Abstract
Background/Aims
Drug-induced and indeterminate Acute Liver Failure (ALF) might be due to an autoimmune-like hepatitis that is responsive to corticosteroid therapy. The aim of this study was to evaluate whether corticosteroids improve survival in fulminant autoimmune hepatitis, drug-induced or indeterminate ALF, and whether this benefit varies according to the severity of illness.
Methods
We conducted a retrospective analysis of autoimmune, indeterminate and drug-induced ALF patients in the Acute Liver Failure Study Group from 1998-2007. The primary endpoints were overall and spontaneous survival (SS, survival without transplant).
Results
361 ALF patients were studied, 66 with autoimmune (25 steroids, 41 no steroids), 164 with indeterminate (21 steroids, 143 no steroids), and 131 with drug-induced (16 steroids, 115 no steroids) ALF. Steroid use was not associated with improved overall survival (61% vs. 66%, p=0.41), nor with improved survival in any diagnosis category. Steroid use was associated with diminished survival in certain subgroups of patients, including those with the highest quartile of MELD (MELD > 40, survival 30% vs. 57%, p=0.03). In multivariable analysis controlling for steroid use and diagnosis, age (OR 1.37 per decade), coma grade (OR 2.02 grade 2, 2.65 grade 3, 5.29 grade 4), MELD (OR 1.07) and pH<7.4 (OR 3.09) were significantly associated with mortality. Though steroid use was associated with a marginal benefit in SS overall (35% v. 23%, p=0.047), this benefit did not persistent in multivariable analysis; mechanical ventilation (OR 0.24), MELD (OR 0.93), and ALT (1.02) were the only significant predictors of SS.
Conclusions
Corticosteroids did not improve overall survival or SS in drug-induced, indeterminate or autoimmune ALF and were associated with lower survival in patients with the highest MELD scores.
Keywords: drug-induced, cryptogenic, autoimmune, liver transplant, steroids
Introduction
Acute liver failure (ALF) is a heterogeneous syndrome resulting in rapid deterioration of liver function with subsequent coagulopathy and encephalopathy in a previously healthy individual.1 Drug-induced (DI)-ALF is the most common etiology, the majority of which is due to the intrinsic hepatotoxin, acetaminophen,2 and the remainder due to idiosyncratic drug reactions, presumably immune-mediated liver injury due to the metabolic generation of a neo-antigen3. Approximately 4% of ALF cases are believed to be secondary to autoimmune acute liver failure (AI-ALF), although diagnostic criteria have not been well developed, and are primarily based upon criteria for patients with classical autoimmune hepatitis (AIH). The etiology of another 17% of ALF cases remains indeterminate after extensive evaluation. Recently, we have suggested that many patients with ALF of indeterminate etiology have AI-ALF, and have proposed histological features suggestive of an autoimmune pathogenesis.4
The prognosis of patients with non-acetaminophen-induced ALF remains very poor, with transplant-free or spontaneous survival (SS) rates of only 44%. Patients with idiosyncratic drug-induced, indeterminate, and AI-ALF have a particularly dismal SS rate of < 25%.2 There are no etiology-specific treatments that have improved the prognosis of non-acetaminophen ALF, and the trend toward increased SS in the last 30 years can be primarily attributed to improved critical care management.
The administration of glucocorticoids in patients with potentially immune-mediated ALF remains controversial after early studies suggested no benefit or even an adverse effect.5-7 AI-ALF may theoretically respond to corticosteroids based upon their efficacy in patients who present with classical AIH and a few case reports of AI-ALF, although the largest series published to date concluded that corticosteroids might be harmful and may increase the risk of infection around the time of liver transplantation. 1,8,9 The use of corticosteroids has also been proposed as a potential treatment for idiosyncratic DI-ALF based upon a presumed immune-mediated pathogenesis6 and the fact that the innate immune system plays a key role in the development of ALF in humans and in animal models.10-12 In specific idiosyncratic drug reactions from HMG-CoA reductase inhibitors--“statins”, antibiotics, and non-steroidal anti-inflammatory drugs, glucocorticoid administration has ameliorated the liver injury. 8-13 While it remains unclear whether these cases of DI-ALF induce bona fide AI-ALF or only mimic it, the frequent appearance of autoantibodies supports the former possibility. Based upon our previous observations that patients with indeterminate ALF have histological features of autoimmunity on liver biopsy, we also postulated that corticosteroids might also benefit this sub-population. The administration of corticosteroids may therefore represent an opportunity to reverse severe immune-mediated injury and obviate the need for liver transplantation.
Consequently, the aim of the present study was to evaluate the efficacy of corticosteroids in improving overall survival and SS in 3 groups of ALF with a potentially autoimmune-mediated pathogenesis: those with AI-ALF, indeterminate ALF, and DI-ALF. Considering the clinical heterogeneity of patients with the ALF syndrome, subgroup analyses were undertaken to determine whether the possible benefit of corticosteroids were associated with the severity of the clinical syndrome at time of presentation.
Materials and Methods
Patients and Study Design
We conducted a retrospective analysis of all patients enrolled in the Acute Liver Failure Study Group (ALFSG) Registry between 1998-2007 who were deemed to have either AI-ALF, indeterminate ALF, or DI-ALF by the site Principal Investigator (PI). The ALFSG study cohort and its outcomes have been previously described. 16 A total of 361 consecutive patients were enrolled with ALF. Inclusion criteria included coagulopathy (international normalized ratio of prothrombin time [INR] ≥1.5) and any degree of hepatic encephalopathy. After informed consent was obtained from next of kin, detailed prospective clinical and laboratory data were collected in an anonymous fashion on admission to the study and for 7 consecutive days until Day 21, or the time of death or liver transplantation. The protocol was IRB approved at all study sites as a prospective cohort and at UT Southwestern as the data-coordinating center. The etiologies of ALF included indeterminate ALF (N = 164), DI-ALF (N = 131), and AI-ALF (N = 66). Each center determined whether a patient met criteria for AIH except where reclassified by histology (see below).
