Abstract
Treatment of acute myeloid leukaemia (AML) is challenging and emerging treatment options include protein phosphatase 2A (PP2A) activators. Fingolimod is a known PP2A activator that inhibits multiple signalling pathways and has been used extensively in patients with multiple sclerosis and other indications. The initial positive results of PP2A activators in vitro and mouse models of AML are promising; however, its safety for use in AML has not been assessed. From human studies of fingolimod in other indications, it is possible to evaluate whether the safety and toxicity profile of the PP2A activators will allow their use in treating AML. A literature review was carried out to assess safety before the commencement of Phase I trials of the PP2A activator Fingolimod in AML. From human studies of fingolimod in other indications, it is possible to evaluate whether the safety and toxicity profile of the PP2A activators will allow their use in treating AML. A systematic review of published literature in Medline, EMBASE and the Cochrane Library of critical reviews was carried out. International standards for the design and reporting of search strategies were followed. Search terms and medical subject headings used in trials involving PP2A activators as well as a specific search were performed for ‘adverse events’, ‘serious adverse events’, ‘delays in treatment’, ‘ side effects’ and ‘toxicity’ for primary objectives. Database searches were limited to papers published in the last 12 years and available in English. The search yielded 677 articles. A total of 69 journal articles were identified as relevant and included 30 clinical trials, 24 review articles and 15 case reports. The most frequently reported adverse events were nausea, diarrhoea, fatigue, back pain, influenza viral infections, nasopharyngitis and bronchitis. Specific safety concerns include monitoring of the heart rate and conduction at commencement of treatment as cardiotoxicity has been reported. There is little evidence to suggest specific bone marrow toxicity. Lymophopenia is a desired effect in the management of multiple sclerosis, but may have implications in patients with acute leukaemia as it may potentially increase susceptibility to viral infections such as influenza. Fingolimod is a potential treatment option for AML with an acceptable risk to benefit ratio, given its lack of bone marrow toxicity and the relatively low rate of serious side effects. As most patients with AML are elderly, specific monitoring for cardiac toxicity as well as infection would be required.
Keywords: acute myeloid leukaemia, cytopenia, fingolimod, infections, PP2A activators, toxicity
Introduction
Treatment of acute myeloid leukaemia (AML) is challenging and clinical trials involving novel approaches are considered the best therapeutic strategy in many patients with AML 1. One potential approach, which has shown promising results in in vitro and mouse models, is pharmacological activation of a serine/threonine phosphatase-negative regulator, protein phosphatase 2A (PP2A) 2.
PP2A is a multimeric enzyme composed of a catalytic subunit (C), a structural subunit (A) and a variable regulatory subunit (B), of which there are at least three different families (B55, B56, B″), each with several isoforms 3. The association of a particular B subunit with the core AC dimer introduces substrate specificity and subcellular targeting of PP2A activity 4–6. PP2A activity is further regulated by post-translational modifications and by endogenous inhibitory proteins such as the SET and CIP2A (cancerous inhibitor of PP2A) oncogenes 7,8. PP2A is involved in numerous cellular processes, including cell cycle regulation, DNA damage response, apoptosis and migration. The tumour-suppressing activities of PP2A depend on its ability to inactivate multiple components of growth and survival signalling pathways required for tumourigenesis, such as the Ras/ERK, PI3K/Akt, JNK, JAK/STAT and Wnt/β-catenin pathways. PP2A also regulates c-Myc, p53 and apoptosis-related proteins such as Bcl-2, Bax and Bad 2. Functional inactivation of PP2A has been observed in a range of myeloid malignancies 6. In AML, the receptor tyrosine kinase c-KIT is mutated in a small subset of patients as seen in those with core binding factor AML, where oncogenic c-KIT signalling requires inhibition of PP2A for leukaemogenesis 9. Impaired PP2A activity has further been reported as a common event in AML 10. In preclinical studies on core binding factor AML, pharmacological activation of PP2A inhibited proliferation, survival and in-vivo leukaemogenesis, suggesting that targeting PP2A activation may be a useful therapeutic strategy 9,11. PP2A inactivation has also been described to be essential for BCR-ABL1-induced leukaemogenesis in chronic myeloid leukaemia and in Jak2(V617F) mutant-driven myeloproliferative neoplasms 12. In all of these studies, preclinical evaluation of the PP2A activating drug, fingolimod (FTY720, Gilenya; Novartis, Basel, Switzerland), showed a substantial antileukaemic effect. A preclinical study has also reported an apoptotic effect of fingolimod in B-cell chronic lymphoid leukaemias 13.
A number of small molecules have been reported to activate PP2A 14,15. To date, the most promising of these for clinical applications is the sphingosine analogue FTY720 (fingolimod; Gilenya; Novartis). It is a water-soluble drug with high oral bioavailability that reversibly arrests lymphocyte trafficking and has recently been approved by the United States Food and Drug Administration (FDA) as an immunomodulator for multiple sclerosis (MS) patients. In vivo, fingolimod is phosphorylated by sphingosine kinase 2 and phosphorylated fingolimod (FTY720-P) binds to sphingosine-1-phosphate receptors (SIP-R), leading to modulation of chemotactic responses and lymphocyte trafficking 16,17. In addition to SIP-R agonism, fingolimod activates PP2A (both in situ and in vitro) and induces apoptosis of a range of cancer cells. PP2A activation is independent of fingolimod phosphorylation and SIP-R binding, and is absolutely essential for its apoptosis-inducing effects 9,13,18,19. Indeed, a number of chiral analogues of fingolimod that cannot be phosphorylated by sphingosine kinase 2 and do not bind SIP-Rs, such as AAL(S) and OSU-2S, are just as effective at activating PP2A complexes in vitro and in mouse models 20–22. The mechanism by which fingolimod activates PP2A is not completely understood, but is likely through its direct interaction with PP2A inhibitory proteins, such as the SET oncogene 23.
The initial positive results of PP2A activators in vitro and mouse models are promising; however, its safety for use in humans with AML has not been assessed 11. Given the extensive experience with fingolimod, a PP2A activator, in MS and other indications, we aimed to study whether the safety and toxicity profile of this PP2A activator will allow their use in treating AML as well as other haematological malignancies.
