Abstract
A human artificial chromosome (HAC) is maintained as an episome within a cell and avoids random integration into the host genome. It can transfer multiple and/or large transgenes along with their regulatory elements thereby resembling native chromosomes. Using this HAC system, we established mesenchymal stem cells (MSCs) that simultaneously expressed hepatocyte growth factor, glial cell line-derived neurotrophic factor, and insulin-like growth factor 1, termed HAC-MSCs. This cell line provides an opportunity for stable transplantation and thorough analyses. We then introduced the cells for the treatment of a neurodegenerative disorder, amyotrophic lateral sclerosis. The HAC-MSCs were transplanted via the fourth cerebral ventricle (CV) or intravenous (i.v.) infusion at various ages of recipient mice. Littermate- and sex-matched mice underwent a sham procedure. Compared to the controls, there was an encouraging trend of increased life span via CV transplantation and delayed onset in i.v. infusion 60 days after transplantation. Further, we confirmed a statistically significant increase in life span via CV transplantation at 100 days. This effect was not seen in mice transplanted with MSCs lacking the HAC. We successfully enhanced the trophic potential of the MSCs using the HAC. This strategy could be a promising direction for the treatment of neurodegenerative disorders.
Keywords: amyotrophic lateral sclerosis, human artificial chromosome, mesenchymal stem cells
Introduction
Stem cell therapy offers great hope for the treatment of intractable or incurable conditions like neurodegeneration. However, replenishing damaged neurons is not necessarily the only strategy of this treatment. Rather, the transplantation could exert its effect through protecting debilitated neurons by modulating the surrounding environment via the secretion of trophic factors or other cytoprotective mechanism(s). In either case, cell transplantation does not appear sufficiently potent for the treatment of many central nervous system (CNS) disorders. It has been anticipated that combination therapy might consist of stem cells and the expression of beneficial growth factors.1 Although this could be realized by using gene transfer systems, e.g., viral vectors, there are still limitations in these systems, including genomic instability and/or dysregulation of gene expression by random insertions into the host genome. Further, genetic engineering is currently unable to manipulate large genomic sequences or multiple genetic regions that remain under normal expression control. To overcome these problems, we used a human artificial chromosome (HAC)2 strategy combined with mesenchymal stem cells (MSCs) expressing three trophic factors: hepatocyte growth factor (HGF), glial cell line-derived neurotrophic factor (GDNF), and insulin-like growth factor 1 (IGF-1). The established cell lines are termed HAC-MSCs. Genomic sizes of each factor are as follows: 2,220 bp for HGF, 460 bp for IGF-1, and 630 bp for GDNF. Although the HAC is called “artificial,” the HAC by itself contains no “synthetic” materials. Rather, the HAC used in this experiment is a truncated version of human chromosome 21, lacking distal p- and q-arms but retaining the full capability of autonomous replication and segregation. Like native chromosomes, the HAC can contain a large genomic region or several genes concurrently without requiring integration into the host genome. In this regard, the HAC system is unique and offers opportunities to use novel approaches to treat a variety of disease conditions. Using this system, we are the first to report the establishment of tissue stem cells containing a HAC that was targeted to treat CNS disorders by cell transplantation. Specifically, the goal is a new therapeutic approach for the treatment of a devastating neurodegenerative disorder, amyotrophic lateral sclerosis (ALS).
ALS affects upper and lower motor neurons.3 It paralyzes the limbs and respiratory muscles, causes difficulties of phonation and swallowing, and inevitably leads to death. At present, no therapy shows satisfactory efficacy. Here, we used transgenic SOD1G93A (glycine to alanine substitution at position 93 of the SOD1 protein) mice, or formally B6SJL-Tg(SOD1G93A)1Gur mice.4 These animals are widely studied as an ALS model for both the analysis of the underlying pathogenesis and possible therapeutic interventions. Like human ALS cases, the mice showed a decline of motor performance leading to death, making it easy to evaluate the efficacy of the transplantation because the endpoint could be set as the onset of the disease and/or overall life span, without requiring pathological or biochemical examination. A significant number of cell transplant experiments have been conducted using this model. However, fundamental parameters such as transplantation route (via the fourth cerebral ventricle (CV) or via intravenous (i.v.) administration) or the best transplantation timing have not been evaluated. Using HAC-MSCs as donor cells allows the generation of stable and reproducible results. Thus, we successfully confirmed the efficacy of the transplantation, and we were further able to determine the best transplantation parameters in our model system.
Results
Construction of HAC-MSCs
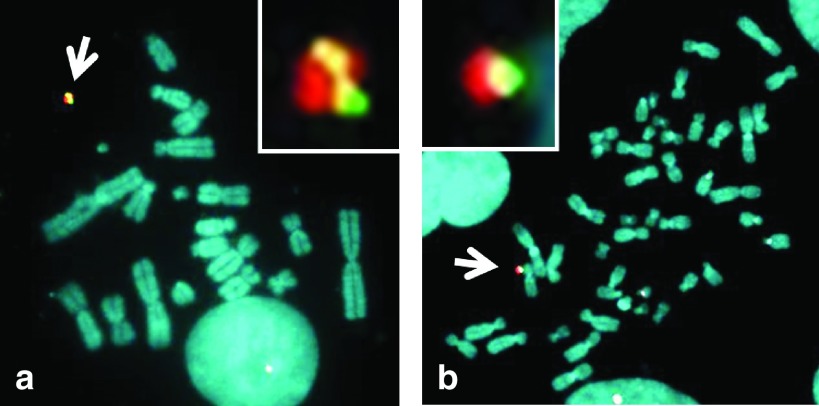
The Cre expression vector and P1 artificial chromosome (PAC) vector containing genes for HGF (hgf), GDNF (gdnf), IGF-1 (igf-1), and luciferase (e-luc)5 were co-transfected into 3′- hypoxanthine phosphoribosyl transferase (HPRT)-deficient Chinese hamster ovary (CHO) (hprt-/-) cells containing an artificial chromosome (21HAC2) (Supplementary Figure S1a).2 Recombinant clones were selected using hypoxanthine-aminopterin-thymidine (HAT) over a period of 10 days. Thirteen out of 38 drug-resistant clones were proven to be positive by PCR (data not shown). Fluorescence in situ hybridization (FISH) analyses showed that the PAC construct was inserted into the 21HAC2 in CHO (hprt-/-) cells in two clones out of the genomic PCR-positive clones (Figure 1a). The 21HAC2 with the PAC was transferred to MSC6 by microcell-mediated chromosome transfer (MMCT) using the CHO clones (Supplementary Figure S1b).2 The MSC (21HAC2-hgf, gdnf, igf-1, e-luc) cells (designated HAC-MSCs) were characterized by PCR and FISH analyses. PCR showed that 30 of the 46 HAC-MSCs clones contained the HAC with the PAC (data not shown). FISH analyses showed that the PAC construct was inserted into the 21HAC2 in MSCs in 12 clones tested (Figure 1b). Two independent HAC-MSCs clones 3–31 and 3–32 were utilized in the following study.