Primary end points were overall survival and spontaneous survival (SS), defined as survival without transplantation or death, by 21 days. The criteria for transplantation varied by center, however listing criteria are standardized. To be listed as Status 1 they had to meet UNOS criteria for ALF, which are similar to study entry criteria, including presence in the ICU, encephalopathy and a INR > 1.5. If a patient was not listed as Status 1, they were listed at their native MELD score.
The primary predictor was corticosteroid use, defined as any dose of prednisone PO or methylprednisolone IV. In half of the cases, treatment had been begun prior to onset of ALF while in the other half of cases the use of prednisone and related compounds began after the diagnosis of ALF had been made. The AI-ALF group was used as the control group for the corticosteroid benefit analyses. Sub-group analyses were performed using quartiles of AST, ALT, MELD and grade of hepatic encephalopathy as measures of the degree of hepatic inflammation and severity of illness. Infection was thought to be present when a positive culture from blood, urine, tracheal aspirates, vascular catheters, wounds or ascites was reported during study day 1-7. These data were initially analyzed based on the etiology of ALF. A diagnosis of AIH was made by histology (when available) and ANA and anti- smooth muscle antibody, as determined by the principal investigator at each center. In addition, for AI-ALF and indeterminate ALF patients, liver histopathology was reviewed specifically to identify features of autoimmunity. The effects of corticosteroids were then analyzed in a sensitivity analysis after group reassignment to AIH or indeterminate etiology according to the overall impression of a single liver pathologist, as previously described.4 The reassignment to AIH was based upon 4 features of autoimmunity: distinctive patterns of massive hepatic necrosis, presence of lymphoid follicles, plasma cell-enriched inflammatory infiltrate and central perivenulitis. Eight patients initially considered to have AI-ALF by the site PI were reclassified as indeterminate based upon absent features of autoimmunity, 2 of whom received corticosteroids. Twenty-six patients initially considered to have indeterminate ALF were found to have autoimmune features on histology and were reclassified as AI-ALF, 4 of whom received corticosteroids. Therefore, after reclassification according to histology, 84 patients were deemed to have AI-ALF (27 of whom received corticosteroids) and 146 were deemed to have indeterminate ALF (19 of whom received corticosteroids). The number of patients who received steroids and had a reclassified diagnosis was very small (2 patients reclassified from AI-ALF to indeterminate and 4 patients reclassified from indeterminate to AI-ALF). Given the small N no statistically significant differences were noted when overall survival or spontaneous survival was calculated with the original diagnosis versus the reclassified diagnosis. Further subgroup analyses were not done given the small N. As no differences were noted when the original diagnosis was used versus the reclassified diagnosis in sensitivity analyses, all analyses reported utilize the original diagnosis (66 with autoimmune [25 steroids, 41 no steroids], 164 with indeterminate [21 steroids, 143 no steroids], and 131 with drug-induced [16 steroids, 115 no steroids] ALF.
Corticosteroid Administration
The decision whether to administer corticosteroids was entirely at the discretion of the site PI, and was based upon an overall impression of an immune pathogenesis; however, the specific criteria used by the site PI to administer corticosteroids were not available. The timing, route of administration, and dosing of corticosteroids was also according to the site PI, and were not uniform.
Statistical analysis
All data were analyzed for normality and were reported as mean [SD] or median [range], as appropriate. The impact of steroid use on the primary endpoints of overall mortality and SS were analyzed using a chi-square analysis. Because of the early time frame for death in ALF, time to event analyses were felt to be inappropriate as an increase in survival time in a twenty-one day period would not be clinically meaningful. Thus, sub-analyses were performed using quartiles of AST, ALT, MELD and grade of hepatic encephalopathy as measures of severity of illness (Quartile values listed in Supplemental Table 1). A sensitivity analyses were performed after reclassifying the etiology of ALF based on histology.
Additionally, univariate and multivariable logistic regression analyses were performed to evaluate predictors of overall mortality and SS. All predictors with p<0.2 in univariate analysis were evaluated in the multivariable model, and then sequentially removed to establish the final model reported. Steroid use and diagnosis category were included in all multivariable models as these were the central predictors evaluated. Diagnosis and coma grade were evaluated as categorical predictors, and pH was analyzed as a dichotomous variable around its median (7.4). In addition, the components of MELD (bilirubin, INR and creatinine) were analyzed but not included in multivariable models along with the MELD score due to collinearity. Statistical analysis was performed using STATA 10.0 (College Station, TX, USA) and SPSS 18 (IBM, USA). A p value of <0.05 was considered statistically significant.
Results
Characteristics of Study Population
A total of 361 patients were studied, 18, 45, and 36% of whom were initially diagnosed with AI-ALF, indeterminate ALF, and DI-ALF, respectively. Clinical characteristics of the 3 groups of patients are depicted in Table 1. Although baseline characteristics were generally similar between groups, patients with AI-ALF tended to be older (p=0.001) and more likely to be female (p=0.001) than those with DI-ALF or indeterminate ALF.
Table 1.