Methods
A systematic review of the published literature in Medline, EMBASE and the Cochrane Library of critical reviews was performed. International standards for the design and reporting of search strategies were followed. Search terms and medical subject headings used in trials involving PP2A activators as well as a specific search were performed for ‘adverse events’, ‘serious adverse events’, ‘delays in treatment’, ‘ side effects’ and ‘toxicity’ for primary objectives. Database searches were limited to papers published in the last 12 years, from January 2003 to December 2014, and available in English.
Search strategy
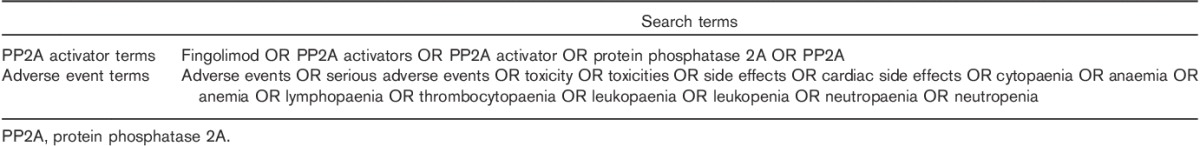
A combination of keywords and subject headings was used to identify eligible publications. Terms relating to fingolimod or PP2A activators were combined with adverse event terms using the ‘AND’ Boolean operator. Each search strategy was tailored to the specifications of the individual database. A list of the search terms used is provided in Table 1.
Table 1.
Search terms used in the systematic review
Inclusion criteria
Publications were eligible for inclusion if the full-text publication could be accessed and if reported rates of adverse events were associated with fingolimod-treated patients. Both qualitative and quantitative studies were included where appropriate. Review publications were included if they contained a systematic analysis of adverse events. Case reports and conference abstracts were included if relevant information relating to adverse events could be extracted.
Exclusion criteria
Studies that included only cell culture, animal model or in-vitro data were excluded. Publications in a language other than English were not included. Commentaries, letters to the editor, and protocol publications were excluded.
Publication analysis
A second reviewer assessed all publications for eligibility, with publications excluded if the title or the abstract clearly indicated that the study did not fulfil the inclusion criteria. The same reviewer then reviewed the abstracts of the remaining publications. As a measure of quality assurance, a second reviewer assessed 20% of all publication titles and abstracts. Any discrepancies were discussed and resolved. Two reviewers assessed all full-text publications for eligibility independently. Data from all eligible publications were analysed and extracted by the two reviewers.
Data coding and extraction
A PRISMA four phase approach was used for selection of eligible publications as shown in Fig. 1 24. Only outcome data relating to medication adverse events in patients on fingolimod were extracted and analysed. The information extracted from each publication included author name, journal, year of publication, country where the study was carried out, patient age group, sample size, response rate, study design, disease type, participant sex, treatment type including the combination of drugs used with fingolimod, systemic complications, complication rates, abnormalities in laboratory investigations and conclusions.
Fig. 1.
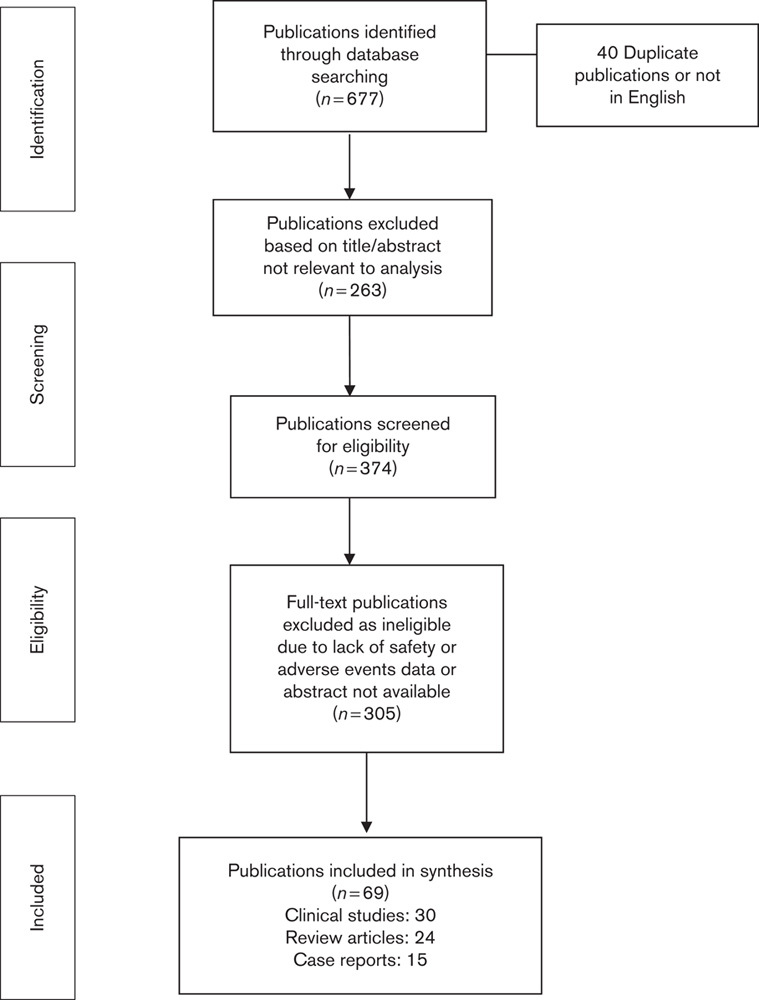
PRISMA four-phase flow diagram describing the selection process of the eligible publications.
Results
Publication screening
A total of 677 publications were initially identified from database searches, out of which 637 were screened following the removal of duplicates and those not in English. Fingolimod was the only PP2A activator reported as being in clinical use and all articles subsequently screened for adverse events reported on the use of fingolimod only. Another 263 publications were excluded on the basis of title and/or abstract that was not relevant to the analysis. After screening the full-length article and/or abstract, an additional 305 articles were excluded on the basis of lack of inclusion of safety or adverse event data in the publication.