Figure 1.
FISH analyses of the HAC with the PAC in CHO cells and MSCs. FISH analyses of CHO (21HAC2-hgf, gdnf, igf-1, e-luc) and MSCs (21HAC2- hgf, gdnf, igf-1, e-luc). Digoxigenin-labeled human COT-1 DNA (red) and p11-4 (red) were used to detect the (a) HAC in CHO cells and (b) MSCs, respectively. Biotin-labeled PAC (green) was used to detect the PAC DNA on the HAC in a and b. Chromosomal DNA was counterstained with 4′,6-diamidino-2-phenylindole. The inset shows an enlarged image of the HAC with the PAC (arrow).
Clonal selection and characterization of HAC-MSCs
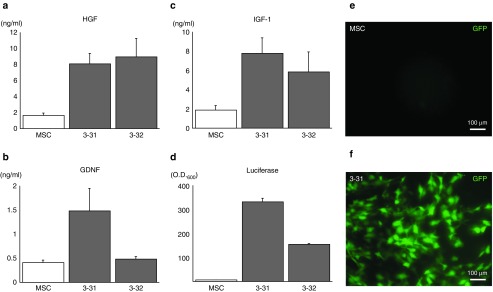
We assayed the concentrations of HGF, GDNF, and IGF-1 in the conditioned media obtained from candidate HAC-MSCs clones 3–31, 3–32, and the original MSCs. Clone 3–31 expressed approximately four times more HGF (Figure 2a), three times more GDNF (Figure 2b), and four times more IGF-1 (Figure 2c) compared to the MSCs. Although clone 3–32 showed higher expression levels of HGF compared to clone 3–31 (Figure 2a), GDNF expression level was insufficient compared to clone 3–31 (Figure 2b). In luciferase assays, background luciferase luminescence was negligible. Clone 3–31 emitted twice as much light as clone 3–32 (Figure 2d). Considering these results, we selected clone 3–31 for transplant experiments. Green fluorescent protein (GFP) expression by clone 3–31 was sufficient by fluorescence microscopy (Figure 2e,f). Lastly, to investigate whether HAC-MSCs and MSCs had different characteristics, we employed flow cytometric analysis. Clone 3–31 expressed low levels of endothelial marker CD31, endothelial progenitor cell marker CD34, and leukocyte marker CD45 as did MSCs (Supplementary Figure S2). Mesenchymal marker CD90 was expressed to similar extents by clone 3–31 and MSCs (Supplementary Figure S2). Hereafter, we term clone 3–31 as simply HAC-MSCs, unless otherwise specifically mentioned.
Figure 2.
Characterization of HAC-MSCs and parental MSCs. Expression levels of (a) HGF, (b) GDNF and (c) IGF-1 were determined by enzyme-linked immunosorbent assay assessment of conditioned media obtained from each cell culture. For HAC-MSCs clone 3–31, the (b) GDNF and (c) IGF-1 expression levels were the highest among the three groups and still good for (a) HGF. Chromosomally introduced luciferase of HAC-MSCs clone 3–31 was about twice as high as that of (d) HAC-MSCs clone 3–32. The HAC vector used in the present study possessed the GFP gene beneath multiple cloning sites; therefore, we also confirmed green fluorescence under ultraviolet (UV) light. The GFP fluorescence was seen in (f) HAC-MSCs (3–31) but not (e) MSC alone.
