Abstract
RNA interference (RNAi) has become a powerful tool for suppressing gene expression in vitro and in vivo. A great deal of evidence has demonstrated the potential for the use of synthetic small interfering RNAs (siRNAs) as therapeutic agents. However, the application of siRNA to clinical medicine is still limited, mainly due to sequence-independent suppression of angiogenesis mediated by Toll-like receptor 3 (TLR3). Here, we describe novel types of synthetic RNA, named nkRNA and PnkRNA, that exhibit sequence-specific gene silencing through RNAi without activating TLRs or RIG-I–like receptor signaling. In addition, we confirmed the therapeutic effect for the novel types of RNA in an animal model of age-related macular degeneration (AMD) without retinal degeneration. These data indicate that nkRNA and PnkRNA are of great potential utility as therapies against blinding choroidal neovascularization due to AMD.
Keywords: innate immune, molecular target therapy, RNAi, TLR
Introduction
Age-related macular degeneration (AMD) is a complex disorder that primarily affects the macula, including the retinal pigment epithelium but also to a certain extent the photoreceptor layer and retinal neurons.1,2 The most common cause of vision loss from AMD is pathologic choroidal neovascularization (CNV), vascular leakage, and subsequent fluid accumulation in the neurosensory retina. Therefore, pharmacologic inhibition of vascular endothelial growth factor-A (VEGF-A) via targeted monoclonal antibody therapy has become the front line treatment for this blinding disease; this approach has high efficacy against AMD.3,4 Several other modalities of VEGF-A–targeted therapies have been investigated including posttranscriptional gene silencing via small interfering RNA (siRNA), an approach that showed great promise in preclinical studies. However, the clinical trials studying the first generation of siRNA-based drugs targeting VEGF-A for treatment of neovascular AMD were abruptly halted because they failed to reach the primary endpoints.4
Toll-like receptors (TLRs) belong to a family of innate immune receptors that detect and clear invading microbial pathogens. Specifically, intracellular TLRs such as TLR3, TLR7/8, and TLR9 recognize microbe-derived nucleic acids such as double-stranded RNA, single-stranded RNA, and CpG DNA, respectively.5,6,7,8 Many siRNAs with a wide range of targets, as well as siRNAs that are not competent for RNA interference (RNAi), suppress CNV in mice.9,10 Moreover, nontargeted (i.e., not targeted against mammalian gene) and targeted siRNAs (against Vegfa or Vegfr1) suppressed CNV via TLR3, its adaptor TRIF, and induction of interferon-γ (IFNγ) and interleukin-12.10
To address some of these issues, we developed two novel types of synthetic RNA named nkRNA and PnkRNA.11 Both of these types of synthetic RNAs are single stranded and not chemically modified. Notably, nkRNA and PnkRNA exhibited sequence-specific gene silencing through RNAi. In addition, we transfected nkRNA and PnkRNA into murine endothelial cells and confirmed that they did not activate TLR3 signaling. Furthermore, we found that nkRNA and PnkRNA against VEGF suppressed CNV in laser-induced mouse models. These findings suggested that nkRNA and PnkRNA represent a suitable technology platform for molecularly targeted therapy.
