Abstract
Interleukin-1β (IL-1β) is a key cytokine involved in inflammatory illnesses including rare hereditary diseases and common chronic inflammatory conditions as gout, rheumatoid arthritis, and type 2 diabetes mellitus, suggesting reduction of IL-1β activity as new treatment strategy. The objective of our study was to assess safety, antibody response, and preliminary efficacy of a novel vaccine against IL-1β. The vaccine hIL1bQb consisting of full-length, recombinant IL-1β coupled to virus-like particles was tested in a preclinical and clinical, randomized, placebo-controlled, double-blind study in patients with type 2 diabetes. The preclinical simian study showed prompt induction of IL-1β-specific antibodies upon vaccination, while neutralizing antibodies appeared with delay. In the clinical study with 48 type 2 diabetic patients, neutralizing IL-1β-specific antibody responses were detectable after six injections with doses of 900 µg. The development of neutralizing antibodies was associated with higher number of study drug injections, lower baseline body mass index, improvement of glycemia, and C-reactive protein (CRP). The vaccine hIL1bQb was safe and well-tolerated with no differences regarding adverse events between patients receiving hIL1bQb compared to placebo. This is the first description of a vaccine against IL-1β and represents a new treatment option for IL-1β-dependent diseases such as type 2 diabetes mellitus (ClinicalTrials.gov NCT00924105).
Introduction
Interleukin-1β (IL-1β) is a key cytokine involved in a spectrum of chronic inflammatory syndromes including orphan diseases such as cryopyrin-familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystem inflammatory disease and in common medical conditions like rheumatoid arthritis, gout, and type 2 diabetes.1 Pathological overproduction of IL-1β can be blocked by IL-1 receptor antagonists or neutralizing antibodies against the protein itself. In orphan hereditary diseases, drugs that block the IL-1β effect have emerged as first-line therapy.2 In type 2 diabetes and prediabetic subjects, inhibition of the IL-1 receptor revealed to be beneficial in terms of glycemic and inflammatory parameters.3,4 A number of monoclonal antibodies directed against IL-1β5,6,7 have shown comparable results. Accordingly, IL-1β antagonism is now in phase 3 of clinical development for diabetes and associated cardiovascular complications.8 However, existing IL-1 antagonists require daily injections of interleukin-1 receptor antagonist (IL-1Ra) and neutralizing antibodies are costly.
An alternative approach to block the IL-1β pathway is the development of active vaccination against endogenous proinflammatory proteins. We have previously described the preclinical evaluation of a murine vaccine against IL-1β9 and shown that active immunization against IL-1β, using the recombinant cytokine chemically conjugated to virus-like particles (VLP) of the bacteriophage Qβ efficiently protected mice from inflammation in a model of rheumatoid arthritis.9 Hence, vaccination against IL-1β may be explored clinically for inflammatory diseases where IL-1β is involved. A subcutaneous injection of wild-type IL-1β has been shown to induce a febrile response in patients already at low doses.10,11,12 Therefore, we developed a human IL-1β mutein with a roughly 10,000-fold lower biological activity compared to wild-type human IL-1β as assessed by IL-1β-induced IL-6 release in HeLa cells.13 A Qβ VLP-based vaccine comprising the murine form of this mutated IL-1β was shown to induce neutralizing antibodies in mice and to protect from diet-induced type 2 diabetes.13 Hence, this IL-1β mutein exhibited the right conformation to induce IL-1β-neutralizing antibodies but with lower proinflammatory activity and was further developed for preclinical and clinical studies. Here, we show the tests for safety and immunogenicity in nonhuman primate studies using human and the corresponding simian IL-1β muteins coupled to Qβ VLPs. The preclinical study was followed by a phase 1/2 clinical trial in patients with type 2 diabetes mellitus using the human version of the vaccine (hIL1bQb) to evaluate its safety, immunogenicity, and preliminary efficacy.
Results
Safety and immune function in nonhuman primates upon vaccination
Rhesus monkeys repeatedly dosed with Alum-adjuvanted Qb (control), rhesus- (rmIL1bQb) or human (hIL1bQb)- vaccine over 10 weeks followed by a 6-week treatment-free recovery period showed no signs of systemic toxicity (Supplementary Tables S1, S2a and S2b). Importantly, analyses of body temperature and cytokine profiles after dosing showed no evidence of acute reactivity to the detoxified IL1β-antigen component of the vaccine at the relatively high dose-level tested. The ability of primates to mount an antigen-specific, T-cell-dependent antibody response upon keyhole limpet hemocyanin challenge was not impaired by immunization (data not shown). Flow cytometry showed no treatment-related changes in any specific immune cell population beyond what might be considered general and expected changes following subcutaneous immunizations with Alum-based vaccines (Supplementary Figure S1). Thus, the vaccine did not negatively impact key immune cell populations of the innate and adaptive immune system.
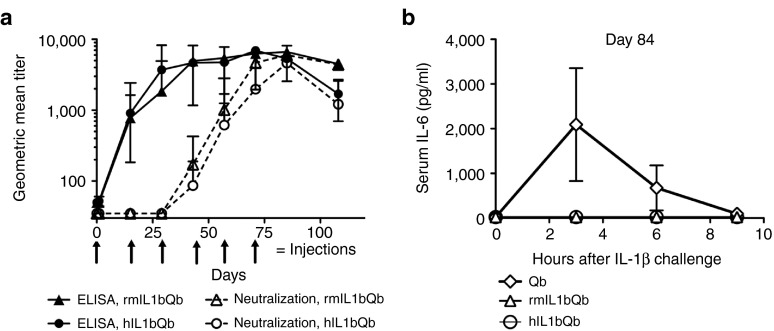
Anti-IL-1β immunoglobulin G (IgG) antibody responses became detectable after a single injection. In contrast, neutralizing antibody responses were delayed and became detectable only after three to four injections (Figure 1a). To test whether the induced antibodies were also able to neutralize IL-1β in vivo, six rhesus monkeys of the control group immunized with Qβ alone and six animals immunized with the simian and the human version of the vaccine were challenged with 1 µg/kg wild-type rhesus IL-1β by intravenous injection on day 84. Serum IL-6, a biomarker of IL-1β activity, was measured 3, 6, and 9 hours after injection. Control animals showed a robust IL-6 response upon IL-1β challenge, while those immunized with rmIL1bQb or hIL1bQb had no detectable IL-6 response, demonstrating that induced antibodies efficiently neutralized IL-1β in vivo (Figure 1b). The favorable safety profile and evidence for induction of neutralizing antibodies obtained in rhesus monkeys supported the decision to commence first-in-human studies.
