Abstract
♦ Background:
Chronic renal failure and aging are suggested as risk factors for cognitive impairment (CI). We studied the prevalence of CI among peritoneal dialysis (PD) patients using Montreal Cognitive Assessment (MoCA), its impact on PD-related peritonitis in the first year, and the potential role of assisted PD.
♦ Methods:
One hundred fourteen patients were newly started on PD between February 2011 and July 2013. Montreal Cognitive Assessment was performed in the absence of acute illness. Data on patient characteristics including demographics, comorbidities, blood parameters, dialysis adequacy, presence of helpers, medications, and the number PD-related infections were collected.
♦ Results:
The age of studied patients was 59±15.0 years, and 47% were female. The prevalence of CI was 28.9%. Patients older than 65 years old (odds ratio [OR] 4.88, confidence interval [CI] 1.79 – 13.28 p = 0.002) and with an education of primary level or below (OR 4.08, CI 1.30 – 12.81, p = 0.016) were independent risk factors for CI in multivariate analysis. Patients with PD-related peritonitis were significantly older (p < 0.001) and more likely to have CI as defined by MoCA (p = 0.035). After adjustment for age, however, CI was not a significant independent risk factor for PD-related peritonitis among self-care PD patients (OR 2.20, CI 0.65 – 7.44, p = 0.20). When we compared patients with MoCA-defined CI receiving self-care and assisted PD, there were no statistically significant differences between the 2 groups in terms of age, MoCA scores, or comorbidities. There were also no statistically significant differences in 1-year outcome of PD-related peritonitis rates or exit-site infections.
♦ Conclusion:
Cognitive impairment is common among local PD patients. Even with CI, peritonitis rate in self-care PD with adequate training is similar to CI patients on assisted PD.
Keywords: Cognitive impairment; peritoneal dialysis, peritonitis
It is increasingly recognized that renal failure is associated with cognitive impairment (CI) (1). More severe renal impairment is associated with more severe CI (1). In addition, the prevalence of CI among patients with end-stage renal failure (ESRF) is 3 times higher than that of the age-matched general population in Western countries, in which the prevalence is around 16 – 38% (2,3). However, most of the previously reported patients with a prevalence of CI in ESRF were put on hemodialysis (HD) (3). Such higher prevalence of CI in dialysis patients was partially related to the age of the studied populations. The prevalence of CI among the elderly increases with age (4). In Hong Kong, the prevalence of dementia increased from 1.7% in the 70 – 74 age group to 18.8% in the 85 – 89 age group (4).
There is an increasing trend of elderly patients suffering from renal diseases in many countries including Canada and the United States (5,6). In Australia, the number of new ESRF patients 65 years of age and over increased by 8% per annum to 1,125 in 2008 from 860 in 2004 (6). In Hong Kong (HK), 36.3% of the patients on peritoneal dialysis (PD) were above 65 years old (7). Since PD is our primary mode of renal replacement therapy, a reasonable degree of cognitive function is of paramount importance for patients to properly handle the complex PD exchange procedures as any error can result in PD-related peritonitis. Li et al. previously reported that the prevalence of CI among elderly PD patients (≥ 65 years old) in HK is 32.1% using the Cantonese version of the Mini-Mental State examination (CMMSE) (8). Unfortunately the CMMSE was not sensitive enough to detect executive dysfunction in ESRF patients with CI and hence may underestimate the actual prevalence in this local study (2). In addition, up to 41% of patients on HD between 55 and 64 years old suffered from severe CI in a western study but the previous local study did not include PD patients younger than 65 years old (3). Most importantly, to our knowledge, there has been no study to explore the impact of CI on PD-related peritonitis.
It is also increasingly common for elderly patients with ESRF to be put on assisted PD because of the presence of comorbidities including CI. Assisted PD refers to PD performed with the assistance of a family member, partner, or healthcare professional (9). There has been no study on whether assisted PD for patients with CI can achieve similar outcomes to those achieved by self-care PD patients.
