Nanomedicine frequently employs single particles ranging from very simple1 to highly multifunctional.2 However, the diversity of barriers to effective transport can limit the global optimization of delivery for such systems. Multistage particles present an effective means to overcome such issues, allowing scientists to better segment the delivery mechanism into discrete steps by combining multiple formulations for sequential utility in vivo. A recent article in Nature Biotechnology highlights such a system,3 delivering nanoformulated doxorubicin (Dox) within larger disc-shaped, mesoporous silica particles (MSPs). Following intravenous injection, the silica particles can accumulate in tumor endothelia and release the nanoparticles for uptake and drug release within tumor cells. Such systems raise age-old questions in formulation development4,5; however, recent work in multifunctional macromolecule,6,7 nanoparticle,2,8 and cell-based9,10 development should be considered instead, in combination with such formulations and concepts11 to dramatically expand the reach of the drug delivery field as a whole.
To succeed, efforts in cancer nanomedicine must surmount an array of barriers to delivery related to processes involved in systemic administration. However, particle-based drug formulations often employ a “one size fits all” approach. Broadly, scientists have tended to focus on singular systems that are either simple to formulate12 but limited in performance (e.g., Myocet, a simple liposome encapsulating Dox1) or extensively modified with multiple components (polymers, ligands, and other conjugates)2,13,14 that potentially dilute the intrinsic impact of each functional moiety. Both approaches can sacrifice a global optimization of various dynamic processes in systemic delivery, including but not limited to drug encapsulation, particle stability, organ distribution, tissue penetration, cell internalization, and molecular release.15 These considerations reflect the similar yet distinct biological barriers to such delivery as well, including enzymatic degradation, reticuloendothelial clearance, vascular permeation, interstitial impedance, and molecular efflux.4 Each perspective informs formulation development, and through such analysis, cancer nanomedicine can be viewed uncommonly as a multiscale mass transfer problem.4 Thus, rather than focusing on what one particle can do to overcome each heterogeneous barrier, selecting at specific barriers the relevant delivery modality better accommodates the diverse physical phenomena occurring at each stage of delivery, with the smooth coalescence of such systems inevitably benefiting overall efficacy.
Such formulations, termed multistage particles, begin to address the single-particle inadequacy by subdividing delivery into discrete and sequential formulations, reducing the codependencies (e.g., between initial particle stability and terminal drug release) that single-particle systems confront among diverse barriers. A variety of multistage delivery systems, including MSPs16 and biodegradable gelatin nanoparticles,17 can encapsulate or surface-decorate quantum dots or superparamagnetic iron oxide nanoparticles18 for imaging and deep tumor penetration, and multistage therapy has been explored with satisfying potency in loading PEGylated (polyethylene glycol–coated) paclitaxel micelles19 as well as liposomes containing small interfering RNA (siRNA).20
The new study, published in Nature Biotechnology, introduces a new multistage system (therein referred to as an “injectable nanoparticle generator,” or iNPG) based on MSPs used for the therapeutic delivery of Dox as a polyglutamic acid conjugate.3 Dox is an anthracycline antitumor antibiotic that acts by intercalating into DNA. The authors effectively conjugated Dox monomers to polyglutamic acid (pDox) and encapsulated this stage 2 system into the stage 1 MSPs, loading to an efficient 25–30% at each stage (7–8% Dox loading overall within MSPs). Intravenous delivery allowed passive iNPG accumulation in tumor metastases within the lung through microvascular attachment. Subsequently, pDox nanoparticles were spontaneously formed upon release from MSPs and internalized by tumor cells, releasing Dox in the perinuclear space in a pH-dependent fashion so as to avoid P-glycoprotein-mediated drug efflux pumps in both naive and drug-resistant tumor models (Figure 1). These methods increased drug exposure within the lung to a mass-normalized extent on par with that of liver exposure, while yielding order-of-magnitude decreases in initial transient Dox exposure within the heart, where Dox is known to be particularly toxic. These effects led to an improved therapeutic outcome for the iNPG system as well as a notably increased maximum tolerated dose, as compared with results using Doxil, a PEGylated liposomal formulation of Dox. The favorable results, coupled with a conceptually complex formulation that is nevertheless developmentally straightforward as a whole, have potent implications for clinical development of related technologies.
