Abstract
Tectorigenin (Tec) is an effective component of the traditional Chinese medicine Belamcanda chinensis, which has been reported to exert beneficial effects in various types of cancer. However, the activity and mechanism of Tec in osteosarcoma (OS) have not been investigated to date. The aim of the present study was to examine the inhibitory effect of Tec on OS and its underlying mechanism of action. OS cells (Saos2 and U2OS) were treated with various concentrations of Tec for 24, 48, and 72 h. Cell proliferation was evaluated using an CCK-8 assay. Cell migration and invasion ability were measured using the Transwell assay. The expressions of MMP1, MMP2, MMP9, and cleaved caspase3 were measured using real-time PCR and/or western blot analysis. We found that Tec inhibited the proliferation of OS cells (Saos2 and U2OS) in a dose-dependent and time-dependent manner. In addition, Tec significantly inhibited migration and invasion in OS cells (P<0.05). Tec upregulated the expression of cleaved caspase3, while downregulating the expression of MMP1, MMP2, and MMP9. Taken together, the present study provided fundamental evidence for the application of Tec in chemotherapy against OS.
Keywords: migration, matrix metalloproteinases, osteosarcoma, tectorigenin
Introduction
Osteosarcoma (OS) is one of most common primary bone cancers that shows cancerous osteoblastic differentiation and malignant osteoid in patients, and its peak incidence occurs during adolescence 1,2. Currently, chemotherapeutic regimens for human OS treatment involve the combination of multiple chemotherapeutic agents, including high-dose methotrexate with leucovorin rescue, doxorubicin, cisplatin, and ifosfamide, either with or without etoposide 3–7. Although these regimens have remained the mainstay of OS chemotherapy for decades, none have led to any major improvement in survival compared with the original combination. Furthermore, these regimens have only been shown to be efficient in the treatment of localized OS, whereas they were shown to perform poorly in the treatment of metastatic and recurrent OS 8–10.
The development of OS is a complex, multistep, and multifactorial process in which numerous factors are released by the tumor microenvironment and neighboring cells of the innate immune system. These cytokines, including some proinflammatory cytokine genes, including interleukin-6, interleukin-8, and transforming growth factor-β, affect tumor cell proliferation, apoptosis, and tumor invasion 11–13.
Since the novel antitumor drugs are currently under investigation, bioactive natural products have emerged and gained considerable research attention. Tectorigenin (Tec), an effective component of the traditional Chinese medicine derived from Belamcanda chinensis, has been reported to exert a beneficial effect in various types of cancer 14, including lung carcinoma 15, testicular germ cell tumors 16, ovarian cancer 17, prostate cancer 18, and so on. In addition, Tec was found to inhibit interferon-γ/lipopolysaccharide-induced inflammatory responses in murine macrophage RAW 264.7 cells 19. However, the effects and underlying mechanism of action of Tec in OS have not been elucidated to date.
The aim of our study was to assess the antitumor effects of Tec against OS and to investigate its underlying biological mechanisms. Furthermore, we used an in-vitro approach shown in Fig. 1 to elucidate the mechanisms underlying these effects.
Fig. 1.
Schematic of the approach used in this study.
Materials and methods
Cell culture
Human Saos2 and U2OS OS cells were purchased from the Chinese Academy of Sciences (Shanghai, China). All cells were grown in Dulbecco’s modified Eagle medium (DMEM; Hyclone, Tauranga, New Zealand) supplemented with 10% fetal bovine serum (Hyclone), 100 U/ml penicillin, and 100 μg/ml streptomycin (Hyclone) in a 5% CO2 humidified atmosphere at 37°C. All cells were used within 20 passages.