Figure 1.
Characterization of the immune response to IL1bQb in rhesus monkeys. To assess immune response in rhesus monkey, 24 animals were injected subcutaneously six times every 14 days (day 1, 15, 29, 43, 57, and 71, see arrows) with a rhesus monkey version (rmIL1bQb, n = 12) or a human version (hIL1bQb, n = 12) of the IL-1β vaccine. Rhesus monkeys developed anti-IL-1β IgG antibody responses after one injection and neutralizing antibody responses after three to four injections. Anti-IL-1β IgG titers and neutralization titers are expressed as the reciprocals of the serum dilutions needed to achieve half-maximal signal in enzyme-linked immunosorbent assay (ELISA) or half-maximal inhibition of the IL-6 response in the cellular assay, respectively. (a) Two weeks after the last injection (day 84), animals were challenged with an intravenous injection of rhesus IL-1β. IL-6 concentrations were determined in serum collected after 3, 6, and 9 hours. Control animals treated with the virus-like particles carrier Qb showed pronounced IL-6 levels, while immunization with rmIL1bQb and hIL1bQb completely protected against the IL-1β challenge (b).
A randomized, double-blind, placebo-controlled phase 1/2 clinical trial of hIL1bQb in patients with type 2 diabetes
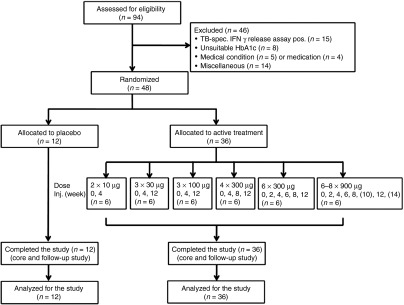
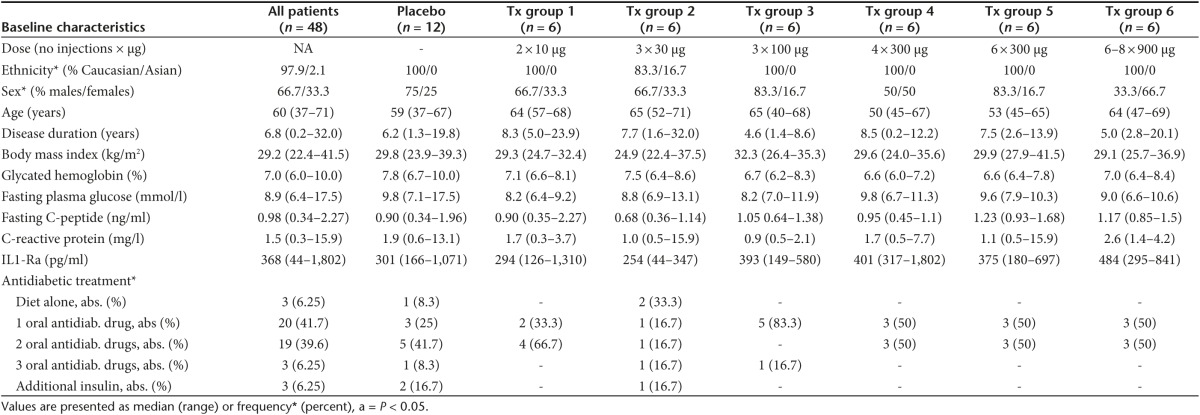
Baseline characteristics. To investigate the safety, immunogenicity, and preliminary efficacy of hIL1bQb, a randomized, double-blind, placebo-controlled phase 1/2 study was initiated in patients with type 2 diabetes. Forty-eight of 94 patients initially screened were randomly assigned to six different treatment groups of vaccination with hIL1bQb or placebo (Figure 2). Most frequent reasons for ineligibility were positivity of a tuberculosis-specific interferon (IFN)γ-release assay (n = 15), unsuitable HbA1c levels (n = 8), medical conditions (n = 5), or medications (n = 4). There were no significant differences concerning baseline characteristics among the groups (Table 1).
Figure 2.
Enrollment and outcomes of phase 1 clinical trial. A total of 94 type 2 diabetic patients were screened in four participating centers. Of those, 46 of patients were not eligible according to entry criteria, most often due to a positive TB-specific test. Forty-eight patients were randomly assigned to active treatment (hIL1bQb) or placebo in ascending treatment groups. All patients completed the study and were analyzed according to the intention-to-treat approach.
Table 1. Baseline characteristics of patients with type 2 diabetes.