Characteristics of included studies
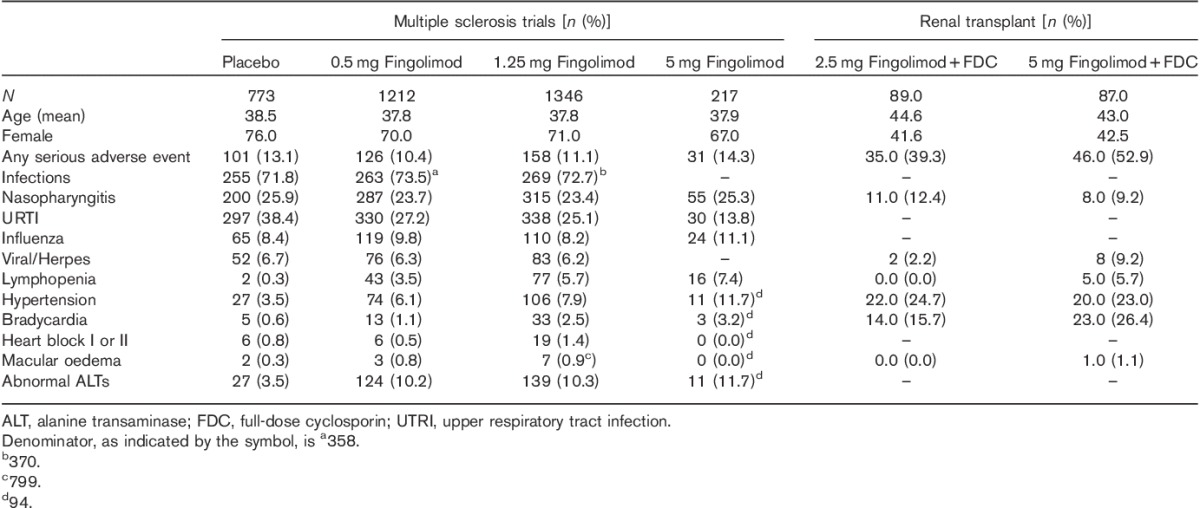
A total of 30 clinical studies, 15 case reports and 24 review articles were analysed. Among the clinical studies, four large randomized control trials (RCTs) in MS patients and one study in the postrenal transplant setting provided the highest-quality evidence as shown in Table 2 25–29. A total of 2951 patients from all RCTs were grouped according to the dose of fingolimod and if there was concomitant use of another drug with specific details of side effects, as shown in Table 3.
Table 2.
Key clinical trials included in the synthesis
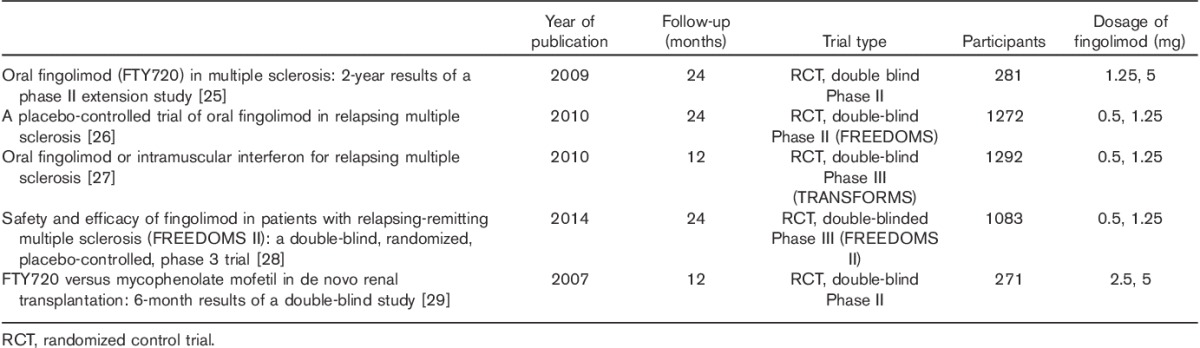
Table 3.
Pooled analysis of adverse events from the major clinical trials
Any adverse event
Overall, serious adverse events ranged from 10.4 to 51.7% depending on the dose and use of other concomitant immunosuppressant medications such as cyclosporine. In contrast, 13.1% of patients on placebo experienced a serious adverse event as shown in Table 3.
Infections and related complications
The most common adverse events reported were nasopharyngitis and upper respiratory tract infections. Inflammatory nasopharyngitis was reported as a separate category in several trials in the range of 23.4–25.3% in MS, with lower rates of 9.2–11.4% in the renal transplant trial. A significant proportion of those on placebo (25.9%) also reported similar symptoms. Lower rates of upper respiratory tract infections were reported by fingolimod (13.8–27.1%) compared with those on placebo (38.4%) as shown in Table 2. Urinary tract infections were also reported in up to 28.1% of patients in renal transplant cohorts – it must be noted that these patients received a full dose of cyclosporine in addition to fingolimod.
Viral infections (apart from influenza) were encountered in about 2.2–9.2%, with 6.7% reported in the placebo arms. A successfully treated varicella zoster infection from the TRANSFORMS study was published as a case report, whereas two fatalities because of viral infections including one because of varicella zoster were reported as part of the main study 27,30. Influenza-like symptoms (presumed to be noninfectious and directly related to the drug) occurred in 9.8% of the patients.
Cardiac side effects
Bradycardia occurred in 1.1–26.4% and hypertension in 7.9–24.7% of the patients. Higher percentages were observed in patients on cyclosporine and these rates are significantly higher than those on placebo alone. Case reports of cardiac toxicity included asystole and sudden cardiac death 31–33. Negative chronotropic effects were also observed in an adolescent population 34. A case report of endocarditis was found in a patient with multiple other medical problems 35.
First dose effects
This was evaluated in an open-label single-arm study of 906 patients where more than 95% of the patients did not experience an adverse event after their first dose 36. Bradycardia was observed in 1.3% and heart blocks in up to 0.2% of the patients. The other cardiovascular events included palpitations, sinus arrhythmia and premature beats. In the Kappos 2006 study, it was observed that all episodes occurred within the first 24 h, with the maximal reductions in heart rate all occurring within 6 h 37.