Cell viability assay
Tec cytotoxicity was assessed using a cell counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan) assay, which measured the metabolic activity of viable cells. The cells were dislodged and suspended in medium, and 8000 cells were seeded into each well of a 96-well plate and treated with 0, 100, 200, and 400 μmol/l Tec for 72 h after adherence. Following treatment with Tec for 24, 48, and 72 h, culture medium was removed and cells were washed with PBS before the addition of 100 µl DMEM and 10 µl CCK-8 solution to each well and incubation at 37°C for 2.5 h. The optical density at 450 nm was determined daily for the subsequent 3 days using a microplate reader (BioTek Instruments Inc., Winooski, Vermont, USA). All the measurements were repeated in triplicate. The cell viability to control was calculated using the following equation:
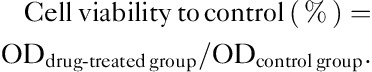 |
Migration and invasion assays
Approximately 1×105 cells were seeded onto a Matrigel-coated polycarbonate membrane insert (6.5 mm diameter, 8.0 μm pores) in a Transwell apparatus (Costar, Cambridge, Massachusetts, USA) and maintained in DMEM containing 0.2% BSA and 0, 100, 200, or 400 μmol/l concentration of Tec. DMEM containing 10% fetal bovine serum was added to the lower chamber. After incubation for 12 h at 37°C in a 5% CO2 atmosphere, the insert was washed with PBS and cells on the top surface of the insert were removed by wiping with a cotton swab. The invasion assay procedure was similar to that of the cell migration assay, except that the Transwell membrane was coated with 1 : 3 diluted Matrigel (BD Biosciences, San Jose, California, USA), and cells were incubated for 32 h at 37°C.
Cells that migrated to the bottom surface of the insert were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet, and subjected to a microscopic examination. Cell counts were based on counts of five fields from digital images taken randomly at ×200 magnification.
Real-time PCR
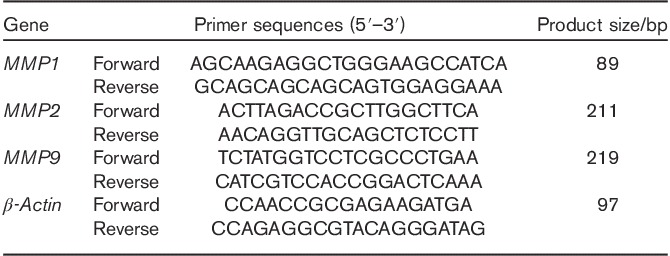
The OS cells (Saos2 and U2OS) were dislodged and suspended in medium, and 1×106 cells were seeded into each well of a six-well plate and treated with 0, 100, 200, and 400 μmol/l Tec for 72 h after adherence. Total RNA was isolated using the AxyPrep Multisource Total RNA Miniprepkit (Axygen, Union City, California, USA). Equivalent amounts of RNA were converted into cDNA using the PrimeScript RT reagent kit (Takara, Shiga, Japan). Real-time PCR was performed using an ABI 7500 Sequencing Detection System and SYBR Premix Ex Taq (Takara). All procedures were performed according to the manufacturer’s protocols. Cycling conditions were 40 cycles of 95°C for 5 s and 60°C for 34 s. Primer sequences are listed in Table 1.
Table 1.
Sequences of primers used in real-time PCR
Western blot analysis
Saos2 OS cells were treated with 0, 100, 200, and 400 μmol/l Tec for 72 h after adherence and cells were washed with PBS and lysed in lysis buffer containing a protease inhibitor cocktail (Roche, Grenzach, Germany). Protein concentration was quantified using the BCA protein assay kit (Santa Cruz Biotechnology Inc., Dallas, Texas, USA). For western blot analysis, equal amounts of total protein were boiled and then separated by SDS-PAGE. Following electrophoresis, proteins were blotted onto PVDF membranes and blocked for 1 h at room temperature. Each membrane was incubated with appropriate primary antibodies at 4˚C overnight. The blots were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Protein bands were detected on radiograph films using an enhanced chemiluminescence detection system. Positive immunoreactive bands were densitometrically quantified and normalized to β-actin.
Statistical analysis
The Statistical Package for the Social Sciences, version 19.0 (IBM, Armonk, New York, USA) was used to analyze the data. The significance of differences between experimental groups and controls were assessed using Student’s t-test or one-way analysis of variance as appropriate. The data are expressed as the mean±SD. A P value less than 0.05 was considered to indicate a statistically significant difference.
