Med (Maywood) 2005;230:413–20. doi: 10.1177/15353702-0323006-09. [DOI] [PubMed] [Google Scholar]
- 5.Breisblatt WM, Stein KL, Wolfe CJ, Follansbee WP, Capozzi J, Armitage JM, et al. Acute myocardial dysfunction and recovery: A common occurrence after coronary bypass surgery. J Am Coll Cardiol. 1990;15:1261–9. doi: 10.1016/s0735-1097(10)80011-7. [DOI] [PubMed] [Google Scholar]
- 6.Lefer DJ, Granger DN. Oxidative stress and cardiac disease. Am J Med. 2000;109:315–23. doi: 10.1016/s0002-9343(00)00467-8. [DOI] [PubMed] [Google Scholar]
- 7.Musacchio E, Rizzoli V, Bianchi M, Bindoli A, Galzigna L. Antioxidant action of propofol on liver microsomes, mitochondria and brain synaptosomes in the rat. Pharmacol Toxicol. 1991;69:75–7. doi: 10.1111/j.1600-0773.1991.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 8.Petros AJ, Bogle RG, Pearson JD. Propofol stimulates nitric oxide release from cultured porcine aortic endothelial cells. Br J Pharmacol. 1993;109:6–7. doi: 10.1111/j.1476-5381.1993.tb13523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathy-Hartert M, Mouithys-Mickalad A, Kohnen S, Deby-Dupont G, Lamy M, Hans P. Effects of propofol on endothelial cells subjected to a peroxynitrite donor (SIN-1) Anaesthesia. 2000;55:1066–71. doi: 10.1046/j.1365-2044.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 10.Mikawa K, Akamatsu H, Nishina K, Shiga M, Maekawa N, Obara H, et al. Propofol inhibits human neutrophil functions. Anesth Analg. 1998;87:695–700. doi: 10.1097/00000539-199809000-00039. [DOI] [PubMed] [Google Scholar]
- 11.Corcoran TB, Engel A, Sakamoto H, O’Callaghan-Enright S, O’Donnell A, Heffron JA, et al. The effects of propofol on lipid peroxidation and inflammatory response in elective coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2004;18:592–604. doi: 10.1053/j.jvca.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Ozaki M. The effects of propofol and midazolam on canine left ventricular contractility. Masui. 2002;51:611–9. [PubMed] [Google Scholar]
- 13.De Hert SG, ten Broecke PW, Mertens E, Van Sommeren EW, De Blier IG, Stockman BA, et al. Sevoflurane but not propofol preserves myocardial function in coronary surgery patients. Anesthesiology. 2002;97:42–9. doi: 10.1097/00000542-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 14.De Hert SG. Study on the effects of six intravenous anesthetic agents on regional ventricular function in dogs (thiopental, etomidate, propofol, fentanyl, sufentanil, alfentanil) Acta Anaesthesiol Belg. 1991;42:3–39. [PubMed] [Google Scholar]
- 15.Royse CF, Liew DF, Wright CE, Royse AG, Angus JA. Persistent depression of contractility and vasodilation with propofol but not with sevoflurane or desflurane in rabbits. Anesthesiology. 2008;108:87–93. doi: 10.1097/01.anes.0000296077.32685.26. [DOI] [PubMed] [Google Scholar]
- 16.Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62:690–701. doi: 10.1111/j.1365-2044.2007.05055.x. [DOI] [PubMed] [Google Scholar]
- 17.Rajda C, Dereczyk D, Kunkel P. Propofol infusion syndrome. J Trauma Nurs. 2008;15:118–22. doi: 10.1097/01.JTN.0000337153.08464.0f. [DOI] [PubMed] [Google Scholar]
- 18.Fransen EJ, Maessen JG, Hermens WT, et al. Perioperative myocardial tissue injury and the release of inflammatory mediators in coronary artery bypass graft patients. Cardiovasc Res. 2000;45:853–9. doi: 10.1016/s0008-6363(99)00403-4. [DOI] [PubMed] [Google Scholar]
- 19.Brasil LA, Gomes WJ, Salomão R, Buffolo E. Inflammatory response after myocardial revascularization with or without cardiopulmonary bypass. The Annals of thoracic surgery. 1998;66:56–9. doi: 10.1016/s0003-4975(98)00181-7. [DOI] [PubMed] [Google Scholar]