Laboratory abnormalities
Fingolimod was associated with lymphopenia in 3.5–7.4% of patients, with the higher proportion occurring in those taking 5 mg. Abnormalities in liver function studies were encountered in up to 11.7% (specifically abnormal alanine transaminase); however, some of the studies using larger doses did not specifically report on liver function test abnormalities.
Case reports, observational and registry-based studies
A total of 15 case reports were reviewed. None of these events reported in these case studies were significant adverse events in any of the larger studies or RCTs. A case report of a patient with Wolf–Parkinson–White syndrome (a cardiac conduction abnormality) and safe use of fingolimod for a period of 1 year has also been reported 38. A registry-based study reported fewer than 10% cardiac events after the first dose, none of which were fatal 39. In another registry-based study, the most common adverse events noted were headaches and lymphopaenia 40. Macular oedema was noted in 0.9% of the larger studies (Table 2), but in 4.7% as a part of a case series whereas haemorrhagic focal encephalitis was observed in a single case report 41–43.
Discussion
PP2A is a serine/threonine phosphatase that negatively regulates a multitude of signal transduction pathways and plays a key role in proliferation and cell survival 44. It is now considered a tumour suppressor 45. In-vitro studies in AML have shown that the pharmacological activation of PP2A with fingolimod subsequently inhibits proliferation, survival and in-vivo leukaemogenesis, suggesting that this approach is a potential therapeutic strategy 9.
Fingolimod has been used in treatment and immunosuppression in a range of clinical settings, with success in MS 46. Given that PP2A activators, such as fingolimod, have potential as antileukaemia treatment agents, this review was carried out with the aim of outlining the safety and toxicity issues that are relevant to AML patients.
This systematic review mainly draws from three RCT double-blinded studies with fingolimod in MS and one phase II study in renal transplant patients, which provide the highest-quality evidence for this review. The data from the RCT for this analysis are particularly important as several of the common side effects reported with fingolimod such as fatigue and headache could be fairly nonspecific. The postrenal transplant group of patients is important to consider as they reflect a more immunosuppressed and complex population as several other immunosuppressive drugs including corticosteroids, cyclosporine and mycophenolate were used in combination with fingolimod in the post-transplantation setting 29. Although the composite end of biopsy-proven acute rejection is not relevant to the current analysis, it is important to note that the profile of infections was comparable across all groups and was as expected in a transplant setting 29.
The mean age of patients in the MS trials ranged from 37.8 to 44.5 years in the pooled analysis of trials. Over 70% of the patients were women, in keeping with the epidemiology of the disease. The renal transplant group was slightly older (the mean age of the cohorts was 44 and 43 years, respectively), with equal proportions of patients of both sexes. The mean age for myelodysplasia or acute myeloid leukaemia is significantly higher.
Any serious adverse event was reported in 10.4–52.9% compared with 13.1% of patients in the placebo group. Nasopharyngitis and upper respiratory tract infections (UTRIs) were reported as common clinical adverse events in many studies and were the most common adverse events in our pooled analysis. In some studies, UTRIs are combined with nasopharyngitis; whether the aetiology was proven to be infective is unclear from the literature. It appears from our analysis that both inflammatory nasopharyngitis as well as UTRIs occurred at similar or lower rates in patients on fingolimod compared with those on placebo. An extension of the Kappos study reported a serious infection rate of 0–3% and serious nasopharyngitis or upper respiratory tract infection in the range of 1–2% 47. These rates are markedly lower than that in our pooled analysis, where the severity or the nature of infections was not taken into consideration. In an open-label study, Hoitsma et al. 48 reported serious infection rates of 14.3% in the fingolimod group compared with 33.3% with mycophenolate when both drugs were combined with tacrolimus in postrenal transplant patients. The fingolimod group had 4.1% serious bacterial and 6.1% serious viral infections compared with 22.2 and 11.1%, respectively, in the mycophenolate group 48. Although fatigue and headaches were reported in a higher proportion, these symptoms were reported in equally higher proportions, even in patients on placebo 47.
Any cytotoxic or immunosuppressive drug used in AML increases the risk of haematological, infective and cardiac side effects. The myelosuppressive therapy that is currently used for elderly AML (using a combination of anthracycline and cytosine) has a 10–15% serious adverse infective risk rate 49. A less intensive regimen such as subcutaneous cytosine reduces the toxicity related to myelosuppression and is a preferable regimen in the elderly, where more toxic regimens may not be tolerable 50. The key concern in the treatment of patients with haematological malignancy is the risk of infections. With traditional chemotherapy, the main risk is myelosuppression, which is the basis for neutropenic infections 49. Myelosuppression is not a side effect with fingolimod 27. In our pooled analysis, the overall risk of upper respiratory tract infections ranged from 11 to 13%, where inflammatory nasopharyngitis was excluded, depending on the dose of fingolimod and combination with other immunosuppression.
Viral infections (apart from influenza) were encountered in 2.2–9.2% of the patients on fingolimod in this analysis. A higher proportion of patients was observed in the group who were on a higher dose of fingolimod and in those concomitantly on cyclosporine, a calcineurin inhibitor that also causes lymphopenia. The impact of lymphopenia on morbidity and mortality in the MDS/AML group of patients is not clearly established, although severe viral infections could be fatal in this population 51. Given the theoretical risk with pneumocystis or viral infections, any clinical trial with fingolimod should involve close monitoring for viral infections. The FREEDOMS study reported a similar incidence of herpes infections across their treatment and placebo groups 26. The TRANSFORMS study specifically reports on herpes zoster and observed a 2.1% (0.5 mg fingolimod group) to 5.5% (1.25 mg fingolimod group) rate compared with a 2.8% rate in the β-interferon group 27. There is a case report of a successfully treated varicella zoster, with intravenous acyclovir, following the two fatalities reported from the TRANSFORMS study because of severe viral infections, including one patient with varicella zoster 27,30. A recent summary analysis shows a lower rate of varicella infections in the fingolimod group compared with placebo and the accompanying expert review suggests that routine prophylaxis may not be required for most patients with MS 52. A consideration for any prospective clinical trial with fingolimod in haematology patients would be prophylaxis to prevent herpes zoster. A single-arm exploratory study using everolimus and fingolimod in combination has been carried out in the renal transplant population, but did not report specifically on fingolimod-related toxicity as relevant to this analysis 53.