Baseline demographics by diagnosis group.
| Variable | AI-ALF N=66 | DI-ALF N=131 | Indeterminate N=164 | P-value |
|---|---|---|---|---|
|
| ||||
| Gender (%) | ||||
| Male | 11 (17) | 40 (31) | 70 (43) | 0.001 |
| Female | 55 (83) | 91 (69) | 94 (57) | |
|
| ||||
| Age, Mean (SD) | 46 (16) | 44 (14) | 39 (15) | 0.001 |
|
| ||||
| Race (%) | ||||
| White | 37 (56) | 91 (69) | 118 (72) | 0.20 |
| African American | 18 (27) | 21 (16) | 25 (15) | |
| Asian | 5 (8) | 10 (8) | 10 (6) | |
| Native American | 0 (0) | 5 (4) | 4 (2) | |
| Other | 6 (9) | 5 (4) | 7 (4) | |
|
| ||||
| Ethnicity (%) | ||||
| Non-Hispanic | 52 (79) | 112 (86) | 146 (89) | 0.13 |
| Hispanic | 14 (21) | 18 (14) | 18 (11) | |
|
| ||||
| Mean MELD (SD) | 30.9 (9.5) | 32.8 (8.8) | 34.5 (9.5) | 0.03 |
|
| ||||
| Laboratory values, mean (SD) | ||||
| AST (U/L) | 619 (560) | 1190 (1997) | 2193 (3640) | <0.001 |
| ALT (U/L) | 601 (510) | 1227 (1799) | 1696 (2235) | <0.001 |
| Total Bilirubin (mg/dL) | 23.2 (9.1) | 20.9 (11.4) | 21.0 (12.7) | 0.37 |
| Creatinine (mg/dL) | 1.5 (1.4) | 2.0 (1.8) | 2.2 (1.8) | 0.02 |
| INR | 3.33 (3.17) | 3.44 (2.37) | 3.76 (3.12) | 0.48 |
|
| ||||
| Positive autoimmune serologies (%)* | ||||
| ASMA | 15/24 (63) | 8/20 (40) | 11/30 (37) | 0.14 |
| ANA | 39/41 (95) | 25/34 (74) | 32/39 (82) | 0.03 |
| AMA | 1/7 (14) | 1/12 (8) | 1/13 (8) | 0.88 |
|
| ||||
| Globulin, mean (SD) | 3.85 (1.72) | 2.99 (1.41) | 3.38 (6.8) | .56 |
|
| ||||
| Coma grade (%) | ||||
| 1 | 20 (30) | 42 (32) | 36 (22) | |
| 2 | 24 (36) | 41 (31) | 46 (28) | 0.12 |
| 3 | 13 (20) | 26 (20) | 38 (49) | |
| 4 | 9 (14) | 22 (17) | 44 (27) | |
|
| ||||
| Ventilatory Support (%) | 28 (42) | 63 (39) | 103 (63) | 0.005 |
|
| ||||
| Steroid Use (%) | 25 (38) | 16 (12) | 21 (13) | |
| Total number of subjects | 25 | 16 | 21 | <0.001 |
| Daily Prednisone Dose**, Median (IQR) | 50 (30-61.3) | 60 (40-62) | 40 (23-50) | |
| Days of Prednisone, Median (IQR) | 24 (6.3-57.5) | 32.5 (24.3-36.3) | 31 (17.3-73) | |
Autoimmune serologies were evaluated in the minority of patients (as indicated) and were considered positive with a titer of ≥ 1:40.
Complete data on prednisone or prednisone equivalent dose was available in 12/25 AI-ALF cases, 7/16 DI-ALF cases and 8/21 indeterminate cases.
Overall, 62 patients (17%) received some form of corticosteroid therapy (Table 1). Patients with an initial diagnosis of AI-ALF had the highest rate of corticosteroid use (38%), higher than those with indeterminate or DI-ALF (13% and 12%, respectively; p<0.001). There were also statistically significant differences in several laboratory values between groups, including AST, ALT, creatinine, and MELD score, all of which tended to be highest in the indeterminate group (Table 1). The minority of patients had anti-nuclear (ANA), anti-mitochondrial (AMA) and anti-smooth muscle antibodies (ASMA) recorded. Among these, ANA was significantly more likely to be positive in patients with AIH-ALF. There were no significant differences in mean globulin levels between the groups.
Corticosteroid dose
Complete data on dose and duration of corticosteroids administered was only available in a proportion of patients (in 72% in AI-ALF, 50% of DI-ALF, and 62% of indeterminate cases). The majority of patients received prednisone (8 AI-ALF, 7 DI-ALF, and 12 indeterminate ALF), The mean daily dose of prednisone in the 3 groups was similar (42.5 mg for AI-ALF, 41.6 mg for indeterminate ALF, and 42.3 mg for DI-ALF). Only a few patients in each group received methylprednisolone (Solumedrol®) (5 AI-ALF, 2 DI-ALF and 1 indeterminate ALF). Methylprednisolone doses were therefore converted to the prednisone equivalents for analysis. Among those with known steroid doses, the mean cumulative steroid dose (in prednisone equivalents) was 1287 ± 1144 mg. The median daily dose (50 mg AI-ALF, 60 mg DI-ALF and 40 mg indeterminate-ALF) and median duration of steroid therapy (24 days AI-ALF, 32.5 days DI-ALF and 31 days indeterminate ALF) were similar between groups. The baseline characteristics of the patients who received steroids versus those that did not were generally similar, though the average age of those who received steroids was higher (Table 2). In addition, admission INR was lower in the patients who received steroids than in the patients that did not receive steroids (2.64 vs. 3.78, p=0.005).
Table 2.