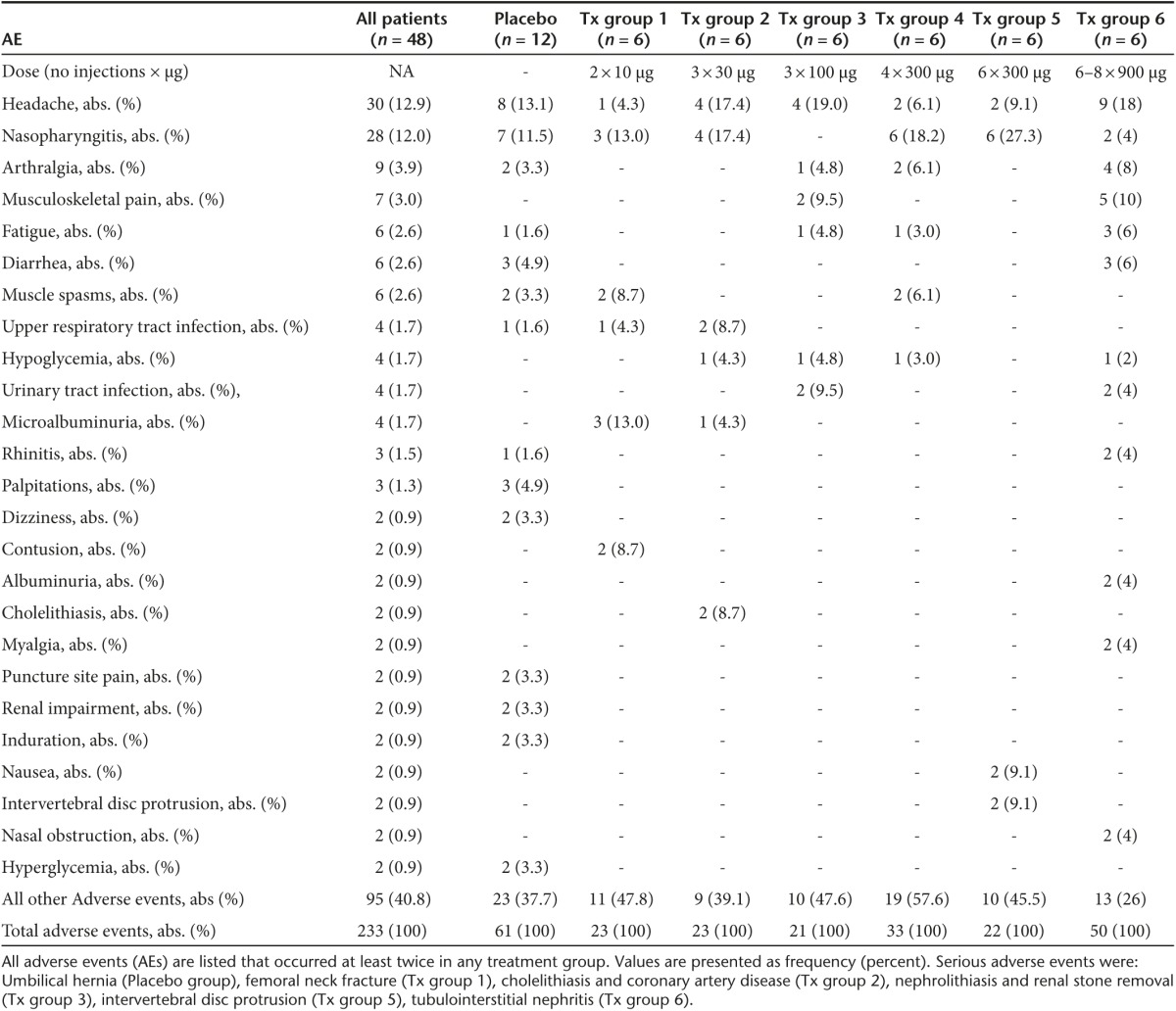
Primary outcome safety. The vaccine was well tolerated with a total of 233 adverse events (AE) in 48 patients during the study period of 48 weeks (Table 2). No dropouts occurred throughout the study. The number of adverse events per subject was similar for patients who received hIL1bQb or placebo, namely 4.8 and 5.1 AEs/subject respectively. Patients in the highest treatment group experienced more adverse events (8.3 AEs/subject), although not statistically significant compared to placebo. Overall, the majority of adverse events was mild in intensity (67.5% mild, 25.3% moderate, 7.2% severe) and only 5.7% of the adverse events were reported to be possibly related to the study drug. There was no significant difference concerning serious adverse events between patients receiving hIL1bQb and those receiving placebo. All eight serious adverse events occurred during the follow-up period (weeks 16–48). Serious adverse events in patients receiving study medication were all classified as being unrelated to the study drug (Table 2).
Table 2. AEs of patients with type 2 diabetes.
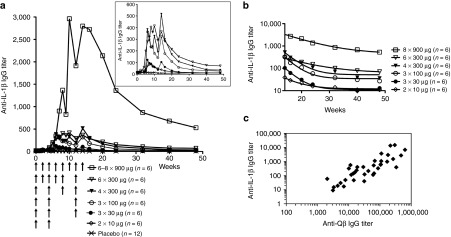
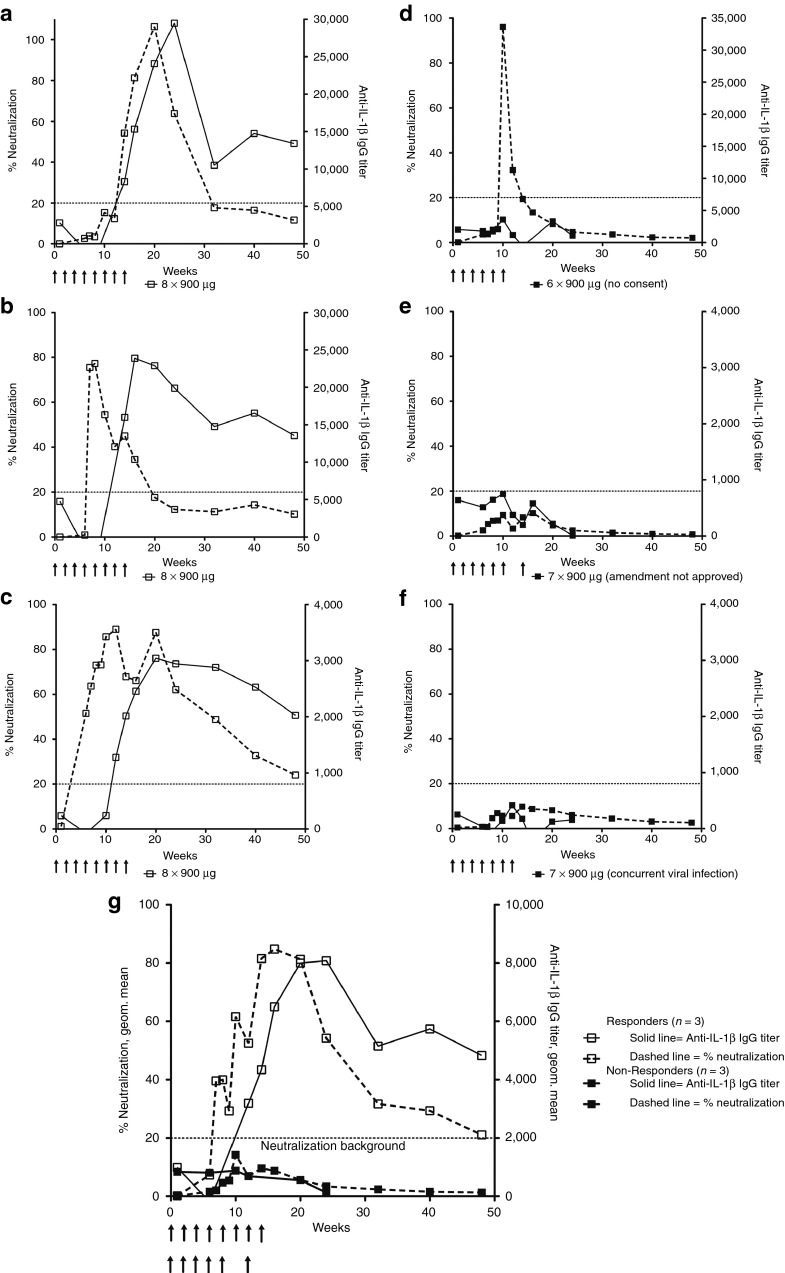
Primary outcome: immunogenicity and neutralizing capacity of hIL1bQb. Before the first immunization, no anti-IL-1β IgG antibodies were detected in any of the patients. Moderate anti-IL-1β IgG antibody responses were observed in the first five treatment groups with dose-dependent antibody response rates and peak titers (Figure 3a inset, Supplementary Figure S2). Antibody response rates were 0/12 for placebo; 3/6 for treatment group 1 (2 × 10 μg); 4/6 for group 2 (3 × 30 μg); 6/6 for group 3 (3 × 100 μg); 5/6 for group 4 (4 × 300 μg); 6/6 for group 5 (6 × 300 μg), and 6/6 for group 6 (6–8 × 900 μg). Geometric mean titers at week 14 in treatment groups 1 through 5 were 39 ± 35, 104 ± 166, 324 ± 73, 517 ± 425, and 364 ± 581, respectively (Figure 3a inset, Supplementary Figure S2). Substantially higher anti-IL-1β IgG antibody titers were observed in the highest treatment group 6 with a geometric mean titer of 2,798 ± 2,631 at week 14 (Figure 3a). After the last vaccine application, anti-IL-1β IgG antibody titers declined in all patients over time: geometric mean titers at week 48 were below detection limit (titer <60) for treatment groups 1 to 4, 71 ± 262 in group 5 and 530 ± 579 in group 6 (Figure 3a). Half-lives of anti-IL-1β IgG antibodies were 7 weeks in treatment groups 5 and 6 compared to 2.6–2.9 weeks in treatment groups 2–4, indicating that higher dosage and increased number of injections resulted in a prolonged antibody response (Figure 3b).
Figure 3.
Induction of IgG antibody responses in type 2 diabetic patients. There was a moderate, dose-dependent anti-IL-1β IgG antibody response in the first 5 dose groups as measured by ELISA assay (inset a). Substantially higher antibody titers were measured in the highest treatment group (a). Decay phase of the anti-IL-1β IgG antibody response shown in (a) with logarithmic y-axis. Half-lives of anti-IL-1β antibodies were about 7 weeks in treatment groups 5 and 6, while in the lower treatment groups half-lives were 2·6-2·9 weeks (b). There was a tight correlation between anti-IL-1β and anti-Qβ IgG antibody titers at week 14 (R2 = 0·71), indicating that the lack of an anti-IL-1β antibody response in some subjects was most likely not due to an IL-1β-specific limitation in the antibody response, but rather due to levels below the detection limit (c). Anti-IL-1β and anti-Qβ IgG antibody titers are expressed as the reciprocal of the serum dilutions needed to achieve half maximal absorbance in ELISA.