Results
Novel types of RNAs induced downregulation of VEGF mRNA expression as effectively as siRNA
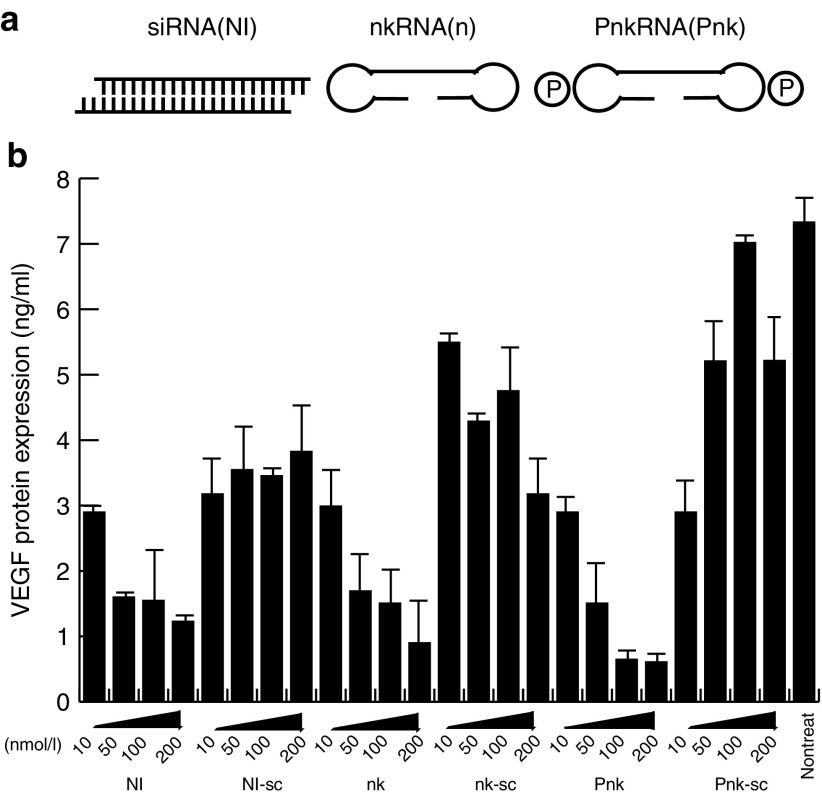
To determine whether our new synthetic RNAs, nkRNA and PnkRNA, could induce knockdown of VEGF gene expression, we transfected these RNAs and conventional siRNA (NI) into the mouse endothelial cell line UVfemale2 and analyzed the expression of VEGF in the culture medium by ELISA. VEGF expression in the culture medium was reduced in a dose-dependent manner in cells transfected with all three types of RNAs. On the other hands, we observed no apparent knockdown of VEGF expression in cells transfected with scrambled RNAs (Figure 1b). These data indicate that nkRNA and PnkRNA can silence gene expression as efficiently as siRNA.
Figure 1.
Novel types of RNAs induced downregulation of VEGF mRNA expression as effectively as siRNA. (a) Structure of novel RNA interference agents. Both nkRNA and PnkRNA were prepared as single-stranded RNA oligomers that underwent self-annealing, as shown. P indicates a proline derivative. (b) 10, 50, 100, and 200 µg of NIRNA, nkRNA, and PnkRNA against VEGF were transfected into mouse endothelial cells. Twenty-four hours after transfection, VEGF expression in the culture medium was measured using a VEGF ELISA kit. siRNA, small interfering RNA; VEGF, vascular endothelial growth factor.
nkRNA and PnkRNA induced TLR3 phosphorylation at low levels
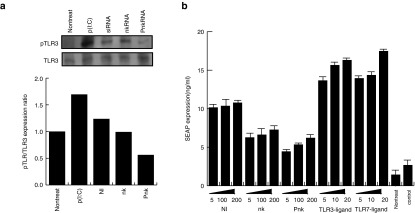
Double-stranded RNA, such as siRNA, is recognized by TLR3 and activates innate immunity via phosphorylation of TLR3 at tyrosine 759.12,13 To determine whether nkRNA and PnkRNA induce TLR3 phosphorylation, we transfected nkRNA, PnkRNA, NIRNA, or p(I: C) to mouse endothelial cells. Western blot analysis of lysates from cells 1 hour after transfection revealed that TLR3 was phosphorylated in cells that received p(I: C) or NIRNA. On the other hand, cells transfected with nkRNA or PnkRNA exhibited lower levels of TLR3 phosphorylation (Figure 2a), indicating that these novel RNAs do not activate TLR3. Phosphorylation of TLR3 leads to the activation of the NF-κB transcription factor.14,15,16 Activation of TLR3 by stimuli, such as viral dsRNA, leads to elevated expression of inflammatory cytokines, mediated by the NF- κB transcription factor. Therefore, to determine whether nkRNA and PnkRNA can induce NF-κB activation, we performed an NF-κB reporter assay using a vector encoding secreted alkaline phosphatase (SEAP) cDNA under the control of NF-κB response element. SEAP expression was lower in nkRNA- or PnkRNA-transfected cells than in NIRNA-transfected cells, and this reduction was dose dependent (Figure 2b). From these data, we concluded that nkRNA and PnkRNA were barely recognized by TLR3.
Figure 2.
nkRNA and PnkRNA induced TLR3 phosphorylation at low levels. (a) Phosphorylated TLR3 was analyzed by western blotting. Ten micrograms of protein was loaded in each lane. The ratio of phosphorylated TLR3 to total TLR3 is shown in the graph. (b) NF-κB activity was measured using an NF-κB reporter in which SEAP cDNA was placed under the control of the κB response element. The numbers on x-axis show RNA concentrations (nM). SEAP, secreted alkaline phosphatase; siRNA, small interfering RNA; TLR, Toll-like receptor.