Baseline characteristics by steroid use.
| Variable | Steroid (n=62) | No steroid (n=299) | P-value |
|---|---|---|---|
|
| |||
| Gender (%) | |||
| Male | 22 (35) | 99 (33) | 0.72 |
| Female | 40 (65) | 200 (67) | |
|
| |||
| Age, mean (SD) | 46 (15) | 41 (15) | 0.02 |
|
| |||
| Race-N(%) | |||
| White | 45 (73) | 201 (67) | 0.68 |
| African American | 10 (16) | 54 (18) | |
| Asian | 2 (3) | 23 (8) | |
| Native American | 1 (2) | 3 (1) | |
| Other | 4 (6) | 18 (6) | |
|
| |||
| Ethnicity (%) | |||
| Non-Hispanic | 55 (89) | 255 (86) | 0.52 |
| Hispanic | 7 (11) | 43 (14) | |
|
| |||
| MELD, mean (SD) | 31.9 (9.9) | 33.5 (9.2) | 0.24 |
|
| |||
| Laboratory values, mean (SD) | |||
| AST (U/L) | 1007 (2459) | 1653 (2871) | 0.10 |
| ALT (U/L) | 897 (1375) | 1414 (1990) | 0.06 |
| Total Bilirubin (mg/dL) | 22.4 (12.6) | 21.2 (11.5) | 0.44 |
| Creatinine (mg/dL) | 2.1 (1.8) | 2.0 (1.7) | 0.53 |
| INR | 2.64 (1.3) | 3.76 (3.08) | 0.005 |
| pH | 7.43 (0.07) | 7.37 (0.62) | 0.51 |
|
| |||
| Autoimmune serologies, positive (%)* | |||
| ASMA | 11/21 (52) | 23/53 (43) | 0.48 |
| ANA | 18/20 (90) | 78/94 (8.3) | 0.43 |
| AMA | 2/7 (29) | 1/25 (4) | 0.05 |
|
| |||
| Coma grade (%) | |||
| 1 | 22 (35) | 76 (25) | 0.41 |
| 2 | 18 (29) | 93 (31) | |
| 3 | 12 (19) | 65 (22) | |
| 4 | 10 (16) | 65 (22) | |
|
| |||
| Ventilatory Support (%) | 28 (45) | 166 (56) | 0.14 |
Autoimmune serologies were evaluated in the minority of patients (as indicated) and were considered positive with a titer of ≥ 1:40.
Effects of corticosteroids on overall survival in ALF
Sixty-six percent (238 of 361 patients) survived, while 34% (123 patients) died, and 44% (159 patients) received a liver transplant. Overall survival among all patients who received corticosteroids was 61%, compared to 66% in those who did not receive corticosteroids (p=0.41; Figure 1). Similarly, corticosteroid administration had no apparent effect on overall survival of patients with an initial diagnosis of AI-ALF (60% with steroids vs. 73% without steroids; p=0.27). Thirty-four patients had liver histology available for analysis of features of autoimmunity. After reclassification of these 34 patients according to histological criteria of AI-ALF,4 overall survival was similar in patients who received corticosteroids compared to those who did not (66% vs. 68%, respectively; p=0.53). In the subgroup of patients with DI-ALF, survival was 69% for those who received corticosteroids versus 66% for those who did not (p= 0.82), and in patients with indeterminate ALF, survival was 57% for those who received steroids versus 66% who did not (p=0.44). Similar results were obtained when the data were analyzed after reclassification according to liver histopathology: overall survival in patients with indeterminate ALF was 79% in those who received corticosteroids compared to 66% who did not (p=0.20).
Figure 1.
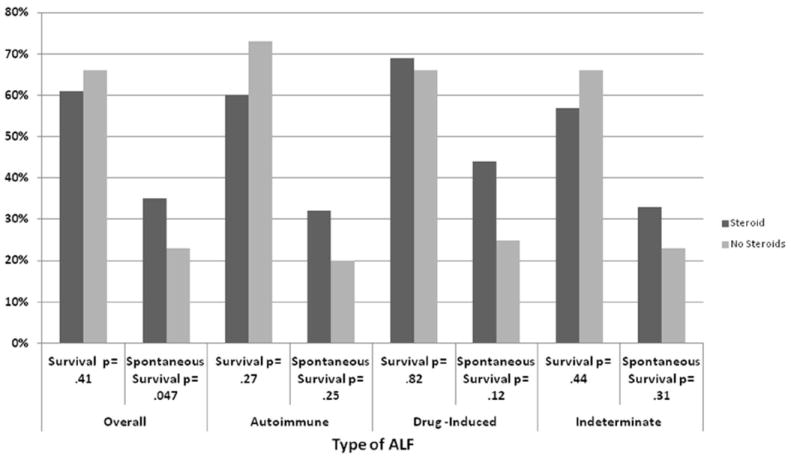
Overall and Spontaneous Survival among different etiologies of ALF
Effects of corticosteroids on spontaneous survival in ALF
SS among patients who received corticosteroids (35%) was significantly higher than in patients who did not receive corticosteroids (23%; p=0.047) (Figure 1). However, there were no significantly differences in SS between steroid groups in any particular diagnosis subgroup, including those with AI-ALF (32% v. 20%, respectively, p=0.25). In addition, reassignment of etiology based upon a histological diagnosis of AI-ALF also yielded no difference (26% in those who received corticosteroids and 21% in those who did not (p=0.41).
Analysis of outcome according to severity of ALF
We hypothesized that the benefit of corticosteroids might be limited to patients with relatively less severe ALF, and subsequently analyzed the effect of steroid administration on overall and spontaneous survival according quartiles of AST, ALT and MELD. There were no statistically significant differences in SS in patients who received corticosteroids among all quartiles of ALT (Figure 2) or AST (data not shown). The highest 50% of ALT values had improved spontaneous survival compared to those not receiving steroid therapy (54% vs 25%, respectively, p=0.003), and numerically higher overall survival (75% vs 61%, respectively, p=0.20). (Figure 2)
Figure 2.