The IgG antibody response against the Qβ VLP part of the vaccine was measured to assess if absent or low IL-1β antibody titers resulted from an inability to respond to the IL-1β part of the vaccine or from a poor overall antibody response. There was a tight correlation between IL-1β- and Qβ-specific IgG antibody levels (Figure 3c, R2 = 0.71), suggesting that individual poor anti-IL-1β responses were not due to specific limitations in the B-cell repertoire, but rather a consequence of generally reduced immune responses in certain individuals. Importantly, these data also indicate that induction of Qβ-specific antibodies does not interfere with the development of an IL-1β-specific response.
No IL-1β-neutralizing antibodies could be detected in groups 1 to 5 (Supplementary Figure S2). Neutralizing antibody responses were observed only with the highest treatment group 6 and only after at least five injections (Figure 4). Three patients developed sustained anti-IL-1β IgG antibody titers as determined by enzyme-linked immunosorbent assay (ELISA; geometric mean titers at week 14 of 8,155 ± 3,830 and 2,107 ± 720 at week 48) and substantial and long-lasting neutralizing responses (single patients Figure 4a–c, average Figure 4g). This group is referred to as responders based on their neutralizing response. In contrast, one patient with a short-lasting peak of anti-IL-1β IgG antibody levels and two patients with only moderate antibody titers (geometric mean titers of 960 ± 2,146 at week 14 and of 133 ± 228 at week 48) did not exhibit a neutralizing response (single patients Figure 4d–f, average Figure 4g) and are thus referred to as nonresponders. The three responders had received eight injections as opposed to the three nonresponders that only got six to seven injections of hIL1bQb. Of note, responders had lower BMIs, lower C-reactive protein (CRP) levels, and lower levels of naturally occurring IL-1 receptor-antagonist at baseline compared to nonresponders (Supplementary Table S3). The differences in weight and IL1-Ra persisted throughout the study and were unaffected by neutralizing antibody responses. In addition, adverse events were more common in responders compared to non-responders (Supplementary Table S4).
Figure 4.
Neutralizing capacity is dependent on strong IL-1β antibody responses. Anti-IL-1β IgG antibody titers measured by ELISA (solid lines) and neutralizing antibody responses (dashed lines) for individual patients in the 900 µg treatment group are shown (open squares for individual subjects with neutralizing response=responders (a–c); closed squares for individual subjects without neutralizing response=nonresponders (d–f)). Anti-IL-1β IgG antibody titers are expressed as outlined in the legend to Figure 1. Neutralizing responses are expressed as % decrease of the absorbance signal detected in the IL-6 quantification assay, when comparing supernatants from HeLa cells incubated with 25 pg/ml IL-1β in the presence of immune sera to supernatants of HeLa cells incubated with 25 pg/ml IL-1β in the presence of the corresponding preimmune serum at a 1:5 dilution. Anti-IL-1β IgG antibody responses measured by ELISA were substantially higher in responders compared to nonresponders. Neutralizing antibody responses were delayed compared to the overall anti-IL-1β IgG antibody response measured by ELISA. (g) Geometric means of anti-IL-1β IgG titers (solid line) and % neutralization (dashed line) are shown for grouped responders (open squares) and nonresponders (closed squares).