Transplantation
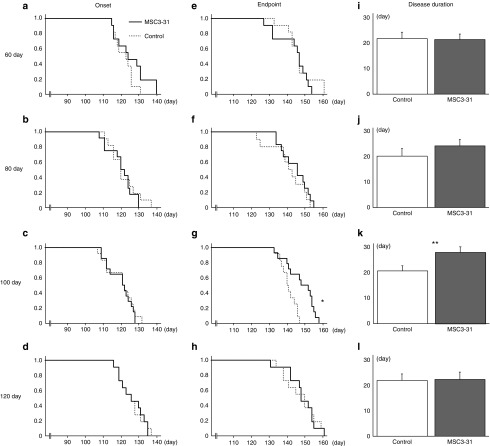
HAC-MSCs were transplanted via the fourth cerebral ventricle (CV) into transgenic SOD1G93A mice7 at the ages of 60 days (n = 12, ♂ = 5, ♀ = 7), 80 days (n = 11, ♂ = 6, ♀ = 5), 100 days (n = 14, ♂ = 5, ♀ = 9), and 120 days (n = 11, ♂ = 5, ♀ = 6). As a control, phosphate-buffered saline (PBS) was injected at 60 days (n = 11, ♂ = 5, ♀ = 6), 80 days (n = 11, ♂ = 6, ♀ = 5), 100 days (n = 12, ♂ = 5, ♀ = 7), and 120 days (n = 11, ♂ = 5, ♀ = 6). The immunosuppressive agent was administered to control mice and transplanted mice. In the mice transplanted at 80 days (Figure 3b,f,j; Supplementary Figure S3b,f) and 120 days (Figure 3d,h,l; Supplementary Figure S3d,h), the transplantation showed no difference in terms of the age of onset, death, disease duration, body weight, nor hind-limb reflex score between transplanted and sham-operated mice. In the mice transplanted at 60 days of age, there were encouraging trends resulting in delayed death (Figure 3a) and increased disease duration (Figure 3i) in the treated mice compared to the controls. If gender was taken into account, there was statistical significance in the female mice of the 60-day-transplanted groups in disease duration (transplanted versus control: 23.3 ± 2.8 versus 13.5 ± 2.6, P < 0.05), whereas in male mice, there was no difference between the groups (transplanted versus control: 25.8 ± 4.7 versus 28.2 ± 3.3). This discrepancy in the transplantation effect on females over males was consistent with our previous report.7 We do not yet have any conclusive explanation whether this gender difference come simply from hormonal disparity, e.g., estrogen or androgen or other intricate mechanism(s). The mice transplanted with HAC-MSCs at 100 days of age showed statistically significant improvements in terms of the age of death (Figure 3g), disease duration (Figure 4k), and body weight (Supplementary Figure S3o). Next, we performed the transplantation with nonchromosomally modified MSCs and compared the outcome to sham-operated mice to see if the effect of HAC-MSC transplantation was attributable to the introduction of HAC. We compared unmodified MSC (n = 15, ♂ = 7, ♀ = 6) and the sham-operated group (n = 13, ♂ = 6, ♀ = 7). The differences between the transplanted and sham-operated groups were not statistically significant: onset, 125.6 ± 1.9 versus 126.8 ± 1.4; life span, 148.0 ± 2.3 versus 149.8 ± 1.5; disease duration, 22.4 ± 2.5 versus 23.0 ± 1.4 (Supplementary Table S1).
Figure 3.
Cell transplantation via the CV to various ages of SOD1G93A transgenic mice. At the ages of 60 (first line), 80 (second line), 100 (third line), and 120 days (last line), SOD1G93A transgenic mice underwent cell transplantation or sham surgery. Figure shows the age of onset (first row), endpoint (second row), and disease duration (last row). There were weak beneficial tendencies such as delayed onset in the group transplanted at (a) 60 days and disease duration in the group transplanted at (j) 80 days. These did not reach statistical significance. Statistical significance was achieved in three instances: the endpoint for transplantation at 100 days (g, log-rank test, *P = 0.0030) as well as disease duration (k, **P = 0.023).
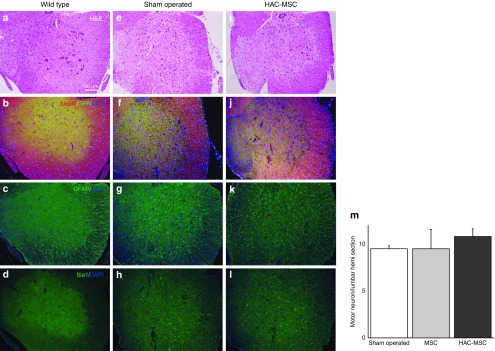
Figure 4.
Histological analyses of mice transplanted with HAC-MSCs or sham-operated mice. Spinal cords were obtained from mice in three groups: wild-type (first line), sham-operated (second line), and HAC-MSCs (third line) transplanted via the CV. (a,b) In wild-type mice, motor neuron numbers in the anterior horn of the spinal cord were preserved and immunostaining for (c) GFAP and for (d) Iba1 was modest. (e,f,i,j) In contrast, the SOD1G93A mice subjected to sham surgery and the mice transplanted with HAC-MSCs showed significant decreases in large motor neurons. Marked augmentation of the immunoreactivity of (g,k) GFAP as well as (h,l) Iba1 was observed in both groups. In spite of improvements such as a longer life span, there was no significant difference between the sham-operated and HAC-MSC groups with regard to the immunohistochemical analyses. (m) The number of remaining motor neurons in the anterior horn of the G93A spinal cords was counted in sham-operated, MSC-transplanted, and HAC-MSC-transplanted mice. We confirmed an advantageous tendency in HAC-MSC transplanted mice over other groups. There were, however, no statistical differences among them.
The mice were i.v. transplanted at 60 days of age (n = 14, ♂ = 7, ♀ = 7) and at 100 days (n = 19, ♂ = 10, ♀ = 9), and controls were sham-operated at 60 days (n = 15, ♂ = 6, ♀ = 9) and at 100 days (n = 18, ♂ = 10, ♀ = 8). In the i.v. groups, significant differences were not found in the 100-day-transplanted group regarding onset time, life span, disease duration, or body weight (Supplementary Figure S4b,d,f,h). In the groups transplanted i.v. at 60 days, there were encouraging trends in the onset, life span, and body weight (Supplementary Figure S4a,c,g) of the treated mice compared to the controls.
Observations of host spinal cords and graft cells after transplantation
Lumbar spinal tissues were obtained from 130-day-old mice that had undergone transplantation at 100 days with MSCs (n = 3) or HAC-MSCs (n = 3) or were subjected to a sham operation (n = 3). As controls, the spinal cord was obtained from wild-type mice at the same age without surgical treatment (n = 3). Immunohistochemical analyses were performed using antibodies targeting anti-neuronal nuclei (NeuN) for motor neuronal loss, glial fibrillary acidic protein (GFAP) for astrocytosis, and ionized calcium binding adaptor molecule 1 (Iba1) for microglial activation. The same degrees of motor neuronal loss (Figure 4a,e,i,b,f,j), astrocytosis (Figure 4c,g,k), and microglial activation (Figure 4d,h,l) were seen in both HAC-MSC transplanted (Figure 4e–h) and sham-operated (Figure 4i–l) groups, compared to wild-type mice (Figure 4a–d). We counted the number of motor neurons remaining 30 days after the transplantation (Figure 4m) using a previously reported method.7 The numbers of remaining motor neurons were not statistically different among sham-operated, MSC-transplanted and HAC-MSC transplanted groups. Consequently, we could not distinguish the HAC-, HAC-MSC-, or sham-operated groups by histological means.