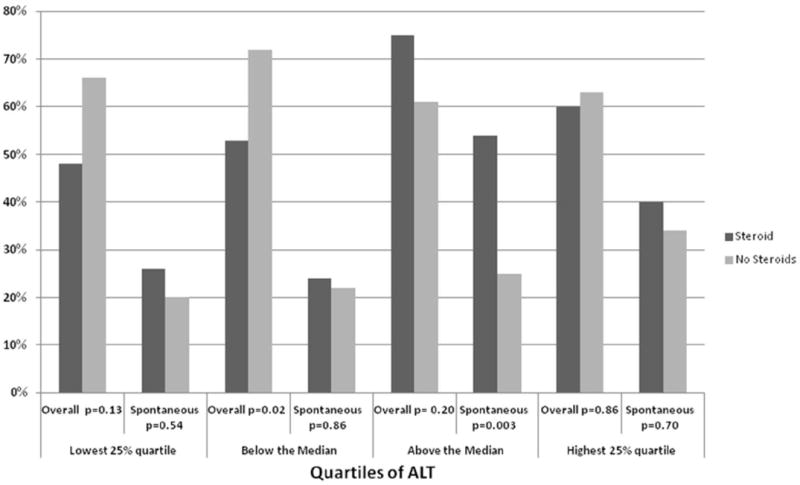
Overall and Spontaneous Survival among Quartiles of ALT
Steroid administration did not result in any difference in overall survival or SS in patients with MELD scores in the lowest three quartiles (Figure 3). In contrast, there was significantly lower overall survival in patients who received steroids in the highest MELD quartile (MELD > 40, 43%% v. 70%, respectively, p=0.03, Figure 3).
Figure 3.
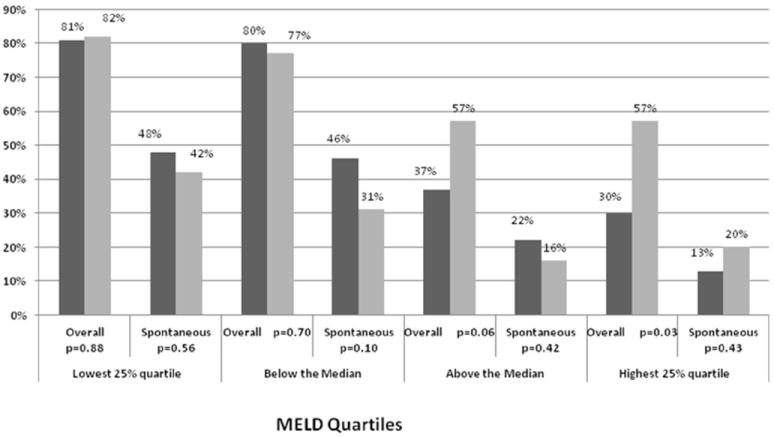
Overall and Spontaneous Survival among Quartiles of MELD
Subgroup analysis based upon encephalopathy stages 1-4 was also performed, and no differences in outcome were observed in patients who received or did not receive corticosteroids (data not shown).
Logistic Regression to Predict Mortality and Spontaneous Survival
Logistic regression was performed to identify independent predictors of overall mortality and SS. Steroid use and diagnosis category were not predictive of overall mortality in uni- or multivariable models (Table 3). In the final model to predict overall mortality, age (OR 1.37 per 10 years), coma grade (OR 2.02 for stage 2, 2.65 for stage 3 and 5.29 for stage 4), MELD (OR 1.07 per MELD point) and pH <7.4 (OR 3.09) were significantly predictive, controlling for steroid treatment and diagnosis category. In addition, steroid use was associated with diminished spontaneous survival (OR 1.80, p=0.049) in univariate analysis. However, in the final model for SS, male gender (OR 1.58), the need for ventilator support (OR 0.21), MELD (0.93) and ALT (1.02 per 100 U/L) were significantly predictive, while steroid use (OR 1.8, p=0.12) and diagnosis category were not significant (Table 4). Though the individual components of MELD were predictive of survival in some univariate and multivariable analyses they did not perform as well as MELD and thus were not included in the final multivariable models (Tables 3 and 4).
Table 3.
Uni- and multivariable analysis to predict overall mortality.
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
| ||||
| Variable | OR | p-value | OR | p-value |
|
| ||||
| Steroid treatment | 1.27 | 0.72 | 1.56 | 0.19 |
|
| ||||
| Diagnosis | ||||
| AI-ALF | -- | -- | -- | -- |
| DI-ALF | 1.10 | 0.78 | 1.22 | 0.59 |
| Indeterminate | 1.17 | 0.51 | 1.00 | 1.00 |
|
| ||||
| Age (per 10 years) | 1.37 | <0.001 | 1.39 | <0.001 |
|
| ||||
| Coma grade | ||||
| 1 | -- | -- | -- | -- |
| 2 | 2.02 | 0.03 | 1.74 | 0.12 |
| 3 | 2.65 | 0.006 | 2.37 | 0.02 |
| 4 | 5.29 | <0.001 | 5.18 | <0.001 |
|
| ||||
| Ventilatory Support | 3.11 | <0.001 | -- | -- |
|
| ||||
| MELD | 1.07 | <0.001 | 1.05 | 0.001 |
|
| ||||
| Creatinine (mg/dL) | 1.29 | <0.001 | -- | -- |
|
| ||||
| pH<7.4 | 3.09 | <0.001 | 2.68 | 0.002 |
Additional variables tested and not significant with a p<0.2 include: gender, AST, ALT, bilirubin, INR.
Table 4.
Uni- and multivariable analysis to predict spontaneous survival.