nkRNA and PnkRNA did not induce an innate immune response, as demonstrated by expression of type I IFNs
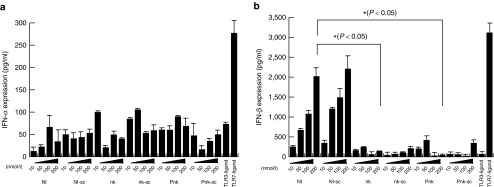
The type I IFN pathway is typically associated with the innate immune response to the adaptors of TLR3.17,18,19 Hence, we analyzed type I IFN expression in nkRNA- and PnkRNA-transfected cells using the IFN ELISA kit. Although IFNα and IFNβ expression increased in a dose-dependent manner, there was no apparent difference between cells transfected with nkRNA, PnkRNA, and NIRNA (Figure 3). Expression of IFNβ was higher in cells transfected with NIRNA than in cells transfected with nkRNA or PnkRNA (Figure 3). According to these data, nkRNA and PnkRNA did not induce IFNβ expression. Therefore, we concluded that nkRNA and PnkRNA do not activate an innate immune response in vitro.
Figure 3.
nkRNA and PnkRNA did not induce an innate immune response, as demonstrated by expression of type I IFNs. (a,b) Levels of IFNα (a) and IFNβ (b) from mouse endothelial cells in culture medium 24 hours after transfection of nkRNA, PnkRNA, NIRNA, or TLR ligands were measured by specific ELISA. The numbers in x-axis showed concentrations (nM) of RNAs. TLR3, TLR7, or TLR9 revealed the ligand against each receptor. IFN, interferon; TLR, Toll-like receptor.
nkRNA and PnkRNA did not induce expression of IFNβ in murine eyes
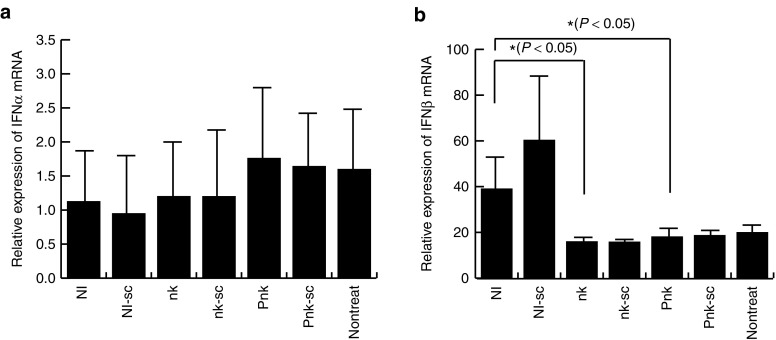
Next, we asked whether administration of nkRNAs and PnkRNA into murine eyes would induce expression of type I IFNs. To address this question, we injected 1 µg of nkRNA, PnkRNA, or NIRNA into murine eyes. Twenty-four hours after injection, we excised the eyes, extracted RNA from them, and analyzed expression of IFNα and IFNβ by real-time quantitative PCR. IFNα was expressed at comparably low levels in all samples, whereas, IFNβ was expressed at lower levels in transfected with nkRNA and PnkRNA than in cells transfected with NIRNA (Figure 4). Thus, nkRNA and PnkRNA barely induced innate immunity in murine eyes.
Figure 4.
nkRNA and PnkRNA did not induce expression of IFNβ in murine eyes. To evaluate of activation for innate immune in vivo by nkRNA and PnkRNA, eyes of C57BL/6J mice were injected 1 µg of nkRNA, PnkRNA, or NIRNA. (a) IFNα and (b) IFNβ mRNAs from murine eyes 24 hours after administration mRNA were quantitated by real-time PCR. IFN, interferon.
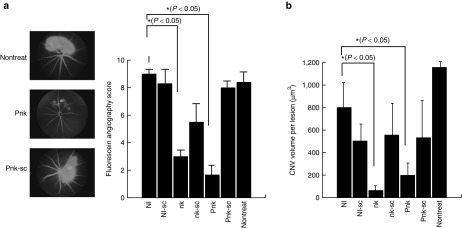
nkRNA and PnkRNA inhibited laser-induced CNV in a mouse model
To determine whether the novel RNAs, which do not extensively activate innate immunity via TLR3, were able to reduce CNV in mice, we evaluated angiogenesis, as determined by leakage of blood, from neovascularization in response to laser coagulation (three shots per eye). The blood leakage scores in CNV mice were scored on a scale from 0 to 3; we estimated score for leakage of blood from vessels in eye. The scores were higher in mice that received NIRNA, nontreated, and scramble RNAs than in mice that received nkRNA or PnkRNA (Figure 5a).