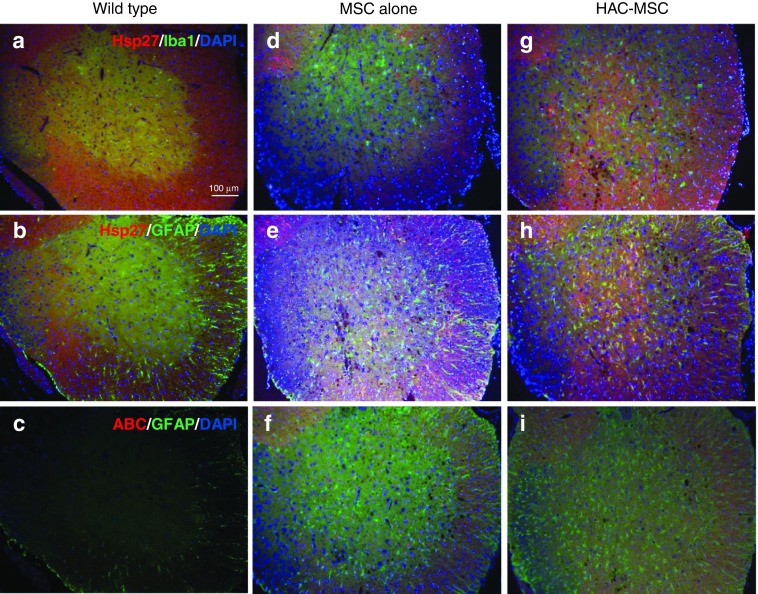
Why was it not possible to detect the pathological changes in spite of the clinical improvements? In chronic denervation conditions like ALS, muscle strength is maintained by compensatory reinnervation until 70–80% of motor units are lost.8 In the present study, animals were transplanted at 100 days and evaluated at 130 days, at which time less than 20–30% of motor neurons remained and subsequent gliosis was progressing. It is possible that the pathological differences between transplanted mice and control groups were too small to be detected by immunohistochemistry. In turn, the efficacy of this late stage therapeutic intervention would suggest the usefulness of our concept of combined therapy as discussed later. On the other hand, when we examined the expression of small heat-shock protein 27 (Hsp 27) in mice transplanted with HAC-MSCs (Figure 5g,h) or unmodified MSCs (Figure 5d,e), we observed explicit upregulation of Hsp27 in HAC-MSC recipients. We could not observe any difference among the groups in their expression of αB crystalline (ABC) (Figure 5c,f,i), a small heat shock protein. In double staining for GFAP or Iba1, upregulation of Hsp27 was found to have occurred in reactive astrocytes.
Figure 5.
Immunohistochemical study of small heat shock proteins. To further investigate the effect of the transplantation of HAC-MSCs, small heat shock proteins, Hsp27, and ABC were examined. Tissues were also stained for (b,c,e,f,h,i) GFAP or (a,d,g) Iba1 to determine which cell populations were responsible for the overexpression of the small heat shock proteins. In wild-type mice, (a,b) Hsp27 and (c) ABC were undetectable. In the group transplanted with HAC-MSCs, we observed marked elevation of the immunoreactivity of (g,h) Hsp27 but not (i) ABC compared to that of (d,e,f) MSCs alone. The Hsp27-positive cells in the group of HAC-MSCs are not (g) Iba1 but (h) GFAP positive indicating these cells are mainly reactive astrocytes.
We next evaluated the state of grafted cells using GFP immunoreactivity. In this way, we observed a small number of GFP-positive cells in the parenchyma of the lumbar spinal cords 30 days after the transplantation (Supplementary Figure S5a,b). To further investigate the expression level of neurotrophic factors in the lumbar spinal cords, we designed human-specific primers to evaluate graft-derived mRNA and performed reverse transcription (RT)-PCR analyses. We successfully observed the expression of human GDNF 1 day after transplantation. However, 7 days after the transplantation, we detected the GDNF signal in only one tissue out of six and none after 30 days (Supplementary Figure S5c).