Results
Proliferation of OS cells
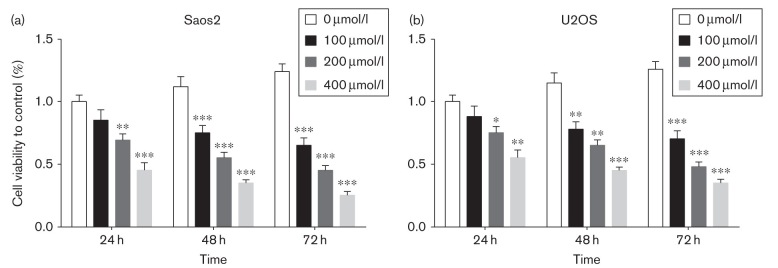
The structure of Tec is shown in Fig. 2. To investigate the effects of Tec, a cell proliferation assay using CCK-8 was performed on OS cells. Proliferation of Saos2 and U2OS cells was inhibited after treatment with 200 μmol/l Tec over 24 h, and then the inhibition of Tec on the proliferation of Saos2 and U2OS OS cells occurred in a dose-dependent and time-dependent manner (Fig. 3a and b). In short, Tec could inhibit the proliferation of OS in a dose-dependent and time-dependent manner.
Fig. 2.
The structure of tectorigenin.
Fig. 3.
Proliferation of OS cells. (a, b). CCK-8 assay showed that tectorigenin could inhibit the proliferation of Saos2 and U2OS OS cells in a dose-dependent and time-dependent manner. Data are presented as the mean±SD. ***P<0.001, **P<0.01, *P<0.05. All data were obtained from at least three independent experiments. OS, osteosarcoma
Tec impairs the migration and invasion of OS cells
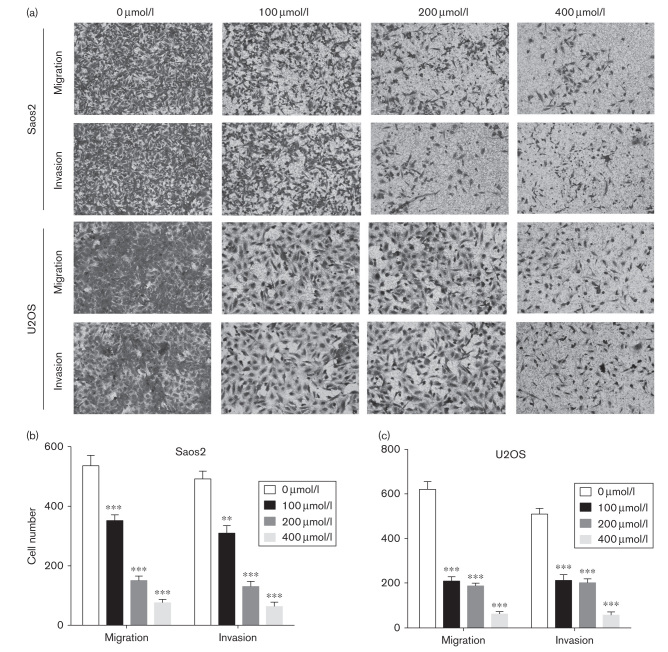
Transwell assay was used to detect the migration and invasion ability of OS after treatment of different concentrations of Tec. Our results showed that the migration and invasion ability of Saos2 and U2OS cells was inhibited by Tec in a dose-dependent manner (Fig. 4a–c). These results indicate that Tec concentration is associated negatively with the migration and invasion of OS cells in vitro.
Fig. 4.
Tec impairs the migration and invasion of OS cells. (a). Transwell assays were used to determine the migration and invasion of Saos2 and U2OS cells. (b). Numbers of migrating and invading cells. Data are presented as the mean±SD. ***P<0.001, **P<0.01. All data were obtained from at least three independent experiments. OS, osteosarcoma; Tec, tectorigenin.