Figure 5.
nkRNA and PnkRNA inhibited laser-induced CNV in a mouse model. (a) Fluorescein angiography. Neovascularization was evaluated based on leakage of blood fluid from neovasculature by fluorescein angiography. (b) Confocal micrograph of laser-induced CNV in mice. The retinas of eyes on RNA-treated mice after laser-induced coagulation were used to generate choroidal flatmounts, which were then examined with a scanning laser confocal microscope to visualize vessels. Vessels in the laser-treated area and superficial to this reference plane were judged as CNV. The area of CNV-related fluorescence was measured. CNV, choroidal neovascularization.
We next examined the ability of nkRNA and PnkRNA to reduce angiogenesis by administering novel RNAs into the vitreous cavity of mice and comparing the CNV volume following laser coagulation 7 days after injection of RNAs. CNV volume was significantly reduced in eyes that received nkRNA and PnkRNA than in eyes that received NIRNA or scramble RNAs (Figure 5b). These data show that nkRNA and PnkRNA suppressed angiogenesis, indicating that the novel RNAs could be useful as therapies against CNV due to AMD.
Discussion
siRNAs have attracted a great deal of attention as a new therapeutic platform for achieving target-specific gene silencing via double-stranded RNA (dsRNA)-mediated RNAi. However, the immune side effects due to TLRs activation are still problematic issues in the context of therapeutic applications. Recent work showed that siRNA activates TLR3, a long double-stranded viral RNA sensor, and activated TLR3 mediates target-independent angiogenesis suppression of CNV in mouse models.10
Recently, we developed a novel class of RNAi agents.11 Each of these agents is prepared as a single-stranded RNA that self-anneals into a unique structure containing a double-stranded RNA with an unpaired site, bound at the right and left ends by an oligonucleotide loop or a non-nucleotide molecule (proline derivative). Here, we showed that these novel RNAi therapeutic agents suppress expression of target genes without inducing type I IFN responses. In general, double-stranded RNAs are recognized by members of the membrane TLR and RIG-I–like receptor families (RIG-I, MDA5, and LGP2).20,21 When double-stranded RNA enters cells, both types of receptors activate NF-κB and/or IRF3/IRF7.20,22,23,24,25 In this study, we confirmed that nkRNA and PnkRNA induced low levels of TLR3 phosphorylation and NF-κB activation. In addition, we examined the type I IFN response. Together, these data demonstrated that nkRNA and PnkRNA can achieve sequence-specific gene silencing without activating the TLRs and RIG-I–like receptor.
How do nkRNA and PnkRNA evade the TLRs and RIG-I–like receptors? TLR3 recognizes double-stranded RNA, and recognition of ligands by TLR3 triggers signaling pathways that lead to the activation of transcription factors such as NF-κB, AP-1, and IRFs, which regulate the production of proinflammatory cytokines and type I IFNs.23,26,27,28,29 Because NIRNA is a double-stranded RNA, it is recognized by TLR3 and triggers the innate immune system. On the other hand, nkRNA and PnkRNA are prepared as single-stranded RNAs, but form double-stranded RNAs via self-annealing11 (Figure 1a). These double-stranded forms may enable the novel RNAs to escape recognition by TLR3. In addition, the ligand that activates TLR3 is double-stranded RNA or oligonucleotides of at least 40–50 base pairs in length.30,31 Although in this study we used oligonucleotides 41 (NIRNA), 62 (nkRNA), and 53 (PnkRNA) base pairs in length, only NIRNA activated TLR3. The structural conformation of the ligand of TLR3 plays an important role in recognition of TLR3.6,32,33,34,35,36,37 Furthermore, mutated virus with an altered conformation can escape the innate immune system, because such viruses are not recognized by TLRs.38,39 Because nkRNA and PnkRNA have loops at both ends, in between the sense and antisense nucleotide sequences, we concluded that the conformations of these unique RNA structures might be altered; therefore, these recognition of these RNAs by TLR3 was significantly impaired. PnkRNAs have prolines in both loops of the nucleotide sequence, and we predicted that the resultant alteration in structural conformation affected recognition of ligand by TLRs. The results of TLR3 phosphorylation indicated at low levels of the phosphorylation in the cell gene transferred with PnkRNA than that in the cell transferred with nkRNA. In addition, the data regarding NF-κB activation and IFNβ expression were consistent with the results described above.