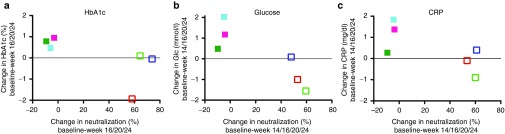
Secondary outcome: preliminary effects on metabolic and inflammatory parameters. As neutralizing responses only developed in the highest-dose group (6–8 × 900 μg), we compared secondary outcomes HbA1c, glucose, and CRP between responders (patients with long-lasting neutralizing response) and nonresponders (patients without neutralizing response). HbA1c levels at baseline compared to the end of the core study (weeks 16, 20, or 24) increased in all nonresponders, while they were unchanged or decreased in responders (Figure 5a, Supplementary Figure S3g–l). Similarly, fasting glucose levels at the end of the core study compared to baseline were reduced in two of three responders compared to increased levels observed in nonresponders (Figure 5b, Supplementary Figure S4g–l). CRP, a surrogate for inflammatory activity, was increased in the majority of nonresponders, while it was stable or decreased in responders from baseline to the end of the core study (Figure 5c, Supplementary Figure S5g–l). This was despite the fact that nonresponders started from higher levels at baseline, making them more susceptible for CRP reductions over time. Time courses of these outcome measures for placebo and all treatment groups are presented in Supplementary Figures S3–S5.
Figure 5.
Changes in metabolic and inflammatory parameters. Secondary outcome measures HbA1c, glucose, and C-reactive protein (CRP) were compared in the highest-dose group (6−8 × 900 μg) as neutralizing responses only developed with this dose level. Changes in neutralization responses are depicted on the x-axis, while changes in HbA1c-levels from baseline (screening and baseline average) to the end of the core study (average for weeks 16, 20, 24) are plotted on the y-axis (a). Changes in fasting glucose levels were related to changes in neutralization in participants from baseline to the end of the core study (average of weeks 14, 16, 20, 24 (b)). Changes in CRP levels were related to changes in neutralization in study participants from baseline to the end of the core study (average of weeks 14, 16, 20, 24 (c)). CRP levels above 30 mg/dl or >3-fold increased compared to baseline as well as HbA1c and glucose levels at time points of increased CRP were not included in the analysis. Colors indicate individual subjects. Nonresponders: closed squares; responders: open squares.
Discussion
In the presented preclinical and clinical study, we demonstrate that neutralizing IL-1β antibodies can be induced using a vaccine against IL-1β. This is the first study demonstrating that vaccination against IL-1β is feasible in humans and apparently safe. This may be a valuable approach to treat patients with type 2 diabetes or other IL-1-mediated inflammatory diseases. The induction of neutralizing antibodies was delayed compared to the overall antibody response, suggesting occurrence of affinity maturation. In humans, the induction of antibodies was more challenging than in animals and neutralizing antibodies were only observed after several injections of the highest dose of the vaccine. Importantly, the induced antibodies neutralized IL-1β without impacting key immune cells of the innate and adaptive immune system. The vaccine was well tolerated and raised no safety concerns in nonhuman primates or humans. Analysis of the patients in the highest treatment group, which developed a neutralizing IL-1β-specific immune response, points to improved glycemic control and inflammatory levels. The responders had a lower BMI as well as lower CRP and IL1-Ra levels at baseline (Supplementary Table S3), indicative for a different immunologic ability to respond to IL-1β vaccination.
In comparison to other established methods inhibiting the IL-1β pathway (e.g., monoclonal antibodies) the use of a vaccine blocking IL-1β activity harbors advantages as well as disadvantages: One advantage of using a vaccine is the favorable cost-to-benefit profile compared to monoclonal antibody therapies. In addition, exogenous antibody treatment comprises the risk of endogenous neutralizing antibody formation, potentially limiting long-term efficacy, which is not the case with vaccination. On the other hand, a current disadvantage of vaccination against IL-1β is that since antibody titers vary upon IL-1β vaccination, the appropriate dosing and monitoring regimen in terms of antibody responses yet needs to be defined. Another disadvantage is that it needs to be investigated whether reversibility of the anti-IL-1β antibody response will also occur at higher/more frequent vaccine doses. So far, the effects of a long-standing persistence of IL-1β antibodies cannot be definitively estimated. However, the effects of long-term (several years) IL-1 antagonism has been extensively studied in different diseases including patients with rheumatoid arthritis in combination with immunosuppressive drugs revealing very limited side effects.8
Although numerous reports have shown preclinical evidence for cytokine-neutralizing antibodies induced by vaccination (for review, see ref. 14), clinical translation has remained difficult due to a number of obstacles. Those include (i) cytokine-specific T-helper (Th) and B-cell tolerance, (ii) acute toxicity exhibited by cytokines, which renders use of vaccines displaying native cytokines potentially difficult, and (iii) antibody responses that need to be of substantial quantity and quality (affinity). The vaccine described in this report addresses most if not all of these obstacles. Conjugation of IL-1β to Qβ-VLPs grafts VLP-specific T helper (Th) cell epitopes onto the cytokine, which readily overcomes Th-cell tolerance.15 Indeed, mice, rhesus monkeys as well as humans responded with Th-cell-dependent IgG responses against the self-protein IL-1β upon vaccination. Potential toxicity of wild-type IL-1β displayed on the vaccine was avoided by using an engineered version of IL-1β. Tolerability of hIL1bQb was comparable to previously tested Qβ-based vaccines.16,17,18,19 This demonstrates that the genetic inactivation of IL-1β had been successful and that the mutated IL-1β on Qβ did not cause local or systemic clinical symptoms while preserving the ability of the vaccine to induce neutralizing antibody responses. In fact, the mutated IL-1β-vaccine readily induced neutralizing antibodies in mice, demonstrating proper conformation for induction of such antibodies (data not shown).