The objectives of the our study were to investigate the prevalence of CI using Montreal Cognitive Assessment (MoCA) in patients of at least 18 years old newly started on PD and to study the impact of CI in PD-related peritonitis. We also studied whether there were any differences in 1-year outcomes between assisted and self-care PD patients with CI.
Materials and Methods
This was a 1-year prospective cohort study. The research protocol was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority, Hong Kong. We recruited patients newly started on PD in Queen Mary Hospital and Tung Wah Hospital between February 2011 and July 2013. Written informed consents were obtained from all study patients or their family members. Eligible patients were at least 18 years old and newly commenced on PD. Patients who cannot understand Chinese language or refused to consent were excluded from the study.
Montreal Cognitive Assessment was performed when the patients were admitted for PD training by a single assessor in each hospital. Montreal Cognitive Assessment is a test of global cognitive function, which includes assessment of visuospatial function, naming, attention, language, reasoning, memory, and orientation. Since we believe that the CI in PD patients is mainly in the form of vascular CI, we had utilized the validated cut-off 21/22 for Hong Kong patients with cerebral small vessel disease (10).
The PD exchange training was on a 1-to-1 basis led by a single registered renal nurse specialist who was blinded from the MoCA results. The 2 hospitals adopted the same training program. At the beginning, patients were briefly assessed for suitability to perform PD exchange, including their visual acuity, hand function, and memory. We recorded the reasons for the need for assisted PD. If patients were found not able to perform PD exchange safely, family members were invited to help. The training included video and live demonstrations, and practice sessions, for a total of 40 hours. Exchange technique and knowledge were tested before the patients were allowed to perform PD exchanges at home.
After the exchange training, patients were regularly followed up at their own training center and monitored for any episode of peritonitis or other PD-related complications. Peritoneal dialysis peritonitis and exit-site infection in the following 12 months were taken as primary study outcomes. Peritonitis was defined by symptoms of abdominal pain with or without fever and a cloudy peritoneal fluid, with white cell (WBC) count > 100/mm3, in which > 50% are polymorphonucleocytes.
Patients' baseline demographic information, comorbidities, use of helper for exchanges, and list of medications were collected. The age-adjusted Charlson Comorbidity Index (CCI), which contained 19 categories of comorbidities, was calculated (11). Any use of sedating medications such as antihistamines, hypnotics, anti-depressants, anti-psychotics, and benzodiazepines was recorded. Both hematological and biochemical laboratory data including hemoglobin, hematocrit, lymphocyte, potassium, creatinine, calcium, phosphate, albumin, parathyroid hormone, low-density lipoprotein and triglyceride levels were obtained within 30 days of the cognitive testing. Dialysis adequacy tests were performed about 8 weeks after completion of PD training, according to international guidelines. Residual renal function was also measured by collection of 24-hour urine and calculated from the average of urea and creatinine clearance. All emergency hospital attendance data in the following 12 months after commencing PD were collected. The last subject completed their 1-year observation on 31 July 2014.
Statistical Analysis
Results were expressed as mean ± standard deviation unless otherwise specified. The frequency of CI was calculated to give the prevalence. Chi-square test or Fisher's exact test were used to compare categorical variables. Independent sample t-test or Mann-Whitney U test were used to compare continuous variables when appropriate. After performing univariate analyses, variables with p ≤ 0.10 were entered into logistic regression analysis with CI by MoCA and PD peritonitis in the first 12 months as the dependent variables. Statistical significance was inferred for a 2-tailed p value of less than 0.05. All statistical analyses were carried out using SPSS (Windows version 18; SPSS Chicago, IL, USA).