Downstream gene expressions
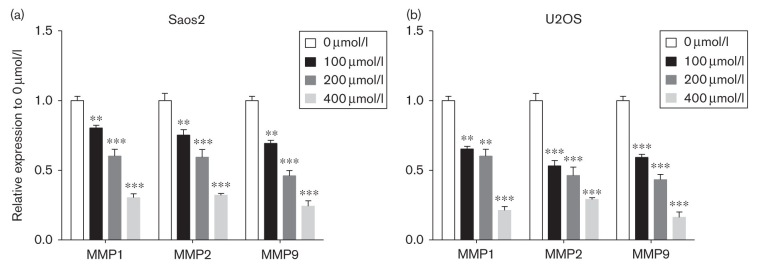
To investigate the depth molecular mechanisms of the inhibition effect of Tec in OS, real-time PCR was used to examine the variation of gene expression. We found that gene expression levels of MMP1, MMP2, and MMP9 were reduced in Tec-treated Saos2 and U2OS OS cells in a dose-dependent manner (Fig. 5a and b). These results indicate that MMP1, MMP2, and MMP9 are potential downstream targets of Tec in OS.
Fig. 5.
Apoptosis-related and migration-related gene expression in Saos2 and U2OS cells. (a, b). Real-time PCR was used to detect the expression of MMP1, MMP2, and MMP9 in Tec-treated Saos2 and U2OS cells. Data are presented as the mean±SD. ***P<0.001, **P<0.01. All data were obtained from at least three independent experiments. Tec, tectorigenin.
MMP1, MMP2, MMP9, and cleaved caspase3 are potential downstream targets of Tec
We used western blotting to analyze protein expression levels of MMP1, MMP2, MMP9, and cleaved caspase3 in Saos2 OS cells following different concentrations of Tec treatment. Our results showed that Tec increased levels of cleaved caspase3 in a dose-dependent manner (Fig. 6a and b). MMP1, MMP2, and MMP9 were inhibited as the concentration of Tec increased (Fig. 6a and c). Taken together, these data suggest that MMP1, MMP2, MMP9, and cleaved caspase3 are potential downstream targets of Tec-induced proliferation, migration, and invasion inhibition of OS cells.
Fig. 6.
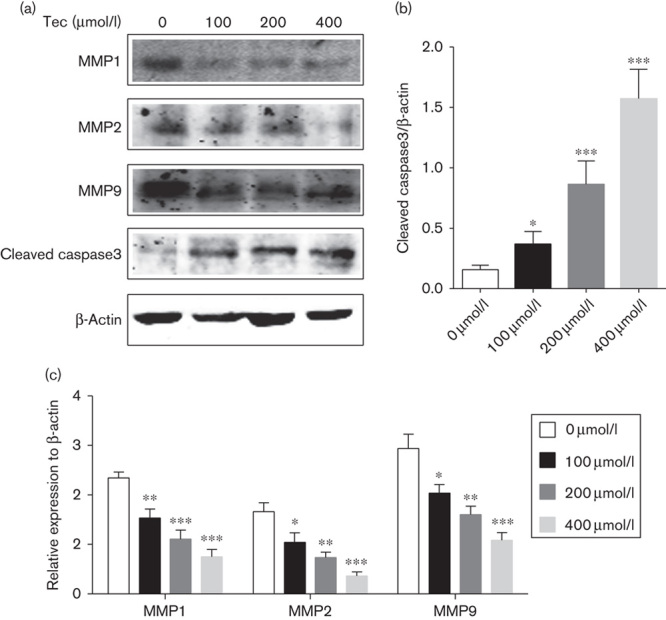
Western blotting of MMP1, MMP2, MMP9, and cleaved caspase3 levels following Tec-induced apoptosis in Saos2 and U2OS cells. (a) Expressions of MMP1, MMP2, MMP9, and cleaved caspase3 in Saos2 and U2OS cells following Tec treatment. (b, c) Levels of MMP1, MMP2, MMP9, and cleaved caspase3 in Saos2 and U2OS cells. Results are expressed as the ratio of MMP1 to β-actin, MMP2 to β-actin, MMP9 to β-actin, and cleaved caspase3 to β-actin. Data are presented as the mean±SD. ***P<0.001, **P<0.01, *P<0.05. All data were obtained from at least three independent experiments. Tec, tectorigenin.