Whereas overall anti-IL1β IgG titers were relatively low, robust IgG responses were detected against the VLP carrier Qβ, indicating that the vaccine can induce a strong T-cell-dependent IgG response in humans (Figure 3c). This might point to the possibility that the antigen-specific B cells rather than the Th cells were limiting in the induction of high-affinity neutralizing antibodies against IL-1β. Earlier studies with different Qβ-based vaccines demonstrated that titers against Qβ were in a similar range as titers against the attached antigen.16,19,20 With our vaccine, more than five to six immunizations were required to induce antibodies that were of sufficient high affinity to exhibit ex vivo IL-1β neutralizing activity. This finding was rather surprising, since soluble proteins generally fail to induce B-cell tolerance.15 While we cannot formally exclude that the point mutation we introduced delayed the development of neutralizing antibodies, this possibility seems rather unlikely. Since essentially all studies on B-cell tolerance against specific antigens have been performed in mice, it is possible that B-cell tolerance in humans is stricter than in mice. In support of this notion is the finding that mice generated neutralizing anti-IL-1β responses after a single immunization with the murine version of the vaccine and that neutralizing titers were not affected by genetic deficiency for IL-1β.13 Compared to other VLP-based anticytokine vaccines it could be noted, however, that IL-1β-neutralizing responses were rather low in mice after the first vaccine injection, and only substantially increased after the second injection.13 Furthermore, primates generated neutralizing antibody responses after three to four injections, exhibiting a phenotype between mice and humans. On the other hand, spontaneously arising cytokine-neutralizing antibodies are frequently found in humans.21 Prominent examples are neutralizing IL-1α and IL-6 antibodies found in up to 10% of humans.22,23 Moreover, in a previous clinical trial, therapeutically effective antibodies could be induced against angiotensin using Qβ VLPs as a carrier.16 Hence the apparent partial B-cell tolerance observed in this study may be specific for IL-1β.
We summarize that a treatment with up to eight subcutaneous injections of 900 µg hIL1bQb was safe and well tolerated in patients with type 2 diabetes. The vaccine stimulated an IL-1β-specific antibody response that was dose-dependent and partially reversible over time. Clinical effectiveness is promising, but larger studies in patients with type 2 diabetes are necessary to evaluate this novel vaccine. Importantly, other chronic common diseases involving IL-1β such as gout, rheumatoid arthritis and in particular hereditary chronic diseases requiring life-long treatment such as Muckle-Wells-syndrome, cryopyrin-familial cold autoinflammatory syndrome and neonatal-onset multisystem inflammatory disease could particularly benefit from a treatment with this novel vaccine. All the more, prospective and long-term follow-up of safety measures are needed to fully understand the clinical potential of this newly developed vaccination approach against endogenous IL-1β.
Materials and Methods
Generation of the IL-1β vaccine. Detailed information on the production strategy of the IL-1β vaccine has been published previously.13 The mature forms of rhesus monkey and human IL-1β differ in 6 out of 153 residues (see Supplementary Figure S6 for sequence alignment). The mature, cleaved 17-kDa forms of rhesus monkey (rmIL1bQb) and human (hIL1bQb) IL-1β were engineered to contain a modified N-terminus, an inactivating D→K mutation at position145 and a cysteine-containing aa linker at the carboxy-terminus. A rhesus (rmIL1bQb) and human (hIL1bQb) version of the IL-1 β vaccine were generated with an identical inactivating mutation of the IL-1β polypeptide. The corresponding rhesus monkey (rmIL1bQb) and human (hIL1bQb) IL-1β vaccines were produced by chemically cross-linking the engineered IL-1β proteins to Qb VLP. Upon coupling, an epitope density of 0.5 IL-1β molecules per Qb monomer was estimated, corresponding to a total of 90 IL-1β molecules per Qb VLP. Coupling densities were comparable between the rhesus monkey and human vaccines.
Nonhuman primate study. As part of a broad safety assessment prior to dosing human subjects, a local tolerance and repeat-dose toxicity study was performed with rhesus monkeys. Animals (n = 12 per group; six males and six females) were repeatedly injected with either Qb carrier (control group) or rhesus- (rmIL1bQb) or human vaccine IL1bQb (hIL1bQb). Animals were dosed six times by subcutaneous administration of 300 μg of the respective vaccine in the presence of Alhydrogel (1.0 mg/dose Al(OH)3) at 14 day-intervals (day 1, 15, 29, 43, 57, and 71). The total in-life phase of the study was 16 weeks. Half of the animals from each group were sacrificed 2 weeks after the final dose and the remaining animals a further 4 weeks later.
Study parameters assessed at defined time intervals throughout the study included: Observations (mortality/ morbidity (injuries), clinical observations (body weights, electrocardiography and body temperature), coagulation, hematology, clinical chemistry, and urinalysis, and terminal studies (organ weights, necropsy, and histopathology) (Supplementary Tables S1, S2a and S2b).
Antibody titers and neutralization titers were determined as described below for human samples. To determine in vivo neutralization capacity of the induced antibodies, six animals per group were challenged 2 weeks after the last injection of vaccines (day 84) with an intravenous injection of 1 µg/kg rhesus monkey IL-1β. IL6 levels in serum were determined 3, 6, and 9 hours after challenge using a cytometric bead assay kit according to the manufacturer's recommendations (BD Biosciences). Basic immune function was assessed by measuring the ability of the immunized animals to mount an antigen-specific, T-cell-dependent, IgG-antibody response following immunization with Keyhole Limpet Hemocyanin. Additionally, immune cell populations (T cells and B cells, monocytes, and natural killer cells) were measured by flow cytometry.
Study design. The clinical study was designed as a double-blind, randomized, placebo-controlled, multicenter trial in 48 patients with type 2 diabetes mellitus. The trial was performed from July 2009 to January 2011 in four different centers (University Hospital Zurich, Zurich; University Hospital Basel, Basel; Outpatient Clinic Roemerhof, Zurich; Momentum Pharma Services GmbH, Hamburg).
Study approval. The study was conducted in accordance with the International Conference on Harmonization-Good Clinical Practice (ICH-GCP) guidelines and the Declaration of Helsinki and approved by investigational regulations committees in Switzerland and Germany. Written informed consent was obtained from all participants before study inclusion. The study was sponsored by CYTOS (Switzerland), the manufacturer of hIL1bQb.