Cardiac toxicity is an important consideration, given that AML is a disease of the elderly, where pre-existing cardiac disease is common. However, it should be noted that postrenal transplant patients are likely to be at a higher risk for vascular complications, given the risks of pre-existing cardiac disease, hypertension and concomitant cyclosporine use. The use of anthracyclines, a common chemotherapy agent used in the treatment of AML, is associated with significant clinical as well as subclinical cardiac toxicity – predominantly cardiomyopathy, resulting in systolic and diastolic dysfunction of the heart 49. A few small healthy volunteer studies report on a single-dose effect of fingolimod on heart rate and lymphocyte count 54.
Bradycardia was encountered in 1.1–25.8% and hypertension in 6.1–22.5% of patients in our analysis of fingolimod. The proportion of patients with bradycardia or hypertension was higher among patients in whom cyclosporine was coadministered. First dose effect (bradycardia) is a well-recognized side effect and is considered to be because of the interaction of fingolimod with sphingosine-1-phosphate receptors, particularly in the cardiac tissue 54. A transient increase in heart rate is directly linked to the expression of sphingosine-1-phosphate receptors in atrial myocytes. The evidence for the subsequent transient decrease in heart rate after the first dose of fingolimod has also been reported in a pharmacodynamics study of a single dose in renal transplant patients 55. The Kappos 2006 study reported that maximal reductions in heart rate occurred within the first 6 h and all episodes within 24 h, followed by spontaneous resolution 37. Holter monitoring, at months after starting the 1.25 mg fingolimod dose, was normal in all patients 37.
The phase III RCT that compared β-interferon and fingolimod was a crossover study 56. Patients on this study switched from interferon β to fingolimod at the end of 12 months of treatment and were continued at two dose cohorts of 0.5 and 1.25 mg for another 12 months. The switch-over group also reported a 1% rate of bradycardia in both dose cohorts as well as a 1% rate of second-degree atrioventricular block and a complete atrioventricular block in the 1.25 mg dose cohort 56. The phase II and III trials in MS patients have also reported bradycardia, which occurs within 6 h of the first dose. This is usually most commonly observed at 4–5 h after the dose is administered and is transient; subsequent complications include first-degree or second-degree heart blocks. Two dose levels at 2.5 and 5 mg fingolimod were studied (administered with cyclosporine) in the postrenal transplant setting (Table 3). The rates of bradycardia as well as viral infection were higher in the group receiving a 5 mg dose (with cyclosporine). This implies that any combination therapy that is planned with fingolimod must take into consideration potential cardiac as well as infective risks. In AML, the concomitant use of anthracycline is more important to consider as cyclosporine or similar drugs are unlikely to be used.
Sudden cardiac death has been reported in a patient with MS on fingolimod 31. The postmortem in this patient and in another case report with sudden death in MS (not on fingolimod) indicated demyelination in the medulla oblongata, indicating autonomic dysfunction because of progressive MS as the major reason for the cardiac death 31,33. Fingolimod has been administered successfully to a patient with Wolf–Parkinson–White syndrome, a cardiac conduction defect that can result in fatal arrhythmias, under close surveillance with no untoward effects 38. It has been observed that autonomic dysfunction as a part of MS may predict cardiac outcomes in patients on fingolimod 57. The negative chronotropic effect has been reported in a patient with no previous cardiac history, but whose medications included risperidone, an antipsychotic drug known to cause cardiac conduction abnormalities 32. The combination of fingolimod and risperidone is likely to have precipitated the bradycardia 58. The significance of screening for drugs that may potentially prolong QT interval has been outlined in the FDA communication 59. The use of atropine has been described to prevent as well as reverse the negative chronotropic effect 60.
One common issue in elderly patients, as with the disease group of AML, is that concomitant medications can significantly alter heart rate and blood pressure. The study by Kovarik et al. 54 specifically evaluated this issue and studied the impact of β-blockers (atenolol) or other antihypertensive drugs (diltiazem) on heart rate and blood pressure when used in combination with fingolimod. They observed that the combination of fingolimod with atenolol, a β-blocker, with fingolimod resulted in a 15% mean lower day time heart rate, whereas if combined with diltiazem, a calcium channel blocker, the mean heart rate was not lower than that when fingolimod was administered alone. The blood pressure was stable irrespective of whether fingolimod was combined with atenolol or dialtiazem 54. The first dose–effect study had a third of patients with other significant medical problems and 15% (136/906) on medications that influenced heart rate and conduction 36. Very significantly, they did not find any association with the use of many medications including β-blockers or antipsychotic drugs (which can prolong QT interval) to significant first dose cardiac adverse events from fingolimod 36. They observed the need for prolonged monitoring with two drugs, topiramate and trazodone; however, the authors could not draw firm conclusions because of small numbers. Another study that looked at predictors of cardiac side effects in MS patients and observed that pre-existing autonomic instability, in patients with MS, is an important indicator of heart rate and blood pressure changes 57. This is unlikely to be a significant issue in most patients with leukaemia.
The FDA (USA) and European Medicines Agency (Europe) independently reviewed safety data from clinical trials as well as postmarketing surveillance in 2012 59,61. They both recommend screening for cardiac conditions, assessing concomitant medications and monitoring heart rate for at least 6 h. The FDA also ruled that the contribution of fingolimod towards the cardiovascular deaths, as reported in the literature, was unclear 59.