Discussion
OS is a heterogeneous group of malignancies characterized by varying degrees of mesenchymal differentiation. Successful clinical cure of OS faces two major challenges. First, the toxic and adverse effects associated with chemotherapy may significantly reduce patient quality of life, although preoperative and postoperative chemotherapy regimens have improved the 5-year survival rate 20,21. Second, OS has a high rate of recurrence and metastasis, which causes the majority of OS-related mortalities 22. Thus, there is an urgent need to identify less toxic and more efficacious treatment alternatives. As a result, increasing attention has been focused on the application of natural products in the treatment of OS.
Tec (molecular weight, 300.26), one of the bioactive components purified from the Chinese herb B. chinensis, has been investigated in the treatment of some tumors and a number of additional pharmacological effects of Tec have been identified 14. Tec has been found to inhibit the inflammation of lipopolysaccharide-induced acute lung injury in mice 23. The antibacterial assay of Tec with detergents or ATPase inhibitors against methicillin-resistant staphylococcus aureus was also useful 24. Tec inhibits tumor growth in hepatocellular malignancies. It mediates this role by augmenting intratumoral apoptosis, while at the same time, it reduces intratumoral proliferation 25. Therefore, Tec may represent a potentially effective option for the treatment of inflammation and tumors. However, the function and underlying mechanisms of Tec in OS have not been elucidated currently.
The aim of our research was to investigate whether Tec had antitumor activity against human OS. Our data showed that Tec suppressed the proliferation of OS cell lines in a dose-dependent and time-dependent manner. These inhibitory effects of Tec were correlated with upregulation of cleaved caspase3. Caspase3 plays an irreplaceable role in cell apoptosis. Caspase3 can be activated by a variety of factors, and it is the main terminal shear enzyme in the process of cell apoptosis 26,27. Taken together, these results indicated that Tec possesses effective antitumor activities against OS through activation of caspase apoptosis axis.
Furthermore, we examined the inhibition of migration and invasion ability of OS induced by Tec. As metastasis is one of the challenges in OS treatment, our study shows the potential role of Tec in the treatment of OS metastasis. In addition, the underlying mechanism research indicated that the MMP1, MMP2, and MMP9 were suppressed under the stimulus of Tec. As MMP1, MMP2, and MMP9 are very important in the metastasis of OS 28–30, MMP1, MMP2, and MMP9 may be the targets of Tec. Altogether, Tec could impair the migration and invasion ability through suppression of MMP1, MMP2, and MMP9.
To our knowledge, we are the first to report a role for Tec in restricting proliferation and limiting the migration and invasion capacities of OS cells. We show that Tec may directly or indirectly affect the activation of MMPs and caspase apoptosis signaling, all of which are key regulators of metastasis and apoptosis during OS treatment. However, additional in-depth investigations should follow this preliminary study. First, the intermediate molecular mechanisms controlling the Tec-mediated changes to MMPs expression and activity of caspase apoptosis signaling pathway should be better clarified. Second, animal experiments should be established to verify the treatment effect of Tec in vivo. Finally, our findings should be validated by patient treatment.
Conclusion
The present study showed that Tec exerted more effective antitumor activities against OS in vitro. Furthermore, Tec impaired the migration and invasion ability and downregulated the protein expression of MMP1, MMP2, and MMP9, while upregulating the protein expression of cleaved caspase3. This may be the mechanism by which Tec exerts it effects in OS. However, further and more comprehensive studies are required to confirm this. These data strongly suggest for the first time that Tec may have a significant potential for application in the treatment of OS.
Acknowledgements
This study was supported by the Foundation of Shanghai Jiao Tong University School of Medicine (grant no. 03xj21001).