- 20.Tárnok A, Hambsch J, Emmrich F, Sack U, van Son J, Bellinghausen W, et al. Complement activation, cytokines, and adhesion molecules in children undergoing cardiac surgery with or without cardiopulmonary bypass. Pediatr Cardiol. 1999;20:113–25. doi: 10.1007/s002469900417. [DOI] [PubMed] [Google Scholar]
- 21.Ascione R, Lloyd CT, Underwood MJ, Gomes WJ, Angelini GD. On-pump versus off-pump coronary revascularization: Evaluation of renal function. Ann Thorac Surg. 1999;68:493–8. doi: 10.1016/s0003-4975(99)00566-4. [DOI] [PubMed] [Google Scholar]
- 22.Chen RM, Wu CH, Chang HC, Wu GJ, Lin YL, Sheu JR, et al. Propofol suppresses macrophage functions and modulates mitochondrial membrane potential and cellular adenosine triphosphate synthesis. Anesthesiology. 2003;98:1178–85. doi: 10.1097/00000542-200305000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Chen RM, Chen TG, Chen TL, et al. Anti inflamatory and antioxidative effects of propofol on lipopolysaccharide-activated macrophages. Ann N Y Acad Sci. 2005:262–71. doi: 10.1196/annals.1338.030. [DOI] [PubMed] [Google Scholar]
- 24.Chang H, Tsai SY, Chang Y, Chen TL, Chen RM. Therapeutic concentrations of propofol protects mouse macrophages from nitric oxide-induced cell death and apoptosis. Can J Anaesth. 2002;49:477–80. doi: 10.1007/BF03017924. [DOI] [PubMed] [Google Scholar]
- 25.Vasile B, Rasulo F, Candiani A, Latronico N. The pathophysiology of propofol infusion syndrome: A simple name for a complex syndrome. Intensive Care Med. 2003;29:1417–25. doi: 10.1007/s00134-003-1905-x. [DOI] [PubMed] [Google Scholar]
- 26.Fudickar A, Bein B. Propofol infusion syndrome: Update of clinical manifestation and pathophysiology. Minerva Anestesiol. 2009;75:339–44. [PubMed] [Google Scholar]
- 27.Hsing CH, Lin MC, Choi PC, Huang WC, Kai JI, Tsai CC, et al. Anesthetic propofol reduces endotoxic inflammation by inhibiting reactive oxygen species-regulated Akt/IKKß/NF-kB signaling. PLoS One. 2011;6:e17598. doi: 10.1371/journal.pone.0017598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy PG, Myers DS, Davies MJ, Webster NR, Jones JG. The antioxidant potential of propofol (2,6-diisopropylphenol) Br J Anaesth. 1992;68:613–8. doi: 10.1093/bja/68.6.613. [DOI] [PubMed] [Google Scholar]
- 29.De La Cruz JP, Sedeño G, Carmona JA, Sánchez de la Cuesta F. The in vitro effects of propofol on tissular oxidative stress in the rat. Anesth Analg. 1998;87:1141–6. doi: 10.1097/00000539-199811000-00031. [DOI] [PubMed] [Google Scholar]
- 30.Mouithys-Mickalad A, Hans P, Deby-Dupont G, Hoebeke M, Deby C, Lamy M. Propofol reacts with peroxynitrite to form a phenoxyl radical: Demonstration by electron spin resonance. Biochem Biophys Res Commun. 1998;249:833–7. doi: 10.1006/bbrc.1998.9235. [DOI] [PubMed] [Google Scholar]
- 31.Hess ML, Okabe E, Kontos HA. Proton and free oxygen radical interaction with the calcium transport system of cardiac sarcoplasmic reticulum. J Mol Cell Cardiol. 1981;13:767–72. doi: 10.1016/0022-2828(81)90258-3. [DOI] [PubMed] [Google Scholar]
- 32.Kokita N, Hara A, Abiko Y, Arakawa J, Hashizume H, Namiki A. Propofol improves functional and metabolic recovery in ischemic reperfused isolated rat hearts. Anesth Analg. 1998;86:252–8. doi: 10.1097/00000539-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Hall RI, Smith MS, Rocker G. The systemic inflammatory response to cardiopulmonary bypass: Pathophysiological, therapeutic, and pharmacological considerations. Anesth Analg. 1997;85:766–82. doi: 10.1097/00000539-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Zhang SH, Wang SY, Yao SL. Antioxidative effect of propofol during cardiopulmonary bypass in adults. Acta Pharmacol Sin. 2004;25:334–40. [PubMed] [Google Scholar]