Laboratory abnormalities were reported in some studies; however, there is heterogeneity in how they were reported as the abnormalities are an expected pharmacodynamic effect of the drug. Lymphopenia was not reported as an adverse event in some studies, unless the value decreased to less than 0.2×109/l. Lymphopenia is a common side effect of fingolimod and this may increase the risk of particular infections such as pneumocystis or respiratory viruses as discussed earlier. Fingolimod inhibits the re-entry of lymphocytes from lymph nodes into circulation by inhibiting SIP receptors on lymphocytes and hence their ability to bind to the respective ligand 62. Data on the recovery period for lymphopenia after the drug is stopped in healthy volunteers suggest that peripheral blood reconstitution occurred within 2–4 weeks of discontinuing the drug 63. Follow-up of patients in clinical trials indicates that there was a 24–30% decrease in the lymphocyte count from baseline 64. This occurred within about 2 weeks from starting therapy and was stable on long-term follow-up of up to 5 years. The kinetics of lymphocytes in the long-term use of fingolimod suggest that following cessation of the drug, reconstitution of counts occurred within about 3 months 64. Infection rate per patient-year was 1.4 with placebo compared with 1.0 in patients treated with fingolimod in the group with the lowest lymphocyte counts (FREEDOMS study phase 3 core group), suggesting that lymphopenia per se may not be a major risk factor for infection 64. The liver function abnormalities were mainly noted to be increases in alanine transaminase. This appeared to return to normal when the drug was stopped and even in some patients who continued study treatment 56. Bilirubin was noted to be stable, with no clinically significant change in any of the studies 56. This is important as an increase in bilirubin may preclude the use of certain types of chemotherapy such as anthracyclines or vinca alkaloids.
Rarer side effects such as macular oedema and haemorrhagic focal encephalitis have been reported as case reports 42,43. However, in a recent publication, macular oedema was more carefully evaluated and observed only in about 0.5% of the patients 65. Fingolimod-associated macular oedema is now considered to be dose dependent with a tenfold increased incidence with dose increases from 0.5 to 5 mg 65. However, in a case series of patients with MS, who were not on fingolimod, 4.7% of the patients were noted to have a microcystic pattern of macular oedema. This related more to disease severity and occurred more commonly in those with previous optic neuritis 42. The coexistence of diabetes-related macular changes as well as difficulty distinguishing from primary optic neuritis, were likely confounders in these studies 65. Patients with a history of eye problems, diabetes or uveitis would need to undergo an ophthalmic assessment before starting fingolimod; the drug would need to be avoided in those with active macular oedema 65.
In summary, an oral antileukaemia drug with potential to reduce the dosage or the intensity of standard chemotherapy is an attractive therapeutic option in AML. Fingolimod or other more specific PP2A activators, in combination with hydroxyurea or subcutaneous cytarabine, may be a potential strategy in the elderly, with low tumour burden AML, or high-risk myelodysplasia. As a potential drug, fingolimod may need to be used with caution in those with active infections or pre-existing cardiac toxicity. A close surveillance monitoring with ECGs as well as having a low threshold for investigation and treatment or prophylaxis for viral infections would be a suggested approach for any phase I trials with fingolimod in patients with acute myeloid leukaemia or high-risk myelodysplasia. Development of more specific PP2A activators, such as the nonphosphorylatable analogues AAL(S) or OSU-2S, may avoid several of the cardiac and infective complications that are likely to occur with the broader effects of fingolimod 2. Given that fingolimod has also shown preclinical efficacy in chronic lymphocytic leukaemia and chronic myeloid leukaemia models, the safety/toxicity profiles outlined here may also be relevant for treating other leukaemias as an investigational agent.
Acknowledgements
This work was supported by a grant from the Hunter Cancer Research Alliance (HCRA) and Cancer Council, NSW. A.E. was supported by Calvary Mater Newcastle research and HCRA research grants; he is also a recipient of an HCRA fellowship grant. N.M.V. is supported by a Cancer Institute NSW fellowship. The authors acknowledge the input of Anita Ariyarajah for critical review of the tables and manuscript.
Author contributions: A.E. and A.D.C.: project overview, analysis and manuscript; K.M. and A.E.: references review, tables and manuscript; N.V. and P.R: manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE. Medical Research Council Adult Leukemia Working Party. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood 2001; 98:1302–1311. [DOI] [PubMed] [Google Scholar]
- 2.Perrotti D, Neviani P. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol 2013; 14:e229–e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho US, Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature 2007; 445:53–57. [DOI] [PubMed] [Google Scholar]
- 4.Kranias G, Watt LF, Carpenter H, Holst J, Ludowyke R, Strack S, et al. Protein phosphatase 2A carboxymethylation and regulatory B subunits differentially regulate mast cell degranulation. Cell Signal 2010; 22:1882–1890. [DOI] [PubMed] [Google Scholar]
- 5.Sim AT, Ludowyke RI, Verrills NM. Mast cell function: regulation of degranulation by serine/threonine phosphatases. Pharmacol Ther 2006; 112:425–439. [DOI] [PubMed] [Google Scholar]
- 6.Smith AM, Roberts KR, Verrills NM. Ser/Thr phosphatases: the new frontier for myeloid leukemia therapy?. In: Koschmieder S, Krug U, editors. Myeloid leukemia – basic mechanisms of leukemogenesis. Intech. 2011. pp. 123–148. DOI: 10.5772/26425. Available at: http://www.intechopen.com/books/myeloid-leukemia-basic-mechanisms-of-leukemogenesis/ser-thr-phosphatases-the-new-frontier-for-myeloid-leukemia-therapy.