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
| ||||
| Variable | OR | p-value | OR | p-value |
|
| ||||
| Steroid treatment | 1.80 | 0.049 | 1.80 | 0.12 |
|
| ||||
| Diagnosis | ||||
| AI-ALF | -- | -- | -- | -- |
| DI-ALF | 1.18 | 0.63 | 1.49 | 0.33 |
| Indeterminate | 1.00 | 0.98 | 1.36 | 0.46 |
|
| ||||
| Male gender | 1.58 | 0.07 | -- | -- |
|
| ||||
| Coma grade | ||||
| 1 | -- | -- | -- | -- |
| 2 | 0.56 | 0.05 | ||
| 3 | 0.50 | 0.04 | ||
| 4 | 0.22 | <0.001 | ||
|
| ||||
| Ventilatory Support | 0.24 | <0.001 | 0.21 | <0.001 |
|
| ||||
| MELD | 0.93 | <0.001 | 0.93 | <0.001 |
|
| ||||
| INR | 0.84 | 0.01 | -- | -- |
|
| ||||
| ALT (per 100 U/L) | 1.02 | <0.001 | 1.04 | <0.001 |
|
| ||||
| AST (per 100 U/L) | 1.01 | 0.004 | -- | -- |
|
| ||||
| Bilirubin | 0.93 | <0.001 | -- | -- |
|
| ||||
| pH<7.4 | 0.60 | 0.13 | -- | -- |
Additional variables tested and not significant with a p<0.2 include: age, creatinine.
Effects of corticosteroids on the incidence of infection
Infection is a frequent complication of ALF, and the risk of increasing the incidence of infection has been cited as a rationale to avoid corticosteroids in this patient population. The overall rate of infection in patients was high but similar in those who received corticosteroids (42%) and those who did not (40%; p=0.733). The rate of infection also did not differ in subgroups according to etiology (Data not shown).
Discussion
In this prospectively collected, non-randomized study of large numbers of patients with ALF we demonstrated that corticosteroids did not improve overall survival in AI-ALF, DI ALF, or indeterminate ALF. In multivariable models, known predictors of poor outcomes in liver failure including age, coma grade, MELD and pH<7.4 were the only significant predictors of mortality, controlling for steroid use and diagnosis category. Interestingly, corticosteroid use was significantly associated with lower overall survival in high MELD patients. Although steroid use was marginally associated improved SS in univariate analysis, this difference did not persist in multivariable analysis after controlling for important clinical predictors of outcomes. In multivariable analysis controlling for steroid use and diagnosis category, the need for mechanical ventilation and MELD were significantly associated with diminished rates of SS, while ALT was significantly associated with increased SS. One potential explanation for the lack of benefit of corticosteroids overall may be selection bias. Patients in our study who received corticosteroids might have been more ill than those who did not, which may have biased the results towards making corticosteroids appear less effective. However, this is unlikely as the average MELD score in patients who received corticosteroids was similar to those who did not, and INR was actually higher in the no steroid group.
Kings College criteria were not assessed as all required components were not available for all patients. However it has previously been shown that MELD score has an excellent utility in the prediction of outcomes in ALF, and correlates equally with Kings College Criteria17. Subgroup analyses based on either MELD or degree of encephalopathy showed equivalent results, suggesting that selection bias based upon disease severity was not the primary factor responsible for our results.
Based upon observations in patients with non-ALF autoimmune hepatitis 9, patients with AI-ALF would reasonably be expected to respond to corticosteroids. However, we did not identify survival benefit in patients with AI-ALF who received corticosteroids, and noted a non-significant trend towards decreased survival in this group. Although this observation remained unchanged even after reclassification of etiology based on histology, it remains possible that some patients were misclassified. Unfortunately, given the retrospective nature of this study and lack of biopsy data in all patients, the “Revised Original Scoring System of the International Autoimmune Hepatitis Group” was unable to be calculated. However, as biopsy is not always possible in the acute setting in clinical practice, the diagnostic approach utilized does reflect the “real world” experience at experienced liver centers in the US. Overall, 38% of the total AI-ALF group were ANA negative. However, when the data for this cohort was reviewed for the study by an independent pathologist and only those with a histopathologically confirmed diagnosis of AIH were analyzed, steroids still showed no benefit. This sensitivity analysis supports our conclusion.
Another possible explanation for the lack of benefit of steroid treatment for AI-ALF patients in this cohort is that AI-ALF may have a different pathogenesis than classical autoimmune hepatitis, or that the condition of patients with AI-ALF is too advanced to allow for rescue with corticosteroids. Indeed, patients with acute presentations of autoimmune hepatitis have been shown to be less steroid responsive than patients with more chronic presentations, and have significant biochemical and histologic differences.4,18,19 Specifically, patients with acute presentations of AI-ALF more frequently have centrilobular necro-inflammatory changes than those with classical AIH, a histological feature of severe, and treatment-refractory AIH and acute cellular rejection in transplant recipients.4,20 Based on the present study, patients with AI-ALF often have irreversible liver damage with or without treatment with corticosteroids, and most will die without liver transplantation. Larger cohorts will be required to determine whether these trends are clinically significant.
Despite the observation that administering corticosteroids was not associated with an overall survival benefit, and potentially increased mortality in high MELD patients, there were no statistically significant differences in the rate of infections in the patients that received corticosteroids compared to the patients who did not. Previous studies have raised concern that corticosteroids increased infectious complications in patients with ALF 8. Thus, the explanation for the decreased survival in patients treated with corticosteroids is unclear.
In our subgroup analysis of patients categorized by disease severity, only patients who received corticosteroids and had ALT in the higher 50% of values had improved spontaneous survival (54% steroid users vs 25% patients who did not receive steroids (p=0.003) and modestly improved overall survival (75%), compared to those not receiving steroid therapy (61%, p=0.2 Figure 2). We postulate that higher ALT may be a surrogate for an inflammatory process that is more corticosteroid-sensitive than other mechanisms of injury.