Discussion
MSCs and trophic factors
MSCs, as adult stem cells, are capable of self-renewal and have the potential to differentiate into multiple lineages with practically no risk for malignant transformation.9 MSCs preferentially migrate to damaged sites or tissues, including the CNS. It is relatively easy to obtain patient's own MSCs, thereby facilitating autologous transplantation. Even in the case of an allograft, MSCs are weakly immunogenic.10 The MSCs release trophic factors of their own,10 and our system intensifies the expression of HGF, GDNF, and IGF-1 through the HAC system. None of these three factors is reported to affect the MSCs' capacity for self-renewal or their pluripotency. In contrast, vascular endothelial growth factor (VEGF), a candidate for ALS treatment, stimulates endothelial differentiation of MSCs if its concentration reaches high levels.11 We neither used neurotrophins like NGF, BDNF, or NT-3 because high level expression of these factors could promote apoptosis in neurons through a low-affinity p75 neurotrophic receptor.12
While neurotrophic factors have long represented a great hope for the treatment of neurodegenerative diseases, none of these have reached clinical use. One of the reasons behind such failures is, no doubt, poor accessibility of these factors to the CNS. HGF, GDNF, and IGF-1 scarcely penetrate the blood–brain barrier, and only GDNF can reach motor neurons by retrograde axonal transport. Because of the inefficiency of blood–brain barrier penetration, peripherally injected factors would cause systemic side effects that would overwhelm any benefits provided for the CNS. Along with many salutary properties of MSCs, we hypothesized that HAC-MSCs could act as useful vectors for the delivery of trophic factors to the CNS. Also, this strategy might be relatively economical for the continuous supply of trophic factors instead of infusing large quantities of purified proteins or peptides. In this regard, cell transplantation procedures via the CV or by i.v. are both feasible modes of delivery. Our results indicated both routes were beneficial but the transplantation via the CV was advantageous compared to i.v. with regard to efficacy. To our surprise, the transplanted cells dramatically decreased with time, and practically none of them survived a week following the CV transplantation. We suggest that this is due in part to the abrupt environmental changes from a culture dish to the cerebrospinal space in rodents; i.e., changes in nutritional composition, trophic support, vascular supply, scaffolds, and so forth. Rationally, a longer period of engraftment and improved survival would enhance the therapeutic effects of transplantation. We hypothesize that modification of transplanted cells and/or the host tissue microenvironment would improve graft cell survival. There are several reports of cell transplantation experiments13,14,15,16,17 using high expresser SOD1G93A mice and a single injection13,14,15,16,17 or serial injections15of donor cells via CV (Supplementary Table S1). Life spans have been used as endpoints of both the transplanted mice and controls. Those studies used MSCs,13,15,17 umbilical cord blood cells,13 neural progenitor cells,14 and neuronal stem cells;16 all were of human origin. In these previous reports, the transplantation efficacy was mostly observed around 60 (refs. 16,17) or 75 days14 of operation. The experiment done by Habisch et al.13 and a single-injection experiment in the article reported by Zhang et al.15 showed no beneficial results from cells transplanted at the ages of 45 and 56 days, respectively. These results indicated that the transplantation should be done far earlier relative to disease onset, while our present results indicated that the transplantation of HAC-MSCs was efficacious as late as 100 days of age, i.e., merely 20 days before the onset, at which time distinct ALS pathology is obvious.8,18 When we transplanted unmodified MSCs at 100 days, we failed to find any efficacy. Furthermore, previous reports indicated that the transplantation efficacy was improved by genetic modification to enhance the expression of GDNF14 or VEGF.16 None of the previous studies confirmed the therapeutic effects as late as 100 days of transplantation, which we stress the marked findings in the present study. We conclude that the therapeutic effects at 100 days transplantation are largely due to trophic effects of chromosomally introduced trophic factors. Considering that few differences were seen in pathological observations among the HAC-, HAC-, MSC-, or sham-operated groups, we conjecture that the trophic factors exert their effects largely by functional maintenance of the remaining motor neurons by affecting the neurons themselves and/or other mechanism(s), e.g., interacting via glial cells. We also propose that the effects were attained by using multiple trophic factors.
Potential clinical use of HAC-MSCs and tailor-made medicine
Like the motor neurons in ALS, substantia nigral neurons are affected in Parkinson's disease and striatal neurons are involved in Huntington's disease. These pathologic conditions lead to neurodegeneration of specific neuronal cell populations in a gradual but progressive manner. In contrast, ischemic stroke precipitates acute damage due to the restriction of the blood supply and thereby devastates neurons as well as glial cells. We suggest that both acute and chronic neurologic damage would be potential targets of HAC-MSCs treatment. To broaden the therapeutic application of HAC-MSCs, other neurotrophic factor combinations could be used. Although MSCs possess low immunogenicity, they do express human leukocyte antigens (HLA). HLA-A, HLA-B, and HLA-DR are known to play major roles in immunological rejection. It is important to note that it is possible to minimize or avoid serious immuno-rejection by obtaining good matches for HLA-A, HLA-B, and HLA-DR. The distributions of these loci are not random. In the Japanese population, for example, a certain strain of HLA homozygous stem cells match three loci in up to 20% of the population.19 Fifty such cell lines could match 90.7% of the population. These facts indicate that it is not necessary to use autologous HAC-MSCs in every case. Future gene-editing technology might be able to modulate the expression of HLAs genes or other important elements of immunogenicity. Further, producing HAC-MSCs requires two steps of single cell selection and expansion, i.e., transferring genes of interest from a PAC to a HAC in CHO cells, and then transferring the HAC from the CHO cells to MSCs by MMCT. Currently, HACs with trophic factors are maintained in CHO cells. Thus, new HAC-MSCs with desirable HLA loci could be obtained on demand by a single MMCT procedure. These approaches would minimize the cost and maximize the therapeutic possibilities.
Phase 1 clinical trials using MSCs in ALS patients have been started and safety has been demonstrated.20 Further studies will demonstrate whether the procedure is effective enough for practical use for the treatment of ALS patients. However, considering the animal experiments which are not necessarily sufficient to demonstrate the efficacy if using unmodified cells13,14,15,16,17 and the present one (nonchromosomally modified MSCs), it might be so optimistic to anticipate the effect on human ALS patients. In such case, to maximize a cell transplantation effect, cell engineering technique like the HAC system would be inevitable for further therapeutic steps.
Materials and methods
Cell culture. We used human immortalized MSCs6 that were immortalized by the combination of human telomerase reverse transcriptase (hTERT) and human papillomavirus E6 and E7 (HPV16E6/E7) genes. The immortalization did not affect the potential for adipogenic, osteogenic, and chondrogenic differentiation.6 The MSCs were maintained in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) plus 10% fetal bovine serum (JRH Biosciences, Lenexa, KS), 100 U/ml penicillin and 100 mg/ml streptomycin.