Patients. Patients were eligible for the study if diagnosis of type 2 diabetes was established according to the American Diabetes Association guidelines for at least 3 months. Further inclusion criteria were HbA1c levels between 6.5 and 9.5%, fasting plasma glucose levels below 13.4 mmol/l (<240 mg/dl), age between 18 and 70 years, and BMI between 23 and 40 kg/m2. Additionally, patients had to be on a stable diet, receiving antidiabetic treatment with diet and exercise alone and/or metformin and/or sulfonylurea.
Patients were excluded if they showed features of type 1 diabetes (positive Glutamic Acid Decarboxylase 65 (GAD65) or Islet Antigen-2 (IA-2) auto-antibodies), had no measurable β-cell function (fasting C-peptide level <400 pmol/l), active infection, immunosuppressive disorders or immunosuppressive therapy, use of systemic anti-inflammatory medication other than aspirin (100 mg/day), malignancy, severe allergy, relevant cardiovascular or hematological disease, renal insufficiency or hepatopathy. Further on, patients were not eligible if they had childbearing potential or if they previously participated in a clinical trial with Qβ-based vaccine.
Study procedures. The study consisted of a screening phase of 2 weeks, a core study of 16 weeks, and a follow-up period up to week 48. In the core study, patients were assessed weekly for 10 weeks and biweekly for another 6 weeks. During the follow-up period, another five visits were performed. Cohorts of eight patients with a treatment allocation ratio of 3:1 (six patients hIL1bQb, two patients placebo) were treated with stepwise increasing doses and number of injections at preset time points (Figure 2 for exact dosing regimens and time points).
In view of the good safety profile, two additional cohorts were added to the preplanned four cohorts to further explore immunogenicity at higher doses and expand the tested safety margin. Once eligible, patients were allocated according to a block randomization list created by Contract Manufacturing Organization (CMO; Fisher Clinical Services AG, Switzerland). Patients as well as study personnel were blinded to the medication allocation. Study medication was injected subcutaneously in the lateral site of the upper arm. Dose escalation was assessed by the Data Review Board (DRB) and only initiated when sufficient safety data of previous cohorts were available.
Study endpoints. Primary outcome measures were safety and immunogenicity of hIL1bQb. Secondary outcome measures were preliminary biological activity on glycemia (HbA1c, fasting glucose) and inflammatory biomarkers (CRP, IL-1Ra).
Safety was assessed by AEs including clinical examination, electrocardiogram, standard laboratory evaluations, as well as immune complexes and antinuclear antibodies.
Immunogenicity was analyzed by ELISA. Eight different dilutions of sera were incubated on ELISA plates that had been coated with 1 μg/ml wild-type human IL-1β (Peprotech). Bound antibodies were detected by incubation with a horseradish peroxidase-conjugated goat anti-human IgG secondary antibody followed by incubation with O-phenylenediamine and hydrogen peroxide as substrates. Absorbance at 492 nm was measured using an ELISA reader (Molecular Probes). IL-1β-specific IgG antibody titers were determined as the reciprocals of those serum dilutions that led to half-maximal absorbance at 492 nm and were calculated using a four-parameter logistic equation (GraphPad Prism).
Neutralizing capacity of antibodies was assessed by IL-1β neutralization assay. Serial dilutions of immune sera were preincubated for 17–19 hours at 4 °C with wild-type human IL-1β. Solutions were then incubated for 3 hours at 37 °C with 5.2 × 104 HeLa cells per well; the IL-1β concentration was 25 pg/ml. IL-6 concentrations in the cell culture supernatants were determined with a sandwich ELISA using a mouse anti-human IL-6 capture Antibody (R&D systems), a biotinylated goat anti-human IL-6 detection-antibody (R&D systems) and horseradish peroxidase-conjugated streptavidin. For the monkey serum samples, neutralization capacity was expressed as neutralization titer, which corresponds to the reciprocal of the serum dilution needed to reduce the absorbance of the IL-6 quantification assay by 50%. For the human samples, the neutralization capacity is given as the % reduction of the IL-6 response obtained with a 1:5 dilution of the immune sera by comparing supernatants from HeLa cells incubated with 25 pg/ml IL-1β in the presence of immune sera to supernatants of HeLa cells incubated with 25 pg/ml IL-1β in the presence of the corresponding preimmune serum. Background IL-6 release was determined with the same dilution of preimmune serum, but in the absence of IL-1β. For the assessment of inflammatory parameters, CRP levels above 30 mg/dl or >3-fold increased compared to baseline were excluded from the analysis as they were interpreted as indicators of acute infections. HbA1c and glucose levels at time points of increased CRP were also not included in the analysis.
Statistics. All 48 patients completed the study and were analyzed according to the intention-to-treat principle. The number of patients per cohort was chosen according to generally accepted procedures for first-in-human studies. A safety interim analysis was performed after all patients had completed the core study. For descriptive statistical analyses, values are expressed as medians/range and geometric means ± SEM for antibody titers due to logarithmic dilution steps in the ELISA. We used the nonparametric t-test (Mann-Whitney) and Student's t-test for statistical significance as indicated. All tests were two tailed; P < 0.05 was defined as significant. Data were analyzed using statistical software (SAS software, version 9.3, SAS Institute, Cary, NC).
SUPPLEMENTARY MATERIAL Table S1. Clinical observations in rhesus monkeys on day 84. Table S2. Laboratory findings in rhesus monkeys on day 84. Table S3. Baseline characteristics of type 2 diabetic patients in highest treatment group. Table S4. Adverse events of type 2 diabetic patients in highest treatment group. Figure S1. Flow cytometry shows no treatment-related changes of specific cell populations in rhesus monkeys. Figure S2. Anti-IL-1β antibody titers and neutralizing capacity of individual type 2 diabetic patients of all treatment groups. Figure S3. Time course of HbA1c (%) and neutralization capacity over time in type 2 diabetic patients in placebo and all treatment groups. Figure S4. Time course of glucose (mg/dl) and neutralization capacity over time in placebo and all treatment groups. Figure S5. Time course of CRP (mg/dl) and neutralization capacity over time in placebo and all treatment groups. Figure S6. Sequence alignment between human and rhesus IL-1β.