Overall, our data suggest that patients with a higher ALT and lower MELD scores may benefit from a liver biopsy since this group may be more likely to respond favorably to steroid administration. In contrast, patients with lower ALT and higher MELD scores are less likely to respond and will usually require OLT. In these cases liver biopsy does not seem warranted as intervention with steroids will not improve survival and may even be detrimental. Our findings are similar to previous studies that have demonstrated that patients with higher ALT values have an increased capacity to regenerate and, consequently, better survival rates.21 In fact, cirrhotic livers have lower elevated liver enzymes, particularly in cases of acute-on-chronic liver failure.22
Finally, it has recently been shown that steroids in higher doses (prednisone 2-20 mg/kg/day over 3-7days) in combination with UDCA might be useful in drug induced liver injury23,24. In that study, patients that received steroids had a decrease in bilirubin and transaminases to less than 50% of peak values in 2 weeks and normalized in 4-8 weeks. The greatest benefit was in patients who had immune–mediated DILI. The number of patients in our study with DI-ALF who received both steroids and ursodiol is too small to perform any meaningful analyses. However, it is possible that the steroid dose and/or duration in this study was not sufficiently large or standardized to see maximal effects, or that steroids could be of greater benefit if used in combination with ursodiol or other agents, and additional studies are warranted in the future.
One major limitation of our study was that analyses were conducted retrospectively and the use of steroids was neither randomized nor standardized. Consequently, the administration of corticosteroids was not uniform in either in formulation, dose, route of administration, and duration of treatment. The use and duration of steroids was completely at the discretion of the provider and this alone could bias or confound our results. However, our findings across almost all subgroups suggest there is no survival benefit with steroid use, even in the AI-ALF group, in whom immunosuppression protocols with corticosteroids may be more likely to be standardized.
In conclusion, this prospectively collected large multicenter cohort study demonstrates no survival benefit with the administration of corticosteroids for patients with ALF of presumed autoimmune or drug induced etiology. Further investigation, if possible, should include a prospective, randomized study of the safety and efficacy of corticosteroids in patients with AI- and DI-ALF. In the interim, based upon the data presented, the risks and benefits of corticosteroid administration should be carefully weighed and early discontinuation considered in the absence of a clear salutary effect, especially in high MELD patients.
Supplementary Material
Acknowledgments
This study was funded by a National Institutes of Health grant (DK U-01 58369) for the Acute Liver Failure Study Group provided by the National Institute of Diabetes and Digestive and Kidney Diseases.
Members and institutions participating in the Acute Liver Failure Study Group 1998-2007are as follows: W.M. Lee, M.D. (Principal Investigator), George A. Ostapowicz, M.D., Frank V. Schiødt, M.D., Julie Polson, M.D., University of Texas Southwestern, Dallas, TX; Anne M. Larson, M.D., University of Washington, Seattle, WA; Timothy Davern, M.D., University of California, San Francisco, CA (current address: California Pacific Medical Center, San Francisco, CA); Michael Schilsky, M.D., Mount Sinai School of Medicine, New York, NY (current address: Yale University, New Haven, CT); Timothy McCashland, M.D., University of Nebraska, Omaha, NE; J. Eileen Hay, M.B.B.S., Mayo Clinic, Rochester, MN; Natalie Murray, M.D., Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, M.D., University of Pittsburgh, Pittsburgh, PA; Andres Blei, M.D., Northwestern University, Chicago, IL (deceased); Atif Zaman, M.D., University of Oregon, Portland, OR; Steven H.B. Han, M.D., University of California, Los Angeles, CA; Robert Fontana, M.D., University of Michigan, Ann Arbor, MI; Brendan McGuire, M.D., University of Alabama, Birmingham, AL; Raymond T. Chung, M.D., Massachusetts General Hospital, Boston, MA; Alastair Smith, M.B., Ch.B., Duke University Medical Center, Durham, NC; Robert Brown, M.D., Cornell/Columbia University, New York, NY; Jeffrey Crippin, M.D., Washington University, St Louis, MO; Edwin Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, M.B.B.S., Medical University of South Carolina, Charleston, SC; Santiago Munoz, M.D., Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, M.D., University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, M.D., Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, M.D., University of California Davis, Sacramento, CA; Raj Satyanarayana, M.D., Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, M.D., University of California, San Diego, CA. The University of Texas Southwestern Administrative Group included Grace Samuel, Ezmina Lalani, Carla Pezzia, and Corron Sanders, Ph.D., and the Statistics and Data Management Group included Joan S. Reisch, Ph.D., Linda S. Hynan, Ph.D., Janet P. Smith, Joe W. Webster, and Mechelle Murray
This research was supported by NIDDK U01-DK-58369
List of abbreviations
- ALF
Acute liver failure
- DI
Drug-induced
- AI
Autoimmune
- SS
Spontaneous survival
- ANA
Anti-nuclear antibodies
- AMA
Anti-mitochondrial antibodies
- ASMA
Anti-smooth muscle antibody
- MELD
Model for End Stage Liver Disease
Footnotes
Financial disclosure: The authors on this paper have no financial relationships to disclose.