Figure 1.
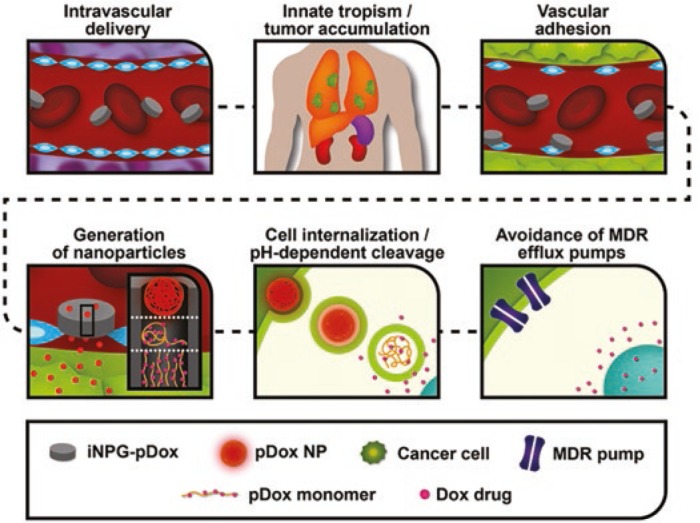
Multistage in vivo delivery mechanism for the iNPG-pDox system. Sequential steps include vascular transport, tumor accumulation of iNPG-pDox in sites of interest due to innate tropism, association with diseased endothelia, in situ generation of pDox nanoparticles, and cellular internalization and transport of pDox that results in release of Dox in the perinuclear region inside the cell. Dox, doxorubicin; iNPG, injectable nanoparticle generator; pDox, doxorubicin monomers conjugated to polyglutamic acid; MDR, multidrug resistance; NP, nanoparticle. Adapted from ref. 12.
The first multistage particles, introduced nearly a decade ago,16 have developed rapidly into advanced systems capable of incorporating an array of both therapeutic19,20 and diagnostic16,17,21 nanotechnologies into the overall carrier design. In particular, MSP delivery systems have been extensively optimized in the oft-neglected areas of particle hemodynamics, margination, and adhesion, in which particle size and shape can strongly influence the frequency of endothelial interactions overall as well as local microvascular adhesion at the tumor.4,5,22 Considerable motivation in this regard stems from complexities and well-known contradictions encountered in multifunctional nanoparticle design, most typically exhibited in the conflict between polymer-mediated steric exclusion for immune evasion (i.e., PEGylation) and specific and active cell internalization (i.e., targeting).4,13,15 Although multifunctional nanoparticle design is frequently disparaged as an illogical compromise in efficacy, such analyses ignore the extraordinary value represented by recent advances in lipid-based delivery systems in particular. The advent of high-throughput nanotechnology, represented most dramatically by the synthetic lipidoids, reveals the strong impact that the physical and chemical properties of lipids can have on the overall efficacy of hepatic transfection.6,23 Indeed, this dependence of performance on four essential components (length of lipid chains, number of lipid chains per lipid, degree of amine substitution, and pKa of the particle surface), when rationally selected before any experimental evaluation, can lead to a near-perfect predictive capability in mediating in vivo siRNA efficacy.7 Furthermore, our own work on hepatic uptake and gene transfer exhibits anything but a contradiction between PEGylation and active targeting. At sufficiently high degrees of PEGylation (15–20 mol%), bilayer lipid-coated nanoprecipitates show a dramatic shift in tropism away from uptake by resident macrophage populations (i.e., Kupffer cells) to an almost exclusive internalization by primary hepatocytes.8
Meanwhile, active targeting via galactose to hepatocyte asialoglycoprotein receptors serves to enhance not only uptake at the cellular level but the overall biodistribution of the nanoparticles as well, facilitating predominant accumulation in the liver (nearly 50% injected dose).8 Coupled with promising clinical development in Alnylam Pharmaceuticals's lipid nanoparticle24 and GalNAc25 systems, complex, functional, and actively targeted platform development can serve superbly in the proper delivery context to enhance formulation efficacy.