Authors contribution: Y. Guo conducted the experiments, and Y.H. Chen, Z.H. Cheng, H.N. Ou-Yang, and C. Luo were involved in the data analysis. Y. Guo helped to draft the manuscript. Z.L. Guo designed the study, analyzed and interpreted the data, and drafted the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Liu P, Sun L, Zhou DS, Zhang P, Wang YH, Li D, et al. Development of alendronate-conjugated poly (lactic-co-glycolic acid)-dextran nanoparticles for active targeting of cisplatin in osteosarcoma. Sci Rep 2015; 5:17387. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Wang Y, Fu Q, Zhao W. Tetramethylpyrazine inhibits osteosarcoma cell proliferation via downregulation of NF-κB in vitro and in vivo. Mol Med Rep 2013; 8:984–988. [DOI] [PubMed] [Google Scholar]
- 3.Duan L, Perez RE, Hansen M, Gitelis S, Maki CG. Increasing cisplatin sensitivity by schedule-dependent inhibition of AKT and Chk1. Cancer Biol Ther 2014; 15:1600–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegyi M, Gulácsi A, Cságoly E, Csordás K, Eipel OT, Erdélyi DJ, et al. Clinical relations of methotrexate pharmacokinetics in the treatment for pediatric osteosarcoma. J Cancer Res Clin Oncol 2012; 138:1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Liu K, Song D, Ding M, Wang J, Jin Q, et al. KLF4 promotes HMGB1-induced chemotherapy resistance in osteosarcoma cells. Cancer Sci 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saitoh Y, Setoguchi T, Nagata M, Tsuru A, Nakamura S, Nagano S, et al. Combination of Hedgehog inhibitors and standard anticancer agents synergistically prevent osteosarcoma growth. Int J Oncol 2016; 48:235–242. [DOI] [PubMed] [Google Scholar]
- 7.Hong CR, Kang HJ, Kim MS, Ju HY, Lee JW, Kim H, et al. High-dose chemotherapy and autologous stem cell transplantation with melphalan, etoposide and carboplatin for high-risk osteosarcoma. Bone Marrow Transplant 2015; 50:1375–1378. [DOI] [PubMed] [Google Scholar]
- 8.Du L, Fan Q, Tu B, Yan W, Tang T. Establishment and characterization of a new highly metastatic human osteosarcoma cell line derived from Saos2. Int J Clin Exp Pathol 2014; 7:2871–2882. [PMC free article] [PubMed] [Google Scholar]
- 9.Fan CL, Jiang J, Liu HC, Yang D. Forkhead box protein M1 predicts outcome in human osteosarcoma. Int J Clin Exp Med 2015; 8:15563–15568. [PMC free article] [PubMed] [Google Scholar]
- 10.Gorlick R, Khanna C. Osteosarcoma. J Bone Miner Res 2010; 25:683–691. [DOI] [PubMed] [Google Scholar]
- 11.Tu B, Du L, Fan QM, Tang Z, Tang TT. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett 2012; 325:80–88. [DOI] [PubMed] [Google Scholar]
- 12.Han XG, Du L, Qiao H, Tu B, Wang YG, Qin A, et al. CXCR1 knockdown improves the sensitivity of osteosarcoma to cisplatin. Cancer Lett 2015; 369:405–415. [DOI] [PubMed] [Google Scholar]
- 13.Tu B, Peng ZX, Fan QM, Du L, Yan W, Tang TT. Osteosarcoma cells promote the production of pro-tumor cytokines in mesenchymal stem cells by inhibiting their osteogenic differentiation through the TGF-β/Smad2/3 pathway. Exp Cell Res 2014; 320:164–173. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor S. Tectorigenin and its inhibitory effects on tumor growth in systemic malignancies. Immunopharmacol Immunotoxicol 2013; 35:533. [DOI] [PubMed] [Google Scholar]
- 15.Amin A, Mokhdomi TA, Bukhari S, Wani SH, Wafai AH, Lone GN, et al. Tectorigenin ablates the inflammation-induced epithelial-mesenchymal transition in a co-culture model of human lung carcinoma. Pharmacol Rep 2015; 67:382–387. [DOI] [PubMed] [Google Scholar]
