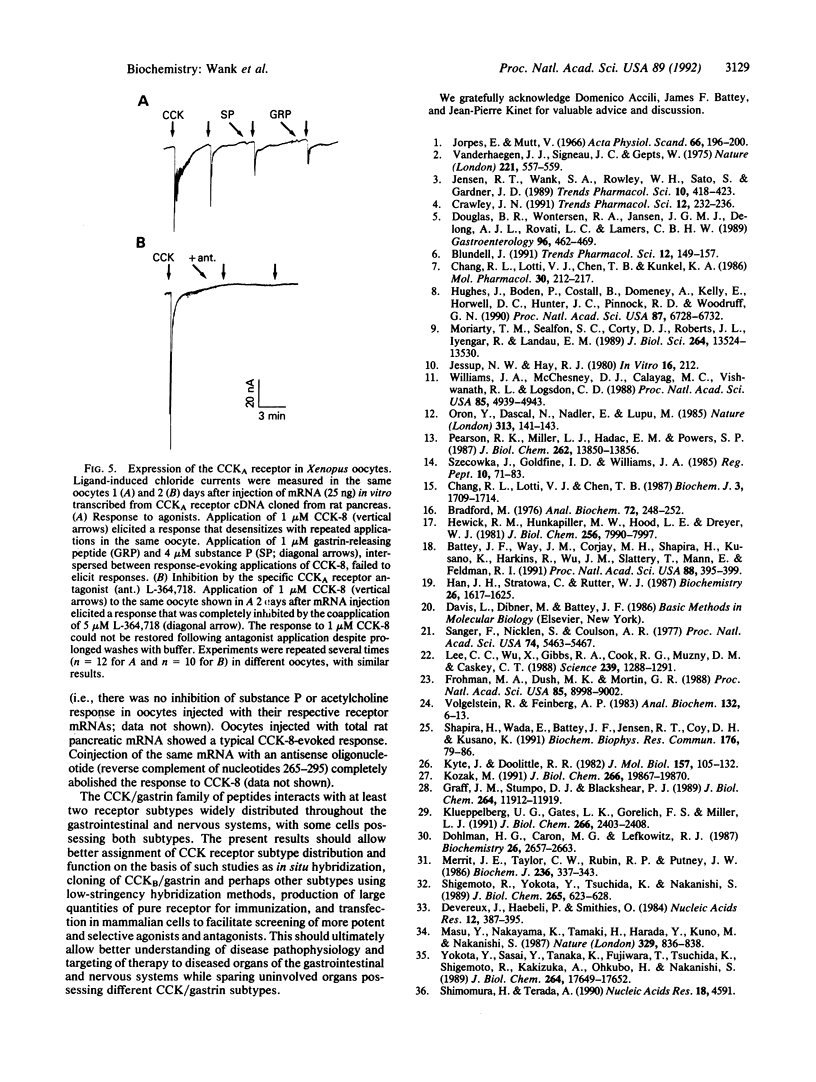
Abstract
The cholecystokinin (CCK) family of peptides and their receptors are widely distributed throughout the gastrointestinal and central nervous systems where they regulate secretion, motility, growth, anxiety, and satiety. The CCK receptors can be subdivided into at least two subtypes, CCKA and CCKB on the basis of pharmacological studies. We report here the purification of the CCKA receptor to homogeneity from rat pancreas by using ion-exchange and multiple affinity chromatographic separations. This allowed partial peptide sequencing after chemical/enzymatic cleavage and synthesis of degenerate oligonucleotide primers. These primers were used for initial cloning of the cDNA from rat pancreas by PCR. The predicted protein sequence of the cDNA clone contained the five partial peptide sequences obtained from the purified protein. Seven putative transmembrane domains suggest its membership in the guanine nucleotide-binding regulatory protein-coupled receptor superfamily. In vitro transcripts of the cDNA clone were functionally expressed in Xenopus oocytes and displayed the expected agonist and antagonist specificity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battey J. F., Way J. M., Corjay M. H., Shapira H., Kusano K., Harkins R., Wu J. M., Slattery T., Mann E., Feldman R. I. Molecular cloning of the bombesin/gastrin-releasing peptide receptor from Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):395–399. doi: 10.1073/pnas.88.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J. Pharmacological approaches to appetite suppression. Trends Pharmacol Sci. 1991 Apr;12(4):147–157. doi: 10.1016/0165-6147(91)90532-w. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Chen T. B. Characterization of [3H](+/-)L-364,718 binding to solubilized cholecystokinin (CCK) receptors of rat pancreas. Biochem Pharmacol. 1987 May 15;36(10):1709–1714. doi: 10.1016/0006-2952(87)90057-8. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Chen T. B., Kunkel K. A. Characterization of the binding of [3H]-(+/-)-L-364,718: a new potent, nonpeptide cholecystokinin antagonist radioligand selective for peripheral receptors. Mol Pharmacol. 1986 Sep;30(3):212–217. [PubMed] [Google Scholar]
- Crawley J. N. Cholecystokinin-dopamine interactions. Trends Pharmacol Sci. 1991 Jun;12(6):232–236. doi: 10.1016/0165-6147(91)90558-a. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., Lefkowitz R. J. A family of receptors coupled to guanine nucleotide regulatory proteins. Biochemistry. 1987 May 19;26(10):2657–2664. doi: 10.1021/bi00384a001. [DOI] [PubMed] [Google Scholar]
- Douglas B. R., Woutersen R. A., Jansen J. B., de Jong A. J., Rovati L. C., Lamers C. B. Influence of cholecystokinin antagonist on the effects of cholecystokinin and bombesin on azaserine-induced lesions in rat pancreas. Gastroenterology. 1989 Feb;96(2 Pt 1):462–469. doi: 10.1016/0016-5085(89)91572-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J. M., Stumpo D. J., Blackshear P. J. Characterization of the phosphorylation sites in the chicken and bovine myristoylated alanine-rich C kinase substrate protein, a prominent cellular substrate for protein kinase C. J Biol Chem. 1989 Jul 15;264(20):11912–11919. [PubMed] [Google Scholar]