- 7.Junttila MR, Puustinen P, Niemelä M, Ahola R, Arnold H, Böttzauw T, et al. CIP2A inhibits PP2A in human malignancies. Cell 2007; 130:51–62. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Damuni Z. I1PP2A and I2PP2A. Two potent protein phosphatase 2A-specific inhibitor proteins. Methods Mol Biol 1998; 93:59–66. [DOI] [PubMed] [Google Scholar]
- 9.Roberts KG, Smith AM, McDougall F, Carpenter H, Horan M, Neviani P, et al. Essential requirement for PP2A inhibition by the oncogenic receptor c-KIT suggests PP2A reactivation as a strategy to treat c-KIT+ cancers. Cancer Res 2010; 70:5438–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cristóbal I, Garcia-Orti L, Cirauqui C, Alonso MM, Calasanz MJ, Odero MD. PP2A impaired activity is a common event in acute myeloid leukemia and its activation by forskolin has a potent anti-leukemic effect. Leukemia 2011; 25:606–614. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Huang Q, Lu Y, Li X, Huang S. Reactivating PP2A by FTY720 as a novel therapy for AML with C-KIT tyrosine kinase domain mutation. J Cell Biochem 2012; 113:1314–1322. [DOI] [PubMed] [Google Scholar]
- 12.Oaks JJ, Santhanam R, Walker CJ, Roof S, Harb JG, Ferenchak G, et al. Antagonistic activities of the immunomodulator and PP2A-activating drug FTY720 (Fingolimod, Gilenya) in Jak2-driven hematologic malignancies. Blood 2013; 122:1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Zhao X, Frissora F, Ma Y, Santhanam R, Jarjoura D, et al. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood 2008; 111:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrotti D, Neviani P. Protein phosphatase 2A (PP2A), a drugable tumor suppressor in Ph1(+) leukemias. Cancer Metastasis Rev 2008; 27:159–168. [DOI] [PubMed] [Google Scholar]
- 15.Switzer CH, Glynn SA, Ridnour LA, Cheng RY, Vitek MP, Ambs S, Wink DA. Nitric oxide and protein phosphatase 2A provide novel therapeutic opportunities in ER-negative breast cancer. Trends Pharmacol Sci 2011; 32:644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen H, Gonzalez-Cabrera P, Marsolais D, Cahalan S, Don AS, Sanna MG. Modulating tone: the overture of S1P receptor immunotherapeutics. Immunol Rev 2008; 223:221–235. [DOI] [PubMed] [Google Scholar]
- 17.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol 2006; 2:434–441. [DOI] [PubMed] [Google Scholar]
- 18.Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, et al. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest 2007; 117:2408–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell 2005; 8:355–368. [DOI] [PubMed] [Google Scholar]
- 20.Collison A, Hatchwell L, Verrills N, Wark PA, de Siqueira AP, Tooze M, et al. The E3 ubiquitin ligase midline 1 promotes allergen and rhinovirus-induced asthma by inhibiting protein phosphatase 2A activity. Nat Med 2013; 19:232–237. [DOI] [PubMed] [Google Scholar]
- 21.Mani R, Mao Y, Frissora FW, Chiang CL, Wang J, Zhao Y, et al. Tumor antigen ROR1 targeted drug delivery mediated selective leukemic but not normal B-cell cytotoxicity in chronic lymphocytic leukemia. Leukemia 2015; 29:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omar HA, Chou CC, Berman-Booty LD, Ma Y, Hung JH, Wang D, et al. Antitumor effects of OSU-2S, a nonimmunosuppressive analogue of FTY720, in hepatocellular carcinoma. Hepatology 2011; 53:1943–1958. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J, et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med 2013; 5:105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, et al. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology 2009; 72:73–79. [DOI] [PubMed] [Google Scholar]
- 26.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362:387–401. [DOI] [PubMed] [Google Scholar]
- 27.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362:402–415. [DOI] [PubMed] [Google Scholar]
- 28.Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13:545–556. [DOI] [PubMed] [Google Scholar]
- 29.Tedesco-Silva H, Szakaly P, Shoker A, Sommerer C, Yoshimura N, Schena FP, et al. FTY720 versus mycophenolate mofetil in de novo renal transplantation: six-month results of a double-blind study. Transplantation 2007; 84:885–892. [DOI] [PubMed] [Google Scholar]
- 30.Uccelli A, Ginocchio F, Mancardi GL, Bassetti M. Primary varicella zoster infection associated with fingolimod treatment. Neurology 2011; 76:1023–1024. [DOI] [PubMed] [Google Scholar]
- 31.Lindsey JW, Haden-Pinneri K, Memon NB, Buja LM. Sudden unexpected death on fingolimod. Mult Scler 2012; 18:1507–1508. [DOI] [PubMed] [Google Scholar]
- 32.Espinosa PS, Berger JR. Delayed fingolimod-associated asystole. Mult Scler 2011; 17:1387–1389. [DOI] [PubMed] [Google Scholar]
- 33.Hengstman GJ, Kusters B. Sudden cardiac death in multiple sclerosis caused by active demyelination of the medulla oblongata. Mult Scler 2011; 17:1146–1148. [DOI] [PubMed] [Google Scholar]
- 34.Ettenger R, Schmouder R, Kovarik JM, Bastien MC, Hoyer PF. Pharmacokinetics, pharmacodynamics, safety, and tolerability of single-dose fingolimod (FTY720) in adolescents with stable renal transplants. Pediatr Transplant 2011; 15:406–413. [DOI] [PubMed] [Google Scholar]
- 35.Cocco G. A patient with Leiden V mutation, multiple sclerosis, psoriasis, and sicca syndrome: could celecoxib and fingolimod adversely affect the heart? Cardiovasc Toxicol 2012; 12:266–272. [DOI] [PubMed] [Google Scholar]
- 36.Laroni A, Brogi D, Morra VB, Guidi L, Pozzilli C, Comi G, et al. Safety of the first dose of fingolimod for multiple sclerosis: results of an open-label clinical trial. BMC Neurol 2014; 14:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006; 355:1124–1140. [DOI] [PubMed] [Google Scholar]
- 38.Huys AC, Lalive PH, Sekoranja L. Fingolimod in a patient with Wolff–Parkinson–White syndrome. Mult Scler 2014; 20:636–637. [DOI] [PubMed] [Google Scholar]
- 39.Fragoso YD, Arruda CC, Arruda WO, Brooks JB, Damasceno A, Damasceno CA, et al. The real-life experience with cardiovascular complications in the first dose of fingolimod for multiple sclerosis. Arq Neuropsiquiatr 2014; 72:712–714. [DOI] [PubMed] [Google Scholar]