The application of RNAi to clinical medicine is still limited, mainly due to activation of the innate immune system via TLRs. The VEGF silencing reagent causes side effects and exhibits sequence-independent suppression of angiogenesis.10
We confirmed the therapeutic effect of the novel RNAs in a mouse model of laser-induced CNV. nkRNA and PnkRNA were not associated with off-target expression of inflammatory cytokines in mice. In vivo data revealed that the novel RNAs, nkRNA and PnkRNA, repressed angiogenesis due to laser-induced coagulation. Thus, these novel RNAs may be effective as therapeutic reagents against CNV. Notably, escape from TLR3 recognition and VEGF mRNA silencing were better for PnkRNA than for nkRNA, indicating that is the superior therapeutic reagent. Accordingly, we concluded that nkRNA and PnkRNA may have great clinical utility as therapies against blinding CNV due to AMD. In particular, PnkRNA may become useful as a therapeutic agent against CNV.
Materials and methods
Cells. The mouse endothelial cell line, UVfemale2,40,41 was purchased from the RIKEN cell bank and cultured in Dulbecco's Modified Eagle's medium (Life Technology, Carlsbad, CA) containing 10% fetal calf serum (FCS) penicillin and streptomycin. siRNA (NIRNA) or novel RNA oligonucleotides (nkRNA and PnkRNA) targeting mouse VEGF were transfected into endothelial cells using HiPerFect (Qiagen, Valencia, CA). One hour after transfection, cells were collected, and proteins were extracted for subsequent investigations.
RNA interference. siRNA (NI) and novel RNAs used in this study were as follows. Mouse VEGF (NI): sense 5′-ACCUCACCAAAGCCAGCACT-3′, antisense 5′- GUGCUGGCUUUGGUGAGGUTT-3′; mouse NI scramble: sense (NIsc) 5′- GCACAACACCCGCUCACAATT-3′, antisense 5′- UUGUGAGCGGGUGUUGUGCTT-3′; mouse VEGF nkRNA (nk): 5′- AACCUCACCAAAGCCAGCACUUCCCCACACCGGAAGUGCUGGCUUUGGUGAGGUUUCUUCGG-3′; mouse nk scramble RNA (nksc): 5′- AGCACAACACCCGCUCACAAUUCCCCACACCGGAAUUGUGAGCGGGUGUUGUGCUUCUUCGG-3′; mouse VEGF PnkRNA (Pnk): 5′-AACCUCACCAAAGCCAGCACUUCC-P5-GGAAGUGCUGGCUUUGGUGAGGUUUC-P5-G-3′; mouse Pnk scramble RNA (Pnksc): 5′- AGCACAACACCCGCUCACAAUUCC-P5-GGAAUUGUGAGCGGGUGUUGUGCUUC-P5-G-3′.
Enzyme-linked immunosorbent assay. IFN and VEGF protein levels were measured in culture medium of cells transfected with nkRNA, PnkRNA, or NIRNA, 24 hours after transfection, using cytokine-specific ELISA kits as follows: IFNα, VeriKine Interferon ELISA kit (PBL Biotechnology, Norwich, UK); IFNβ, EN424001 (Thermo Fisher Scientific, Waltham, MA); IFNγ, EM1011 (Thermo Fisher Scientific); and VEGF, Quantikine Mouse VEGF kit (R&D Systems, Minneapolis, MN).
SEAPorter assay. NF-κB activation by nkRNA, PnkRNA, or NIRNA transfection in cells was assayed using the SEAPorter assay kit (IMK-515; IMGENEX, San Diego, CA). Twenty-four hours after transfection, culture media from the oligo-transfected endothelial cells were analyzed.
Real-time quantitative PCR. RNAs were isolated from mouse eyes 24 hours after inoculation of 1 µg nkRNA, PnkRNA, or NIRNA per body using the Isogen reagent (Nippon Gene, Tokyo, Japan). Complementary DNA was synthesized using SuperScript II and Random Hexamers (Life Technology). Quantitative PCR (FastStart Universal SYBR Green Master; Roche, Basel, Switzerland) reactions were run on a Stratagene MX3000P thermocycler and analyzed using MxPro (Stratagene; Agilent Technologies, Santa Clara, CA). Glyceraldehyde 3-phosphate dehydrogenase mRNA was used as an internal control. IFN primers used in this study were as follows: IFNα (forward) 5′-TCTGATGCAGCAGGTGGG-3′, (reverse) 5′-AGGGCTCTCCAGACTTCTCGC-3′; IFNβ (forward) 5′-AGCACTGGGTGGAATGAGAC-3′, (reverse) 5′- TCCCACGTCAATCTTTCCTC-3′; IFNγ (forward) 5′- CACGGCACAGTCATTGAAAG-3′, (reverse) 5′-TTTTGCCAGTTCCTCCAGAT-3′; glyceraldehyde 3-phosphate dehydrogenase (forward) 5′-AAAATGGTGAAGGTCGGTGTG-3′, (reverse) 5′- TGGCAACAATCTCCACTTTG-3′.