Construction of HAC-MSCs. P1 artificial chromosome (PAC) vector-containing cDNAs for the HGF, GDNF, IGF-1, and E-Luc were constructed as described previously.5 The cDNA clones were purchased from the RIKEN DNA Bank (Ibaraki, Japan). The cDNAs were subcloned into a CAG expression vector (pCX-EGFP), and then, the cassettes were cloned into a PAC vector with loxP and hprt. The PAC vector was transfected into hprt-deficient CHO (hprt-/-) cells containing 21HAC2 (ref. 2) using GeneJuice (Novagen, Madison, WI) in accordance with the manufacturer's protocol. After genomic PCR and FISH analyses, the HAC with the PAC vector were transferred to MSCs via MMCT as described previously.2
FISH analysis. FISH analyses were performed using fixed metaphase spreads of each cell hybrid using digoxigenin-labeled (Roche, Basel, Switzerland) human COT-1 DNA (Invitrogen), digoxigenin-labeled p11-4 (hChr.21-derived alpha-satellite clone),21 and biotin-labeled PAC DNA, essentially as described previously.2
Polymerase chain reaction. PCR was performed using standard methods. Primer pairs for the detection of the region of the PAC were as follows: hHGF-61S/hHGF-2251AS (2220bp), 5′-ccgtccagcagcaccatgtgggtgaccaaactc-3′and 5′-cagacacacttacttcagctatgactgtgg-3′; IGF(XhoI)S/IGF(XhoI)AS (460 bp), 5′-aactcgaggtacttcagaagcaatgggaaaaatcagcagtc-3′ and 5′-ttctcgagactcctcaggagggtcttcctacatcctgtag-3′; GDNF(XhoI)S/GDNF(XhoI)AS (630bp), 5′-aactcgagcaccatgaagttatgggatgtcgtggctgtctgc-3′ and 5′-ttctcgagtcagatacatccacaccttttagcggaatgc-3′. The primer pairs for detecting the Cre-loxP mediated insertion of the PAC into 21HAC2 were as follows: TRANSL1/TRANSR1 (409 bp), 5′-tggaggccataaacaagaagac-3′ and 5′-ccccttgacccagaaattcca-3′. CHO (hprt-/-) and MSC cells were used as negative controls.
Enzyme-linked immunosorbent assays. The concentrations of HGF, GDNF, and IGF-1 in conditioned media from the original MSCs and HAC-MSCs were measured using sandwich enzyme-linked immunosorbent assays. The following capturing and detection antibodies were used: anti-human HGF monoclonal antibodies (MAB694; R&D Systems, Minneapolis, MN) and biotinylated antihuman HGF antibodies (BAF294; R&D Systems) for HGF; antihuman GDNF (500-P81; PeproTech, Rocky Hill, NJ) and biotinylated rabbit antihuman GDNF antibodies (9500-p81BT; PeproTech) for GDNF; and antihuman IGF-1 monoclonal antibodies (27E10; Cosmo Bio, Tokyo, Japan) and antihuman IGF-1 monoclonal antibodies (16G4; Cosmo Bio) for IGF-1. The detection antibodies for IGF-1 are labeled with peroxidase using peroxidase Labeling Kit -NH2 (Dojindo Molecular Technologies, Kumamoto, Japan).
Luciferase assay and GFP fluorescence. Luciferase assays (Luciferase assay kit; Stratagene, La Jolla, CA) were performed according to the manufacturer's protocol. Briefly, cultured HAC-MSCs and their original MSCs were washed and removed from the plates. After brief homogenization, the supernatant fraction of each cell lysate was assessed for the measurement of the light produced from the reaction. The autofluorescence of GFP was observed using a fluorescence microscope.
Flow cytometric analysis. The HAC-MSCs and MSCs were incubated with allophycocyanin (APC)-conjugated antibodies against CD31 (BioLegend, Tokyo, Japan), phycoerythrin (PE)-conjugated antibodies against CD34 (BioLegend), PE/cyanine 7 (Cy7)-conjugated antibodies against CD45 (BioLegend), or APC/Cy7-conjugated antibodies against CD90 (BioLegend) for 30 minutes at 4 °C in 0.5% bovine serum albumin and 2 mmol/l EDTA in PBS. Labeled cells were run on a BD FACSCantoII flow cytometer (BD Biosciences, San Jose, CA). Data were analyzed using BD FACSDiva software (BD Biosciences).
Animals. All of the studies were carried out in accordance with the guidelines for animal experimentation at the Faculty of Medicine, Tottori University. We used transgenic SOD1G93A with high level expression of the mutant form of the SOD1 protein.4 They were purchased from Jackson laboratory (Bar Harbor, ME). Male SOD1G93A mice are cross-bred with female nontransgenic C57BL/6CR and maintained with a C57BL/6 background. Transgenic progenies were identified by PCR of mouse tail DNA.
Transplantation. Because the MSCs were of human origin, an immunosuppressive agent FK506 (3 mg/kg/day, kindly provided from Astellas Pharma, Tokyo, Japan) was administered orally to the mice a week prior to the operation. The transplantation procedures were performed in two different ways: intrathecal injection via CV and i.v. administration. In both the experiments, littermates were distributed equally into cell-transplanted and sham-operated mice, with the groups balanced with regard to date of birth, body weight, and gender. The body weight of each mouse was measured at 17, 18, 19, and 20 weeks of age. Weight loss was expressed as a percentage of the 17-week weight.
For CV administration, transplantation was performed with stereotaxic coordinates through the forth ventricle.7 A 30G needle was inserted from the site 6 mm caudal to the Bregma suture on the midline to 3.75 mm depth from the cerebellar surface, where the fourth ventricle was located. Twenty-five microliters of cell suspension in PBS (about 1 × 106 cells) was injected slowly over 10 minutes. To determine the optimal transplantation period, we used mice that were 60, 80, 100, and 120 days old. Sham controls were injected with 25 µl PBS without cells. For the comparison of HAC-MSCs to unmodified MSCs, about 1 × 106 MSCs were injected in the same manner as previously described. In i.v. transplantations, the right side jugular vein was exposed in deeply anesthetized SOD1 mice. One million HAC-MSCs in 200 μl PBS were injected through the jugular vein over 10 seconds. Control mice received 200 μl of PBS in the same manner.