- 40.Al-Hashel J, Ahmed SF, Behbehani R, Alroughani R. Real-world use of fingolimod in patients with relapsing remitting multiple sclerosis: a retrospective study using the national multiple sclerosis registry in Kuwait. CNS Drugs 2014; 28:817–824. [DOI] [PubMed] [Google Scholar]
- 41.Leypoldt F, Münchau A, Moeller F, Bester M, Gerloff C, Heesen C. Hemorrhaging focal encephalitis under fingolimod (FTY720) treatment: a case report. Neurology 2009; 72:1022–1024. [DOI] [PubMed] [Google Scholar]
- 42.Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain 2012; 135 (Pt 6):1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saab G, Almony A, Blinder KJ, Schuessler R, Brennan DC. Reversible cystoid macular edema secondary to fingolimod in a renal transplant recipient. Arch Ophthalmol 2008; 126:140–141. [DOI] [PubMed] [Google Scholar]
- 44.Arnold HK, Sears RC. Protein phosphatase 2A regulatory subunit B56alpha associates with c-myc and negatively regulates c-myc accumulation. Mol Cell Biol 2006; 26:2832–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janssens V, Goris J, Van Hoof C. PP2A: the expected tumor suppressor. Curr Opin Genet Dev 2005; 15:34–41. [DOI] [PubMed] [Google Scholar]
- 46.Yiu EM, Banwell B. Update on emerging therapies for multiple sclerosis. Expert Rev Neurother 2010; 10:1259–1262. [DOI] [PubMed] [Google Scholar]
- 47.Comi G, O’Connor P, Montalban X, Antel J, Radue EW, Karlsson G, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler 2010; 16:197–207. [DOI] [PubMed] [Google Scholar]
- 48.Hoitsma AJ, Woodle ES, Abramowicz D, Proot P, Vanrenterghem Y. FTY720 combined with tacrolimus in de novo renal transplantation: 1-year, multicenter, open-label randomized study. Nephrol Dial Transplant 2011; 26:3802–3805. [DOI] [PubMed] [Google Scholar]
- 49.Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, et al. Dutch-Belgian Cooperative Trial Group for Hemato-Oncology (HOVON); German AML Study Group (AMLSG), Swiss Group for Clinical Cancer Research (SAKK) Collaborative Group. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med 2009; 361:1235–1248. [DOI] [PubMed] [Google Scholar]
- 50.Sekeres MA, Lancet JE, Wood BL, Grove LE, Sandalic L, Sievers EL, Jurcic JG. Randomized phase IIb study of low-dose cytarabine and lintuzumab versus low-dose cytarabine and placebo in older adults with untreated acute myeloid leukemia. Haematologica 2013; 98:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lech-Maranda E, Seweryn M, Giebel S, Holowiecki J, Piatkowska-Jakubas B, Wegrzyn J, et al. Infectious complications in patients with acute myeloid leukemia treated according to the protocol with daunorubicin and cytarabine with or without addition of cladribine. A multicenter study by the Polish Adult Leukemia Group (PALG). Int J Infect Dis 2010; 14:e132–e140. [DOI] [PubMed] [Google Scholar]
- 52.Arvin AM, Wolinsky JS, Kappos L, Morris MI, Reder AT, Tornatore C, et al. Varicella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. JAMA Neurol 2015; 72:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tedesco-Silva H, Lorber MI, Foster CE, Sollinger HW, Mendez R, Carvalho DB, et al. FTY720 and everolimus in de novo renal transplant patients at risk for delayed graft function: results of an exploratory one-yr multicenter study. Clin Transplant 2009; 23:589–599. [DOI] [PubMed] [Google Scholar]
- 54.Kovarik JM, Lu M, Riviere GJ, Barbet I, Maton S, Goldwater DR, Schmouder RL. The effect on heart rate of combining single-dose fingolimod with steady-state atenolol or diltiazem in healthy subjects. Eur J Clin Pharmacol 2008; 64:457–463. [DOI] [PubMed] [Google Scholar]
- 55.Budde K, L Schmouder R, Nashan B, Brunkhorst R, W Lücker P, Mayer T, et al. Pharmacodynamics of single doses of the novel immunosuppressant FTY720 in stable renal transplant patients. Am J Transplant 2003; 3:846–854. [DOI] [PubMed] [Google Scholar]
- 56.Khatri B, Barkhof F, Comi G, Hartung HP, Kappos L, Montalban X, et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol 2011; 10:520–529. [DOI] [PubMed] [Google Scholar]
- 57.Rossi S, Rocchi C, Studer V, Motta C, Lauretti B, Germani G, et al. The autonomic balance predicts cardiac responses after the first dose of fingolimod. Mult Scler 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 58.Marti V. Sudden cardiac death due to risperidone therapy in a patient with possible hypertrophic cardiomyopathy. Ann Pharmacother 2005; 39:973. [DOI] [PubMed] [Google Scholar]
- 59.FDA.GOV. FDA Drug Safety Communication: revised recommendations for cardiovascular monitoring and use of multiple sclerosis drug Gilenya (fingolimod): Food and Drug Administration, USA; 2012. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm303192.htm. [Accessed 10 June 2015].
- 60.Kovarik JM, Slade A, Riviere GJ, Neddermann D, Maton S, Hunt TL, Schmouder RL. The ability of atropine to prevent and reverse the negative chronotropic effect of fingolimod in healthy subjects. Br J Clin Pharmacol 2008; 66:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.EM Agency (European Medicines Agency). Find medicine – Gilenya: questions and answers on the review of Gilenya; 2011. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002202/human_med_001433.jsp&mid=WC0b01ac058001d124. [Accessed 10 June 2015].
- 62.Chiba K, Matsuyuki H, Maeda Y, Sugahara K. Role of sphingosine 1-phosphate receptor type 1 in lymphocyte egress from secondary lymphoid tissues and thymus. Cell Mol Immunol 2006; 3:11–19. [PubMed] [Google Scholar]
- 63.Kovarik JM, Schmouder R, Barilla D, Wang Y, Kraus G. Single-dose FTY720 pharmacokinetics, food effect, and pharmacological responses in healthy subjects. Br J Clin Pharmacol 2004; 57:586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Francis G, Kappos L, O'Connor P, Collins W, Tang D, Mercier F, Cohen JA. Temporal profile of lymphocyte counts and relationship with infections with fingolimod therapy. Mult Scler 2014; 20:471–480. [DOI] [PubMed] [Google Scholar]
- 65.Jain N, Bhatti MT. Fingolimod-associated macular edema: incidence, detection, and management. Neurology 2012; 78:672–680. [DOI] [PubMed] [Google Scholar]