Results
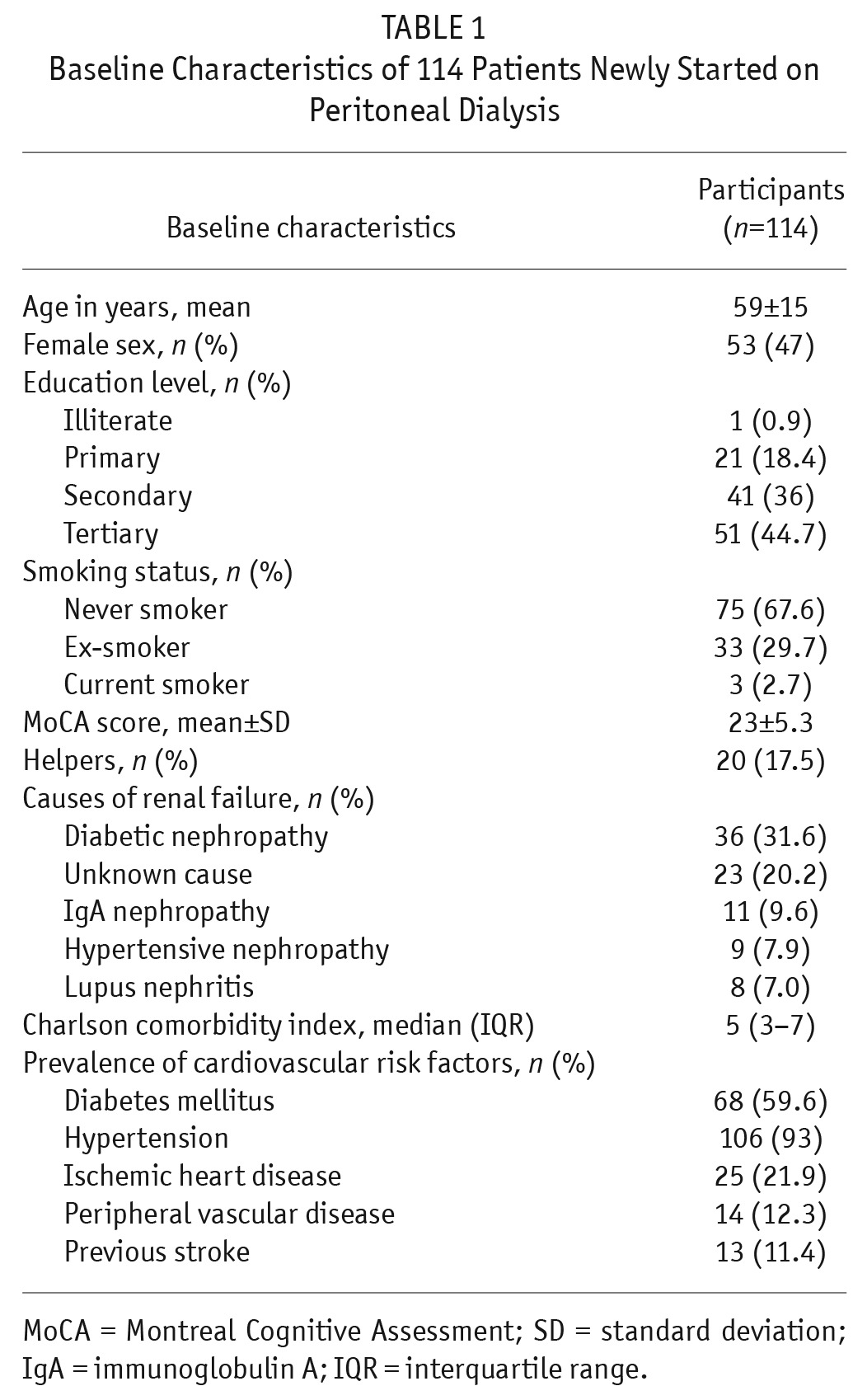
The baseline demographic and clinical characteristics of our patients are summarized in Table 1. One hundred fourteen patients were recruited. Their mean age was 59 ± 15.0 years and 53 (47%) were female. Diabetes mellitus nephropathy was the leading cause of ESRF (31.6%). The median CCI score was 5 (interquartile range [IQR] 3 – 7). The mean total Kt/V was 2.16 ± 0.52 and mean residual renal function (glomerular filtration rate [GFR] based on 24-hour urine) was 4.0 ± 3.0 mL/min. A total of 91.2% (n = 104) had continuous ambulatory PD (CAPD) and 8.8% (n = 10) used automated PD (APD). The 1-year survival was 98.2% (n = 112). The 1-year technique survival and survival free of transplantation were 97.3% (n = 111) and 98.2% (n = 112), respectively. The survival free of peritonitis at 1 year was 11.4 months (95% confidence interval [CI] 10.99 – 11.9).