Western blot method. Protein samples were suspended in sodium dodecyl sulfate loading buffer. After boiling, equal amounts (10 µg) of the proteins were run on 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and then transferred to Immobilon membranes (Millipore, Bedford, MA) by semidry blotting. The membranes were probed with anti-TLR3 (Sc-86912; Santa Cruz Biotechnology, Dallas, TX) or anti-phospho-TLR3 (IMG-5348A; Imgenex/Novus Biologicals, Littleton, CO) antibodies using standard techniques. The signals were visualized using the ECL Plus Western Blotting Detection System (GE Healthcare, Piscataway, NJ) and detected on a LAS-3000 mini (Fujifilm, Tokyo, Japan).
Animals. All animal experiments were in accordance with the guide lines of Tokyo Medical University Institutional Animal Care and Use Committee. Female C57BL/6J mice (Japan SLC, Hamamatsu, Japan) between the age of 6 and 8 weeks were used to minimize variability. For all procedures, anesthesia was achieved by injection of under ketamine–xylazine anesthesia (120 and 6 mg/kg, respectively, i.p.).
Induction of CNV. Laser photocoagulation (532 nm, 200 mW, 100 ms, 75 µm, GYC-2000 Green/Dye; Nidek, Gamagori, Japan) was performed in three spots on one eye of each mouse. The laser spots were evaluated for the presence of CNV on day 7 by fluorescence angiography.
Injection of siRNA or novel RNAs into mice. On day 7 after laser photocoagulation, mice were anesthetized, and we administrated 1 µg of NIRNA, nkRNA, or PnkRNA into the vitreous cavity using a 32-gauge needle attached to a Hamilton syringe (Hamilton, Reno, NV).
CNV volume. Laser-induced CNV volume was measured by fluorescence angiography of Phoenix Micron III Retinal Imaging Microscope (Phoenix Research Lab, Pleasanton, CA) following i.p. injection of 0.1 ml of 1% FLUORESCITE (Alcon, Tokyo, Japan) into each mouse. CNV of retina was evaluated with fluorescence leakage of three categories (by grading score: I–III). Mice were anesthetized (ketamine/xylazine) and perfused through the heart with 1 ml of phosphate-buffered saline containing 50 µg fluorescein-labeled dextran (FITC-Dextran; Sigma-Aldrich, St. Louis, MO). The eyes were removed and fixed with 10% buffered formalin. The cornea and the lens were removed and the neurosensory retina was carefully dissected from the eyecup. Four relaxing radial incisions were made, and the remaining retinal pigment epithelium–choroid–sclera complex was flatmounted, Flatmounts were examined with a scanning laser confocal microscope (LMS710; Carl Zeiss, Oberkochen, Germany). Vessels were visualized by laser excitation and capture and capturing. About 1-µm step of horizontal optical sections were obtained from the surface of the retinal pigment epithelium–choroid–sclera complex. The area of CNV-related fluorescence was measured by computerized image analysis using the microscope software (ZEN; Carl Zeiss). The sum of the all area of CNV-related fluorescence in each horizontal section was used as an index of the CNV volume.
Statistical analysis. Statistical evaluation was performed on the basis of Student's t-test or Welch's t-test. P values less than 0.05 were considered significant.
Acknowledgments
This research was supported in part by Grants-in-Aid for scientific research (B) and Grant-in-Aid for Exploratory Research from Japan Society for the Promotion of Science (JSPS) and supported in part by Strategic Research Foundation Grant-aided Project for Private Universities from the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT).