Preparation of tissue sections. Mice that were transplanted with HAC-MSCs, unmodified MSCs, or sham-operated mice were histologically evaluated. In this set of experiments, the transplantations were performed only via CV at 100 days of age, and the tissues were obtained 30 days after the transplantation.
Animals were deeply anesthetized by intraperitoneal injection of pentobarbital sodium (Abbott Laboratories, North Chicago, IL) and transcardially perfused with normal saline solution, followed by 4% paraformaldehyde in 0.1 mol/l phosphate-buffered solution. A lumbar spinal cord segment was immersed in the same fixative and cryoprotected in a series of sucrose solutions (10, 15, and 20%) in PBS at 4 °C for 2 days. After tissue was embedded in Tissue-Tec (Sakura Finetek, Tokyo, Japan), the samples were frozen in liquid nitrogen cooled isopentane. Ten-micrometer-thick sections were cut transversely using a cryostat.
Histopathological and immunohistochemical staining. Selected sections were stained with hematoxylin and eosin and immunostained for NeuN, GFAP, Iba1, Hsp 27, ABC, or GFP. For immunohistochemical study, the sections were permeabilized with 0.2% Triton X-100 and 4% paraformaldehyde and blocked with 1 mg/ml bovine serum albumin and 1% normal goat serum (Funakoshi, Tokyo, Japan) in PBS. We used the following primary antibodies and final dilutions: mouse anti-NeuN monoclonal antibody (1:100, MAB377; Chemicon, Temecula, CA), rabbit polyclonal antibody against GFAP (prediluted; Dako, Troy, MI), rabbit anti-Iba1 (1:100; Wako Pure Chemical Industries, Osaka, Japan), mouse anti-Hsp27 (1:100, M-20; Santa Cruz Biotechnology, Dallas, TX), and mouse anti-αB crystalline monoclonal antibody (1:100, 1B6.1-3G4; Stressgen, Ann Arbor, MI), and mouse anti-GFP monoclonal antibody (1:250, MAB3580; Chemicon). The secondary antibody was goat antimouse antibody IgG fluorescein isothiocyanate (1:100; Santa Cruz Biotechnology), goat antirabbit antibody IgG fluorescein isothiocyanate (1:100; Santa Cruz Biotechnology), and goat antirabbit antibody IgG Texas Red (1:100; Santa Cruz Biotechnology). Specimens were incubated with primary antibodies overnight at 4 °C, followed by incubation with secondary antibodies for 1 hour at room temperature.
Quantitative RT-PCR analyses. To analyze gene expression specific for grafted cells, we designed PCR primers specific for human forms of GDNF, HGF, and IGF-1. The HAC-MSCs were transplanted into the mice at 100 days of age, and the spinal cords were removed 1 day (n = 6), 7 days (n = 6), and 30 days (n = 6) after the transplantation. Spinal cords from nontransplanted mice were combined with 1.0 × 104 cultured HAC-MSCs and were used as internal positive controls for normalization, while cDNA from C57BL/6 mouse tissues was used as a negative control. Using a previously reported method,22 total RNA was prepared from the lumbar spinal cords using ISOGEN (Nippon Gene, Tokyo, Japan), purified using RNeasy columns (Qiagen, Hilden, Germany) according to the manufacturer's instructions, and treated with RNase-free DNase I (Wako Pure Chemical Industries). First-strand cDNA synthesis was performed using random hexamers and SuperScript III reverse transcriptase (Invitrogen). Quantitative RT-PCR was performed using an Applied Biosystems 7900HT Fast Real-Time PCR system and EXPRESS qPCR SuperMix with premixed ROX (Life Technologies, Carlsbad, CA). Mouse β-actin was used as an endogenous control gene to normalize target genes. The following primers were used for the analyses: β-actin, 5′-ggatgcagaaggagattactgc-3′ and 5′-ccaccgatccacacagagta-3′; human GDNF, 5′-gtctgcctggtgctgctc-3′ and 5′-ggataatcctctggcatatttgag-3′; human HGF, 5′-gaaggatcagatctggttttaatga-3′ and 5′-tgcatccataattaggtaaatcaatc-3′; human IGF-1, 5′-tgtggagacaggggctttta-3′ and 5′-atccacgatgcctgtctga-3′. TaqMan probes #63, #70, #56, and #67 were used to detect mouse β-actin, human forms of GDNF, HGF, and IGF-1, respectively. To perform relative quantification, the comparative threshold cycle (CT) method was used. The fold change in gene expression profile was referred to an internal positive control.
Statistical analysis. The quantitative data were expressed as means ± SEM. Statistical analysis was performed with repeated-measures analysis of variance or with the Kaplan–Meier method (log-rank test) using Statview software (Abacus Concepts, Piscataway, NJ).
SUPPLEMENTARY MATERIAL Figure S1. Schematic diagram illustrating the transfer of the desired genes into MSCs using HAC. Figure S2. FACS analysis of MSCs and HAC-MSCs (3-31). Figure S3. Motor scores after CV transplantation. Figure S4. i.v. cell transplantation to SOD1G93A transgenic mice at 60 and 100 days. Figure S5. Immunohistochemical and RT-PCR analyses of SOD1G93A mice transplanted with HAC-MSCs. Table S1. Comparison with previous transplantation research using high expresser SOD1G93A mice and injection(s) via cerebroventricular space.
Acknowledgments
We thank Masaru Okabe at Osaka University for providing pCX-EGFP; Yoshihito Ohmiya at National Institute of Advanced Industrial Science and Technology for providing the E-luc vector; Junya Toguchida at Kyoto University for providing MSCs; Saori Tsuji at Tottori University for technical assistance. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Y.W.), the 21st Century Center of Excellence program from Japan Society for the Promotion of Science (M.O., Y.K., and Y.W.) and by a Grant from the Research Committee of CNS Degenerative Diseases, Ministry of Health, Labour and Welfare of Japan (K.N.).