- 16.Hasibeder A, Venkataramani V, Thelen P, Radzun HJ, Schweyer S. Phytoestrogens regulate the proliferation and expression of stem cell factors in cell lines of malignant testicular germ cell tumors. Int J Oncol 2013; 43:1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang YI, Lee KT, Park HJ, Kim TJ, Choi YS, IeM Shih, Choi JH. Tectorigenin sensitizes paclitaxel-resistant human ovarian cancer cells through downregulation of the Akt and NFκB pathway. Carcinogenesis 2012; 33:2488–2498. [DOI] [PubMed] [Google Scholar]
- 18.Thelen P, Scharf JG, Burfeind P, Hemmerlein B, Wuttke W, Spengler B, et al. Tectorigenin and other phytochemicals extracted from leopard lily Belamcanda chinensis affect new and established targets for therapies in prostate cancer. Carcinogenesis 2005; 26:1360–1367. [DOI] [PubMed] [Google Scholar]
- 19.Pan CH, Kim ES, Jung SH, Nho CW, Lee JK. Tectorigenin inhibits IFN-gamma/LPS-induced inflammatory responses in murine macrophage RAW 264.7 cells. Arch Pharm Res 2008; 31:1447–1456. [DOI] [PubMed] [Google Scholar]
- 20.Ma H, He C, Cheng Y, Li D, Gong Y, Liu J, et al. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials 2014; 35:8723–8734. [DOI] [PubMed] [Google Scholar]
- 21.He BC, Chen L, Zuo GW, Zhang W, Bi Y, Huang J, et al. Synergistic antitumor effect of the activated PPARgamma and retinoid receptors on human osteosarcoma. Clin Cancer Res 2010; 16:2235–2245. [DOI] [PubMed] [Google Scholar]
- 22.Zhang P, Dong L, Yan K, Long H, Yang TT, Dong MQ, et al. CXCR4-mediated osteosarcoma growth and pulmonary metastasis is promoted by mesenchymal stem cells through VEGF. Oncol Rep 2013; 30:1753–1761. [DOI] [PubMed] [Google Scholar]
- 23.Ma CH, Liu JP, Qu R, Ma SP. Tectorigenin inhibits the inflammation of LPS-induced acute lung injury in mice. Chin J Nat Med 2014; 12:841–846. [DOI] [PubMed] [Google Scholar]
- 24.Joung DK, Mun SH, Lee KS, Kang OH, Choi JG, Kim SB, et al. The antibacterial assay of tectorigenin with detergents or ATPase Inhibitors against methicillin-resistant staphylococcus aureus. Evid Based Complement Alternat Med 2014; 2014:716509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu JH, Wang YR, Huang WY, Tan RX. Anti-proliferative and pro-apoptotic effects of tectorigenin on hepatic stellate cells. World J Gastroenterol 2010; 16:3911–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Li M, Li Z, Gao P, Zhou X, Zhang J. Bufalin induces apoptosis in the U‑2OS human osteosarcoma cell line via triggering the mitochondrial pathway. Mol Med Rep 2016; 13:817–822. [DOI] [PubMed] [Google Scholar]
- 27.Ye K, Wang S, Yang Y, Kang X, Wang J, Han H. Aplasia Ras homologue member overexpression inhibits tumor growth and induces apoptosis through inhibition of PI3K/Akt survival pathways in human osteosarcoma MG-63 cells in culture. Int J Mol Med 2015; 36:776–782. [DOI] [PubMed] [Google Scholar]
- 28.Yao P, Wang ZB, Ding YY, Ma JM, Hong T, Pan SN, Zhang J. Regulatory network of differentially expressed genes in metastatic osteosarcoma. Mol Med Rep 2015; 11:2104–2110. [DOI] [PubMed] [Google Scholar]
- 29.Kimura R, Ishikawa C, Rokkaku T, Janknecht R, Mori N. Phosphorylated c-Jun and Fra-1 induce matrix metalloproteinase-1 and thereby regulate invasion activity of 143B osteosarcoma cells. Biochim Biophys Acta 2011; 1813:1543–1553. [DOI] [PubMed] [Google Scholar]
- 30.Shang HS, Chang JB, Lin JH, Lin JP, Hsu SC, Liu CM, et al. Deguelin inhibits the migration and invasion of U-2 OS human osteosarcoma cells via the inhibition of matrix metalloproteinase-2/-9 in vitro. Molecules 2014; 19:16588–16608. [DOI] [PMC free article] [PubMed] [Google Scholar]