Furthermore, the general concept that has long driven the field—that of passive drug accumulation at sites of inflammation (e.g., tumors), and that underpins the above multistage systems—has recently been superseded by advanced, cell-mediated delivery platforms.9,10,26,27 Along with an extraordinary ability to overcome the blood–brain barrier,9 the capabilities of these platforms in “active targeting” to inflamed tissues in vivo more accurately represent the meaning of the phrase than the ligand-based mechanisms that nanoparticles frequently employ. Cell-mediated systems have proved capable of delivering highly complex cargoes such as nanoformulated enzymes (or nanozymes),9 as well as penetrating rapidly and chemotropically across diverse tissue barriers toward their target site of inflammation.10 Furthermore, such cell-based systems represent a truly ideal multifunctional delivery system in their ability to self-express a diverse array of therapeutic and diagnostic agents. Although cell-mediated delivery remains in its relative adolescence compared with particle-based technologies, innumerable sufferers of currently intractable diseases await the seminal advances that will drive these systems forward.
So, where do these frequently disparate conceptual areas leave the drug delivery field as a whole? In essence, despite complex and extraordinarily cross-disciplinary bases, the theoretical toolbox for formulation scientists has never before been so well stocked, and the coalescence of such technologies is already under way.11 Hierarchical efforts toward tackling one barrier at a time, multiple barriers at once, and the entireties of drug delivery mechanisms in vivo must be developed in concert between multifunctional as well as multistage systems, with more detailed and better-segmented analyses of specific transfer and mass balance at the levels of each discrete barrier.5,13,15 The outlook for the future of nanomedicine remains as strong as ever.
Acknowledgments
Our lab is supported by NIH grants CA149387, CA198999, and DK100664.
References
- Swenson, CE, Bolcsak, LE, Batist, G, Guthrie, THJ, Tkaczuk, KH, Boxenbaum, H et al. (2003). Pharmacokinetics of doxorubicin administered i.v. as Myocet (TLC D-99; liposome-encapsulated doxorubicin citrate) compared with conventional doxorubicin when given in combination with cyclophosphamide in patients with metastatic breast cancer. Anti-Cancer Drugs 14: 239–246. [DOI] [PubMed] [Google Scholar]
- Hu, Y, Haynes, MT, Wang, Y, Liu, F and Huang, L (2013). A highly efficient synthetic vector: nonhydrodynamic delivery of DNA to hepatocyte nuclei in vivo. ACS Nano 7: 5376–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, R, Zhang, G, Mai, J, Deng, X, Segura-Ibarra, V, Wu, S et al. (2016). An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat Biotechnol 34: 414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari, M (2010). Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol 28: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco, E, Shen, H and Ferrari, M (2015). Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 33: 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinc, A, Zumbuehl, A, Goldberg, M, Leshchiner, ES, Busini, V, Hossain, N et al. (2008). A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol 26: 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead, KA, Dorkin, JR, Vegas, AJ, Chang, PH, Veiseh, O, Matthews, J et al. (2014). Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat Commun 5: 4277–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y, Hu, Y and Huang, L (2014). Influence of polyethylene glycol density and surface lipid on pharmacokinetics and biodistribution of lipid-calcium-phosphate nanoparticles. Biomaterials 35: 3027–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyachko, NL, Haney, MJ, Zhao, Y, Manickam, DS, Mahajan, V, Suresh, P et al. (2014). Macrophages offer a paradigm switch for CNS delivery of therapeutic proteins. Nanomedicine (Lond) 9: 1403–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bago, JR, Alfonso-Pecchio, A, Okolie, O, Dumitru, R, Rinkenbaugh, A, Baldwin, A S et al. (2016). Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma. Nat Commun 7: 10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X, Zhang, F, Wang, H, Niu, G, Choi, KY, Swierczewska, M et al. (2013). Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials 34: 1772–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien, M-Y, Liu, L-C, Wang, H-C, Yeh, M-H, Chen, C-J, Yeh, S-P et al. (2014). Safety and efficacy of pegylated liposomal doxorubicin-based adjuvant chemotherapy in patients with stage I–III triple-negative breast cancer. Anticancer Res 34: 7319–7326. [PubMed] [Google Scholar]
- Haynes, MT and Huang, L (2014). Hepatic RNA interference: delivery by synthetic vectors. Drug Deliv Transl Res 4: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S-D and Huang, L (2009). Nanoparticles evading the reticuloendothelial system: role of the supported bilayer. Biochim Biophys Acta 1788: 2259–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, L and Liu, Y (2011). In vivo delivery of RNAi with lipid-based nanoparticles. Ann Rev Biomed Eng 13: 507–530. [DOI] [PubMed] [Google Scholar]
- Tasciotti, E, Liu, X, Bhavane, R, Plant, K, Leonard, AD, Price, BK et al. (2008). Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnology 3: 151–157. [DOI] [PubMed] [Google Scholar]
- Wong, C, Stylianopoulos, T, Cui, J, Martin, J, Chauhan, VP, Jiang, W et al. (2011). Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci USA 108: 2426–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serda, RE, Mack, A, Pulikkathara, M, Zaske, AM, Chiappini, C, Fakhoury, J et al. (2010). Cellular association and assembly of a multi-stage delivery system. Small 6: 1329–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco, E, Sangai, T, Hsiao, A, Ferrati, S, Bai, L, Liu, X et al. (2013). Multistage delivery of chemotherapeutic nanoparticles for breast cancer treatment. Cancer Lett 334: 245–252. [DOI] [PubMed] [Google Scholar]
- Xu, R, Huang, Y, Mai, J, Zhang, G, Guo, X, Xia, X et al. (2013). Multistage vectored siRNA targeting ataxia–telangiectasia mutated for breast cancer therapy. Small 9: 1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasciotti, E, Godin, B, Martinez, JO, Chiappini, C, Bhavane, R, Liu, X et al. (2011). Near-infrared imaging method for the in vivo assessment of the biodistribution of nanoporous silicon particles. Mol Imaging 10: 56–68. [PMC free article] [PubMed] [Google Scholar]
- Lee, SY, Ferrari, M and Decuzzi, P (2009). Design of bio-mimetic particles with enhanced vascular interaction. J Biomech 42: 1885–1890. [DOI] [PubMed] [Google Scholar]
- Love, KT, Mahon, KP, Levins, CG, Whitehead, KA, Querbes, W, Dorkin, JR et al. (2010). Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA 107: 1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho, T, Adams, D, Silva, A, Lozeron, P, Hawkins, PN, Mant, T et al. (2013). Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 369: 819–829. [DOI] [PubMed] [Google Scholar]
- Nair, JK, Willoughby, JLS, Chan, A, Charisse, K, Alam, MR, Wang, Q et al. (2014). Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc 136: 16958–16961. [DOI] [PubMed] [Google Scholar]
- Roger, M, Clavreul, A, Venier-Julienne, M-C, Passirani, C, Montero-Menei, C and Menei, P (2011). The potential of combinations of drug-loaded nanoparticle systems and adult stem cells for glioma therapy. Biomaterials 32: 2106–2116. [DOI] [PubMed] [Google Scholar]
- Young, JS, Morshed, RA, Kim, JW, Balyasnikova, IV, Ahmed, AU and Lesniak, MS (2014). Advances in stem cells, induced pluripotent stem cells, and engineered cells: delivery vehicles for anti-glioma therapy. Expert Opin Drug Deliv 11: 1733–1746. [DOI] [PubMed] [Google Scholar]