References
- 1.Polson J, Lee W. AASLD Position Paper: The Management of Acute Liver Failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 2.Ostapowicz G, Fontana R, Schiodt F, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;237:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 3.Smith GC, Kenna JG, Wolf CR. Autoantibodies to hepatic microsomal carboxylesterase in halothane hepatitis. Lancet. 1993 Oct 16;342(8877):963–4. doi: 10.1016/0140-6736(93)92005-e. [DOI] [PubMed] [Google Scholar]
- 4.Stravitz RT, Lefkowitch JH, Fontana RJ, Gershwin ME, Leung PS, Sterling RK, Manns MP, Norman GL, Lee WM Acute Liver Failure Study Group. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology. 2011 Feb;53(2):517–2. doi: 10.1002/hep.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ware AJ, Jones RE, Shorey JW, Combes B. A controlled trial of steroid therapy in massive hepatic necrosis. Am J Gastroenterol. 1974 Aug;62(2):130–133. [PubMed] [Google Scholar]
- 6.Randomised trial of steroid therapy in acute liver failure. Report from the European Association for the Study of the Liver (EASL) Gut. 1979 Jul;20(7):620–623. doi: 10.1136/gut.20.7.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakela J, Mosley JW, Edwards VM, Govindarajan S, Alpert E. A double-blinded, randomized trial of hydrocortisone in acute hepatic failure. The Acute Hepatic Failure Study Group Dig Dis Sci. 1991 Sep;36(9):1223–1228. doi: 10.1007/BF01307513. [DOI] [PubMed] [Google Scholar]
- 8.Ichai P, Duclos-Vallée J, Guettier C, Hamida S, Antonini T, Delvart V, et al. Usefulness of corticosteroids for the treatment of severe and fulminant forms of autoimmune hepatitis. Liver Transpl. 2007;13:996–1003. doi: 10.1002/lt.21036. [DOI] [PubMed] [Google Scholar]
- 9.Czaja AJ. Corticosteroids or not in severe acute or fulminant autoimmune hepatitis: therapeutic brinksmanship and the point beyond salvation. Liver Transpl. 2007 Jul;13(7):953–955. doi: 10.1002/lt.21088. [DOI] [PubMed] [Google Scholar]
- 10.Giannattasio A, D’Ambrosi M, Volpicelli M, Iorio R. Steroid therapy for a case of severe drug-induced cholestasis. Ann Pharmacother. 2006 Jun;40(6):1196–1199. doi: 10.1345/aph.1G345. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Odriozola P, Gutierrez-Macias A, Ibarmia-Lahuerta J, Munoz-Sanchez J. Meloxicam as a Cause of Drug-Induced Autoimmune Hepatitis. Dig Dis Sci. 2009 Apr 28; doi: 10.1007/s10620-009-0805-5. [DOI] [PubMed] [Google Scholar]
- 12.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006 Jan 5;354(1):54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein NS, Bayati N, Silverman AL, Gordon SC. Minocycline as a cause of drug-induced autoimmune hepatitis. Report of four cases and comparison with autoimmune hepatitis. Am J Clin Pathol. 2000 Oct;114(4):591–598. doi: 10.1309/KV2J-VX6Q-L95V-VDE4. [DOI] [PubMed] [Google Scholar]
- 14.Alla V, Abraham J, Siddiqui J, Raina D, Wu GY, Chalasani NP, et al. Autoimmune hepatitis triggered by statins. J Clin Gastroenterol. 2006 Sep;40(8):757–761. doi: 10.1097/00004836-200609000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Van Heyningen C. Drug-induced acute autoimmune hepatitis during combination therapy with atorvastatin and ezetimibe. Ann Clin Biochem. 2005 Sep;42(Pt 5):402–404. doi: 10.1258/0004563054890105. [DOI] [PubMed] [Google Scholar]
- 16.Gregory B, Larson AM, Reisch J, Lee WM Acute Liver Failure Study Group. Acetaminophen dose does not predict outcome in acetaminophen-induced acute liver failure. J Investig Med. 2010 Jun;58(5):707–10. doi: 10.231/JIM.0b013e3181db8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prakash O, Mumtaz K, Hamid S, Ali Shah SH, Wasim Jafri SM. MELD score: utility and comparison with King’s College criteria in non-acetaminophen acute liver failure. J Coll Physicians Surg Pak. 2012 Aug;22(8):492–6. [PubMed] [Google Scholar]
- 18.Nikias G, Batts K, Czaja A. The nature and prognostic implications of autoimmune hepatitis with an acute presentation. J Hepatol. 1994;21(5):866–871. doi: 10.1016/s0168-8278(94)80251-3. [DOI] [PubMed] [Google Scholar]
- 19.Kessler W, Cummings O, Eckert G, Chalasani N, Lumeng L, Kwo P. Fulminant hepatic failure as the initial presentation of acute autoimmune hepatitis. Clin Gastroenterol Hepatol. 2004;2(7):625–631. doi: 10.1016/s1542-3565(04)00246-0. [DOI] [PubMed] [Google Scholar]
- 20.Turlin B, Slapak GI, Hayllar KM, Heaton N, Williams R, Portmann B. Centrilobular necrosis after orthotopic liver transplantation: a longitudinal clinicopathologic study in 71 patients. Liver Transpl Surg. 1995;1:285–289. doi: 10.1002/lt.500010503. [DOI] [PubMed] [Google Scholar]
- 21.Canbay, et al. Overweight patients are more susceptible for acute liver failure. Hepatogastroenterology. 2005;52(65):1516–20. [PubMed] [Google Scholar]
- 22.Schreiter T, Marquitan G, Darnell M, Sowa JP, et al. An ex vivo perfusion system emulating in vivo conditions in non cirrhotic and cirrhotic human liver. J Pharmacol Exp Ther. 2012;342(3):730–41. doi: 10.1124/jpet.112.194167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant Lafaine, Rockey Don, et al. Drug Induced Liver Injury. Curr Opin Gastroenterol. 2012;28:198–202. doi: 10.1097/MOG.0b013e3283528b5d. [DOI] [PubMed] [Google Scholar]
- 24.Wree Alexander, Dechene, Herzer Kerstin, et al. Steroid and Ursodesoxycholic Acid combination Therapy in Severe Drug-Induced Liver Injury. Digestion. 2011;84:54–59. doi: 10.1159/000322298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