References
- John, S, Natarajan, S, Parikumar, P, Shanmugam P, M, Senthilkumar, R, Green, DW et al. (2013). Choice of cell source in cell-based therapies for retinal damage due to age-related macular degeneration: a review. J Ophthalmol 2013: 465169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q, Dinculescu, A, Shan, Z, Miller, R, Pang, J, Lewin, AS et al. (2008). Downregulation of p22phox in retinal pigment epithelial cells inhibits choroidal neovascularization in mice. Mol Ther 16: 1688–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, JG, Gammons, MV, Damodaran, G, Churchill, AJ, Harper, SJ and Bates, DO (2015). The carboxyl terminus of VEGF-A is a potential target for anti-angiogenic therapy. Angiogenesis 18: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisbourd, M, Loewenstein, A, Goldstein, M and Leibovitch, I (2007). Targeting vascular endothelial growth factor: a promising strategy for treating age-related macular degeneration. Drugs Aging 24: 643–662. [DOI] [PubMed] [Google Scholar]
- Alexopoulou, L, Holt, AC, Medzhitov, R and Flavell, RA (2001). Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732–738. [DOI] [PubMed] [Google Scholar]
- Botos, I, Liu, L, Wang, Y, Segal, DM and Davies, DR (2009). The toll-like receptor 3:dsRNA signaling complex. Biochim Biophys Acta 1789: 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenert, PS (2010). Classification, mechanisms of action, and therapeutic applications of inhibitory oligonucleotides for Toll-like receptors (TLR) 7 and 9. Mediators Inflamm 2010: 986596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, JN, Bell, JK and Segal, DM (2009). Predicting Toll-like receptor structures and characterizing ligand binding. Methods Mol Biol 517: 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, Y, Nozaki, M, Sugitani, K, Ashikari, M, Hirano, Y and Ogura, Y (2013). Efficient delivery of siRNA by atelocollagen in a murine laser-induced choroidal neovascularization model. Ophthalmologica 230: 215–221. [DOI] [PubMed] [Google Scholar]
- Kleinman, ME, Yamada, K, Takeda, A, Chandrasekaran, V, Nozaki, M, Baffi, JZ et al. (2008). Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 452: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki, T, Suzuki, H, Shirohzu, H, Matsumoto, T, D'Alessandro-Gabazza, CN, Gil-Bernabe, P et al. (2012). Efficacy of a novel class of RNA interference therapeutic agents. PLoS One 7: e42655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, MY, Chang, MY, Chen, YJ, Li, YK, Chuang, TH, Yu, GY et al. (2014). The inducible nitric-oxide synthase (iNOS)/Src axis mediates Toll-like receptor 3 tyrosine 759 phosphorylation and enhances its signal transduction, leading to interferon-β synthesis in macrophages. J Biol Chem 289: 9208–9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, SN, Elco, CP, Peters, KL, Chattopadhyay, S and Sen, GC (2007). Two tyrosine residues of Toll-like receptor 3 trigger different steps of NF-kappa B activation. J Biol Chem 282: 3423–3427. [DOI] [PubMed] [Google Scholar]
- Ando, T, Ito, H, Ohtaki, H and Seishima, M (2013). Toll-like receptor agonists and alpha-galactosylceramide synergistically enhance the production of interferon-gamma in murine splenocytes. Sci Rep 3: 2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duluc, D, Tan, F, Scotet, M, Blanchard, S, Frémaux, I, Garo, E et al. (2009). PolyI:C plus IL-2 or IL-12 induce IFN-gamma production by human NK cells via autocrine IFN-beta. Eur J Immunol 39: 2877–2884. [DOI] [PubMed] [Google Scholar]
- Cross, JL, Kott, K, Miletic, T and Johnson, P (2008). CD45 regulates TLR-induced proinflammatory cytokine and IFN-beta secretion in dendritic cells. J Immunol 180: 8020–8029. [DOI] [PubMed] [Google Scholar]
- Matsumoto, M, Oshiumi, H and Seya, T (2011). Antiviral responses induced by the TLR3 pathway. Rev Med Virol 21: 67–77. [DOI] [PubMed] [Google Scholar]
- Seya, T, Matsumoto, M, Ebihara, T and Oshiumi, H (2009). Functional evolution of the TICAM-1 pathway for extrinsic RNA sensing. Immunol Rev 227: 44–53. [DOI] [PubMed] [Google Scholar]
- Hoebe, K and Beutler, B (2004). LPS, dsRNA and the interferon bridge to adaptive immune responses: Trif, Tram, and other TIR adaptor proteins. J Endotoxin Res 10: 130–136. [DOI] [PubMed] [Google Scholar]
- Szabo, A and Rajnavolgyi, E (2013). Collaboration of Toll-like and RIG-I-like receptors in human dendritic cells: tRIGgering antiviral innate immune responses. Am J Clin Exp Immunol 2: 195–207. [PMC free article] [PubMed] [Google Scholar]
- Kawai, T and Akira, S (2008). Toll-like receptor and RIG-I-like receptor signaling. Ann NY Acad Sci 1143: 1–20. [DOI] [PubMed] [Google Scholar]