Acknowledgments
The authors thank Patrizia Zala, Grace Gorden, Miriam Bez, and Kirsten Roubicek for expert technical assistance. G.T.J., P.Müller, and M.Y.D. designed the study. C.C.-W., K.T., E.S., C.K., M. O., and P.Maurer performed experiments. C.C.-W., G.T.J., U.L., G.S., P.Müller, and P.S. researched and analyzed data. C.C.-W., P.Müller, P.S., M.F.B., and M.Y.D. wrote the manuscript. C.C.-W., K.T., E.S., C.K., G.S., P.M., G.T.J., U.L., P.M., J.W., P.S., M.F.B., and M.Y.D. contributed to the discussion, reviewed, and edited the manuscript. All authors affiliated with Cytos owned Cytos shares and/or options.
Supplementary Material
References
- Dinarello, CA, Simon, A and van der Meer, JW (2012). Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 11: 633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamae, T (2012). Cryopyrin-associated periodic syndromes: diagnosis and management. Paediatr Drugs 14: 109–117. [DOI] [PubMed] [Google Scholar]
- Larsen, CM, Faulenbach, M, Vaag, A, Vølund, A, Ehses, JA, Seifert, B et al. (2007). Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356: 1517–1526. [DOI] [PubMed] [Google Scholar]
- van Asseldonk, EJ, Stienstra, R, Koenen, TB, Joosten, LA, Netea, MG and Tack, CJ (2011). Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 96: 2119–2126. [DOI] [PubMed] [Google Scholar]
- Ridker, PM, Howard, CP, Walter, V, Everett, B, Libby, P, Hensen, J et al.; CANTOS Pilot Investigative Group. (2012). Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 126: 2739–2748. [DOI] [PubMed] [Google Scholar]
- Cavelti-Weder, C, Babians-Brunner, A, Keller, C, Stahel, MA, Kurz-Levin, M, Zayed, H et al. (2012). Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care 35: 1654–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan-Lancaster, J, Abu-Raddad, E, Polzer, J, Miller, JW, Scherer, JC, De Gaetano, A et al. (2013). Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1β antibody, in patients with type 2 diabetes. Diabetes Care 36: 2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath, MY (2014). Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 13: 465–476. [DOI] [PubMed] [Google Scholar]
- Spohn, G, Keller, I, Beck, M, Grest, P, Jennings, GT and Bachmann, MF (2008). Active immunization with IL-1 displayed on virus-like particles protects from autoimmune arthritis. Eur J Immunol 38: 877–887. [DOI] [PubMed] [Google Scholar]
- Elkordy, M, Crump, M, Vredenburgh, JJ, Petros, WP, Hussein, A, Rubin, P et al. (1997). A phase I trial of recombinant human interleukin-1 beta (OCT-43) following high-dose chemotherapy and autologous bone marrow transplantation. Bone Marrow Transplant 19: 315–322. [DOI] [PubMed] [Google Scholar]
- Gershanovich, ML, Filatova, LV, Ketlinsky, SA and Simbirtsev, AS (2001). Recombinant human interleukin-1 beta: new possibilities for the prophylaxis and correction of toxic myelodepression in patients with malignant tumors. II. Phase II study of the protective effect of recombinant human interleukin-1 beta on myelodepression induced by chemotherapy in cancer patients. Eur Cytokine Netw 12: 671–675. [PubMed] [Google Scholar]
- Gershanovich, ML, Filatova, LV, Ketlinsky, SA and Simbirtsev, AS (2001). Recombinant human interleukin-1 beta: new possibilities for the prophylaxis and correction of toxic myelodepression in patients with malignant tumors. I. Phase I-II clinical trials of recombinant human interleukin-1 beta as a leukopoiesis stimulator in cancer patients receiving combination chemotherapy. Eur Cytokine Netw 12: 664–670. [PubMed] [Google Scholar]
- Spohn, G, Schori, C, Keller, I, Sladko, K, Sina, C, Guler, R et al. (2014). Preclinical efficacy and safety of an anti-IL-1β vaccine for the treatment of type 2 diabetes. Mol Ther Methods Clin Dev 1: 14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhn, TA and Bachmann, MF (2010). Vaccines against non-communicable diseases. Curr Opin Immunol 22: 391–396. [DOI] [PubMed] [Google Scholar]
- Bachmann, MF and Dyer, MR (2004). Therapeutic vaccination for chronic diseases: a new class of drugs in sight. Nat Rev Drug Discov 3: 81–88. [DOI] [PubMed] [Google Scholar]
- Tissot, AC, Maurer, P, Nussberger, J, Sabat, R, Pfister, T, Ignatenko, S et al. (2008). Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet 371: 821–827. [DOI] [PubMed] [Google Scholar]
- Ambühl, PM, Tissot, AC, Fulurija, A, Maurer, P, Nussberger, J, Sabat, R et al. (2007). A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens 25: 63–72. [DOI] [PubMed] [Google Scholar]
- Maurer, P, Jennings, GT, Willers, J, Rohner, F, Lindman, Y, Roubicek, K et al. (2005). A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur J Immunol 35: 2031–2040. [DOI] [PubMed] [Google Scholar]
- Cornuz, J, Zwahlen, S, Jungi, WF, Osterwalder, J, Klingler, K, van Melle, G et al. (2008). A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One 3: e2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kündig, TM, Senti, G, Schnetzler, G, Wolf, C, Prinz Vavricka, BM, Fulurija, A et al. (2006). Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J Allergy Clin Immunol 117: 1470–1476. [DOI] [PubMed] [Google Scholar]
- Bendtzen, K, Hansen, MB, Ross, C and Svenson, M (1998). High-avidity autoantibodies to cytokines. Immunol Today 19: 209–211. [DOI] [PubMed] [Google Scholar]
- Hansen, MB, Svenson, M, Diamant, M and Bendtzen, K (1991). Anti-interleukin-6 antibodies in normal human serum. Scand J Immunol 33: 777–781. [DOI] [PubMed] [Google Scholar]
- Svenson, M, Poulsen, LK, Fomsgaard, A and Bendtzen, K (1989). IgG autoantibodies against interleukin 1 alpha in sera of normal individuals. Scand J Immunol 29: 489–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.