Supplementary Material
References
- Lunn, JS, Hefferan, MP, Marsala, M and Feldman, EL (2009). Stem cells: comprehensive treatments for amyotrophic lateral sclerosis in conjunction with growth factor delivery. Growth Factors 27: 133–140. [DOI] [PubMed] [Google Scholar]
- Kazuki, Y, Hoshiya, H, Takiguchi, M, Abe, S, Iida, Y, Osaki, M et al. (2011). Refined human artificial chromosome vectors for gene therapy and animal transgenesis. Gene Ther 18: 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht, W and Philips, T (2013). The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci 14: 248–264. [DOI] [PubMed] [Google Scholar]
- Gurney, ME, Pu, H, Chiu, AY, Dal Canto, MC, Polchow, CY, Alexander, DD et al. (1994). Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264: 1772–1775. [DOI] [PubMed] [Google Scholar]
- Hiratsuka, M, Uno, N, Ueda, K, Kurosaki, H, Imaoka, N, Kazuki, K et al. (2011). Integration-free iPS cells engineered using human artificial chromosome vectors. PLoS One 6: e25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, T, Aoyama, T, Nakayama, T, Nakamata, T, Hosaka, T, Nishijo, K et al. (2002). Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun 295: 354–361. [DOI] [PubMed] [Google Scholar]
- Morita, E, Watanabe, Y, Ishimoto, M, Nakano, T, Kitayama, M, Yasui, K et al. (2008). A novel cell transplantation protocol and its application to an ALS mouse model. Exp Neurol 213: 431–438. [DOI] [PubMed] [Google Scholar]
- Swash, M and Ingram, D (1988). Preclinical and subclinical events in motor neuron disease. J Neurol Neurosurg Psychiatry 51: 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo, ME, Zaffaroni, N, Novara, F, Cometa, AM, Avanzini, MA, Moretta, A et al. (2007). Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 67: 9142–9149. [DOI] [PubMed] [Google Scholar]
- Mazzini, L, Ferrero, I, Luparello, V, Rustichelli, D, Gunetti, M, Mareschi, K et al. (2010). Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a phase I clinical trial. Exp Neurol 223: 229–237. [DOI] [PubMed] [Google Scholar]
- Wang, N, Zhang, R, Wang, SJ, Zhang, CL, Mao, LB, Zhuang, CY et al. (2013). Vascular endothelial growth factor stimulates endothelial differentiation from mesenchymal stem cells via Rho/myocardin-related transcription factor–a signaling pathway. Int J Biochem Cell Biol 45: 1447–1456. [DOI] [PubMed] [Google Scholar]
- Sedel, F, Béchade, C and Triller, A (1999). Nerve growth factor (NGF) induces motoneuron apoptosis in rat embryonic spinal cord in vitro. Eur J Neurosci 11: 3904–3912. [DOI] [PubMed] [Google Scholar]
- Habisch, HJ, Janowski, M, Binder, D, Kuzma-Kozakiewicz, M, Widmann, A, Habich, A et al. (2007). Intrathecal application of neuroectodermally converted stem cells into a mouse model of ALS: limited intraparenchymal migration and survival narrows therapeutic effects. J Neural Transm 114: 1395–1406. [DOI] [PubMed] [Google Scholar]
- Park, S, Kim, HT, Yun, S, Kim, IS, Lee, J, Lee, IS et al. (2009). Growth factor-expressing human neural progenitor cell grafts protect motor neurons but do not ameliorate motor performance and survival in ALS mice. Exp Mol Med 41: 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C, Zhou, C, Teng, JJ, Zhao, RL, Song, YQ and Zhang, C (2009). Multiple administrations of human marrow stromal cells through cerebrospinal fluid prolong survival in a transgenic mouse model of amyotrophic lateral sclerosis. Cytotherapy 11: 299–306. [DOI] [PubMed] [Google Scholar]
- Hwang, DH, Lee, HJ, Park, IH, Seok, JI, Kim, BG, Joo, IS et al. (2009). Intrathecal transplantation of human neural stem cells overexpressing VEGF provide behavioral improvement, disease onset delay and survival extension in transgenic ALS mice. Gene Ther 16: 1234–1244. [DOI] [PubMed] [Google Scholar]
- Kim, H, Kim, HY, Choi, MR, Hwang, S, Nam, KH, Kim, HC et al. (2010). Dose-dependent efficacy of ALS-human mesenchymal stem cells transplantation into cisterna magna in SOD1-G93A ALS mice. Neurosci Lett 468: 190–194. [DOI] [PubMed] [Google Scholar]
- Feeney, SJ, McKelvie, PA, Austin, L, Jean-Francois, MJ, Kapsa, R, Tombs, SM et al. (2001). Presymptomatic motor neuron loss and reactive astrocytosis in the SOD1 mouse model of amyotrophic lateral sclerosis. Muscle Nerve 24: 1510–1519. [DOI] [PubMed] [Google Scholar]
- Nakatsuji, N, Nakajima, F and Tokunaga, K (2008). HLA-haplotype banking and iPS cells. Nat Biotechnol 26: 739–740. [DOI] [PubMed] [Google Scholar]
- Mazzini, L, Mareschi, K, Ferrero, I, Miglioretti, M, Stecco, A, Servo, S et al. (2012). Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy 14: 56–60. [DOI] [PubMed] [Google Scholar]
- Ikeno, M, Masumoto, H and Okazaki, T (1994). Distribution of CENP-B boxes reflected in CREST centromere antigenic sites on long-range alpha-satellite DNA arrays of human chromosome 21. Hum Mol Genet 3: 1245–1257. [DOI] [PubMed] [Google Scholar]
- Kazuki, K, Takehara, S, Uno, N, Imaoka, N, Abe, S, Takiguchi, M et al. (2013). Highly stable maintenance of a mouse artificial chromosome in human cells and mice. Biochem Biophys Res Commun 442: 44–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.