- Al-Salleeh, F and Petro, TM (2008). Promoter analysis reveals critical roles for SMAD-3 and ATF-2 in expression of IL-23 p19 in macrophages. J Immunol 181: 4523–4533. [DOI] [PubMed] [Google Scholar]
- Pietilä, TE, Veckman, V, Lehtonen, A, Lin, R, Hiscott, J and Julkunen, I (2007). Multiple NF-kappaB and IFN regulatory factor family transcription factors regulate CCL19 gene expression in human monocyte-derived dendritic cells. J Immunol 178: 253–261. [DOI] [PubMed] [Google Scholar]
- Osterlund, P, Veckman, V, Sirén, J, Klucher, KM, Hiscott, J, Matikainen, S et al. (2005). Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J Virol 79: 9608–9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, KA, Rowe, DC, Barnes, BJ, Caffrey, DR, Visintin, A, Latz, E et al. (2003). LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med 198: 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, WJ, Upton, JW and Mocarski, ES (2008). Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J Immunol 181: 6427–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada, K, Sato, Y, Itatsu, K, Isse, K, Ikeda, H, Yasoshima, M et al. (2007). Innate immune response to double-stranded RNA in biliary epithelial cells is associated with the pathogenesis of biliary atresia. Hepatology 46: 1146–1154. [DOI] [PubMed] [Google Scholar]
- Gautier, G, Humbert, M, Deauvieau, F, Scuiller, M, Hiscott, J, Bates, EE et al. (2005). A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med 201: 1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, M, Sato, S, Hemmi, H, Hoshino, K, Kaisho, T, Sanjo, H et al. (2003). Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301: 640–643. [DOI] [PubMed] [Google Scholar]
- Leonard, JN, Ghirlando, R, Askins, J, Bell, JK, Margulies, DH, Davies, DR et al. (2008). The TLR3 signaling complex forms by cooperative receptor dimerization. Proc Natl Acad Sci USA 105: 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bouteiller, O, Merck, E, Hasan, UA, Hubac, S, Benguigui, B, Trinchieri, G et al. (2005). Recognition of double-stranded RNA by human toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J Biol Chem 280: 38133–38145. [DOI] [PubMed] [Google Scholar]
- Qi, R, Singh, D and Kao, CC (2012). Proteolytic processing regulates Toll-like receptor 3 stability and endosomal localization. J Biol Chem 287: 32617–32629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, T, Gong, J, Rössle, SC, Jamitzky, F, Heckl, WM and Stark, RW (2011). A leucine-rich repeat assembly approach for homology modeling of the human TLR5-10 and mouse TLR11-13 ectodomains. J Mol Model 17: 27–36. [DOI] [PubMed] [Google Scholar]
- Kubarenko, A, Frank, M and Weber, AN (2007). Structure-function relationships of Toll-like receptor domains through homology modelling and molecular dynamics. Biochem Soc Trans 35(Pt 6): 1515–1518. [DOI] [PubMed] [Google Scholar]
- Bell, JK, Botos, I, Hall, PR, Askins, J, Shiloach, J, Davies, DR et al. (2006). The molecular structure of the TLR3 extracellular domain. J Endotoxin Res 12: 375–378. [DOI] [PubMed] [Google Scholar]
- Bell, JK, Askins, J, Hall, PR, Davies, DR and Segal, DM (2006). The dsRNA binding site of human Toll-like receptor 3. Proc Natl Acad Sci USA 103: 8792–8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahira, S, Nishikawa, F, Nishikawa, S, Akazawa, T, Seya, T and Matsumoto, M (2005). Interferon-beta induction through toll-like receptor 3 depends on double-stranded RNA structure. DNA Cell Biol 24: 614–623. [DOI] [PubMed] [Google Scholar]
- Asmal, M, Sun, Y, Lane, S, Yeh, W, Schmidt, SD, Mascola, JR et al. (2011). Antibody-dependent cell-mediated viral inhibition emerges after simian immunodeficiency virus SIVmac251 infection of rhesus monkeys coincident with gp140-binding antibodies and is effective against neutralization-resistant viruses. J Virol 85: 5465–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosopoulos, WC, Rezende, LF, Wainberg, MA and Prasad, VR (1998). Virtues of being faithful: can we limit the genetic variation in human immunodeficiency virus? J Mol Med (Berl) 76: 604–612. [DOI] [PubMed] [Google Scholar]
- Yazama, H, Kitatani, K, Fujiwara, K, Kato, M, Hashimoto-Nishimura, M, Kawamoto, K et al. (2015). Dietary glucosylceramides suppress tumor growth in a mouse xenograft model of head and neck squamous cell carcinoma by the inhibition of angiogenesis through an increase in ceramide. Int J Clin Oncol 20: 438–446. [DOI] [PubMed] [Google Scholar]
- Ishitsuka, T, Ikuta, T, Ariga, H and Matsumoto, K (2009). Serum tenascin-X strongly binds to vascular endothelial growth factor. Biol Pharm Bull 32: 1004–1011. [DOI] [PubMed] [Google Scholar]