- Han J. H., Stratowa C., Rutter W. J. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987 Mar 24;26(6):1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hughes J., Boden P., Costall B., Domeney A., Kelly E., Horwell D. C., Hunter J. C., Pinnock R. D., Woodruff G. N. Development of a class of selective cholecystokinin type B receptor antagonists having potent anxiolytic activity. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6728–6732. doi: 10.1073/pnas.87.17.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. T., Wank S. A., Rowley W. H., Sato S., Gardner J. D. Interaction of CCK with pancreatic acinar cells. Trends Pharmacol Sci. 1989 Oct;10(10):418–423. doi: 10.1016/0165-6147(89)90192-2. [DOI] [PubMed] [Google Scholar]
- Jorpes E., Mutt V. Cholecystokinin and pancreozymin, one single hormone? Acta Physiol Scand. 1966 Jan-Feb;66(1):196–202. doi: 10.1111/j.1748-1716.1966.tb03185.x. [DOI] [PubMed] [Google Scholar]
- Klueppelberg U. G., Gates L. K., Gorelick F. S., Miller L. J. Agonist-regulated phosphorylation of the pancreatic cholecystokinin receptor. J Biol Chem. 1991 Feb 5;266(4):2403–2408. [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty T. M., Sealfon S. C., Carty D. J., Roberts J. L., Iyengar R., Landau E. M. Coupling of exogenous receptors to phospholipase C in Xenopus oocytes through pertussis toxin-sensitive and -insensitive pathways. Cross-talk through heterotrimeric G-proteins. J Biol Chem. 1989 Aug 15;264(23):13524–13530. [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Pearson R. K., Miller L. J., Hadac E. M., Powers S. P. Analysis of the carbohydrate composition of the pancreatic plasmalemmal glycoprotein affinity labeled by short probes for the cholecystokinin receptor. J Biol Chem. 1987 Oct 5;262(28):13850–13856. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira H., Wada E., Battey J. F., Jensen R. T., Coy D. H., Kusano K. Distinguishing bombesin receptor subtypes using the oocyte assay. Biochem Biophys Res Commun. 1991 Apr 15;176(1):79–86. doi: 10.1016/0006-291x(91)90892-b. [DOI] [PubMed] [Google Scholar]
- Shigemoto R., Yokota Y., Tsuchida K., Nakanishi S. Cloning and expression of a rat neuromedin K receptor cDNA. J Biol Chem. 1990 Jan 15;265(2):623–628. [PubMed] [Google Scholar]
- Shimomura H., Terada A. Primary structure of the rat beta-1 adrenergic receptor gene. Nucleic Acids Res. 1990 Aug 11;18(15):4591–4591. doi: 10.1093/nar/18.15.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szecòwka J., Goldfine I. D., Williams J. A. Solubilization and characterization of CCK receptors from mouse pancreas. Regul Pept. 1985 Mar;10(2-3):71–83. doi: 10.1016/0167-0115(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Williams J. A., McChesney D. J., Calayag M. C., Lingappa V. R., Logsdon C. D. Expression of receptors for cholecystokinin and other Ca2+-mobilizing hormones in Xenopus oocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4939–4943. doi: 10.1073/pnas.85.13.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y., Sasai Y., Tanaka K., Fujiwara T., Tsuchida K., Shigemoto R., Kakizuka A., Ohkubo H., Nakanishi S. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem. 1989 Oct 25;264(30):17649–17652. [PubMed] [Google Scholar]