TABLE 1.
Baseline Characteristics of 114 Patients Newly Started on Peritoneal Dialysis
Prevalence of CI and Its Risk Factors
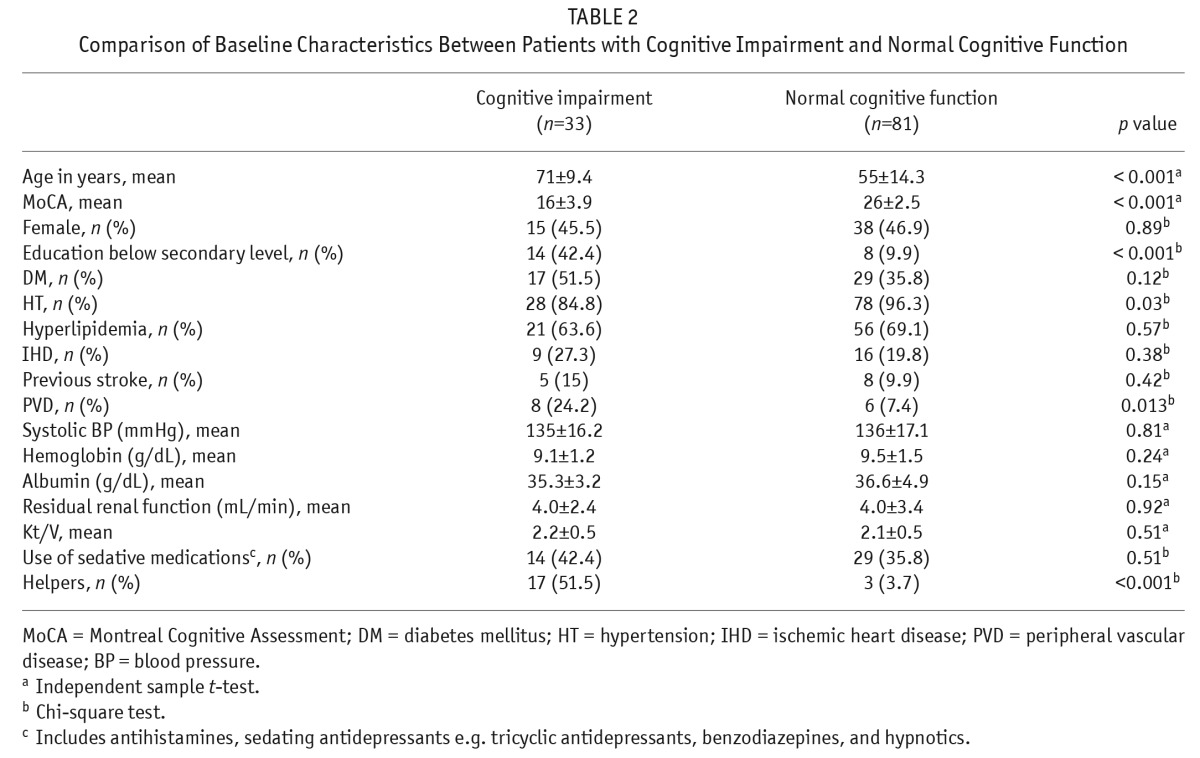
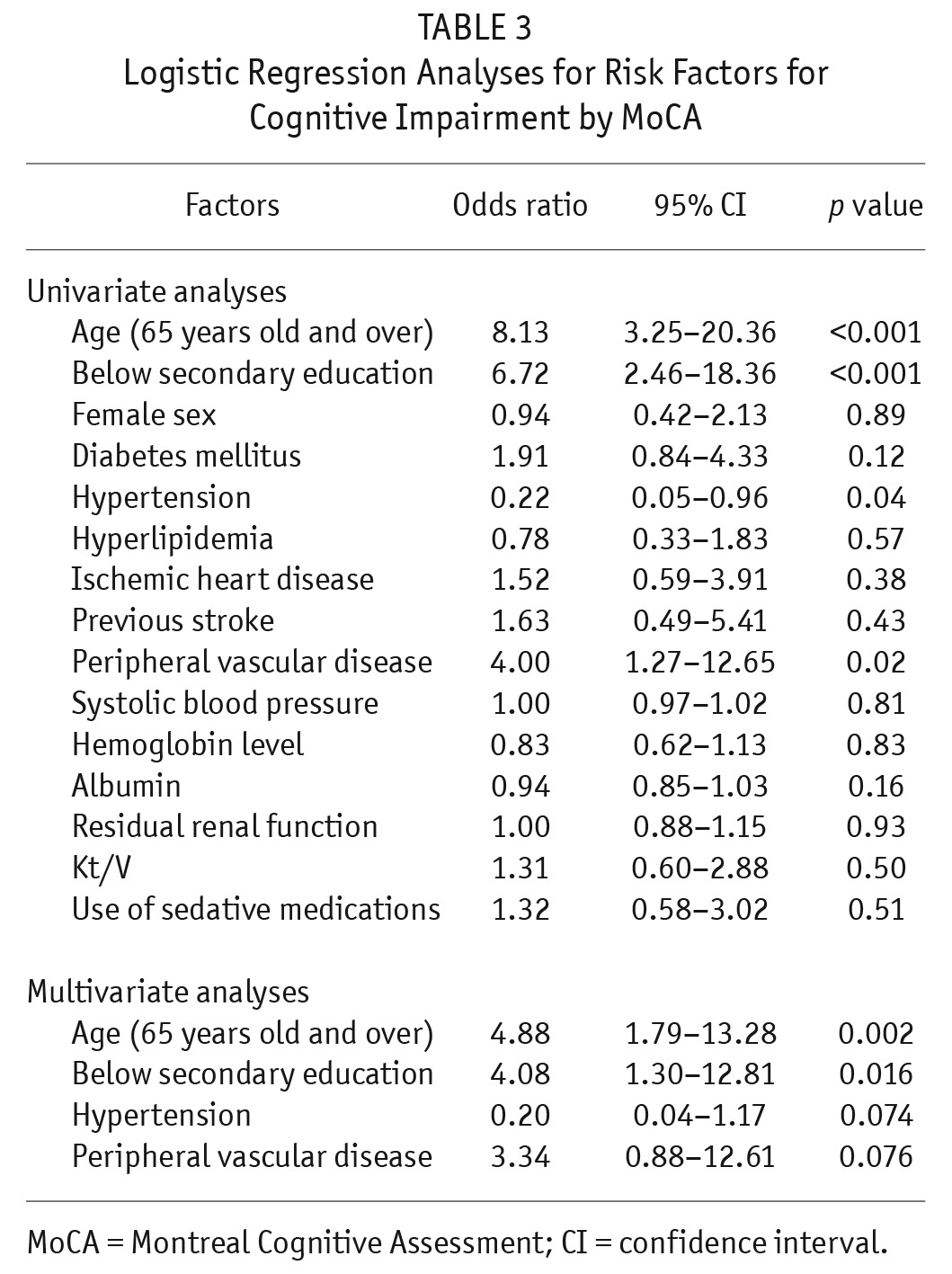
The mean MoCA score was 23 ± 5.3. The prevalence of CI was 28.9%. A higher prevalence of CI was found in those ≥ 65 years old (n = 24, 54.5%) than in those < 65 years old (n = 9, 12.9%) (p < 0.001). In MoCA sub-score analyses, patients with CI scored lower in all aspects (i.e. visuospatial, naming, attention, language, reasoning, memory, and orientation) when compared with patients without CI (all p < 0.001, Supplementary Table 1). Patients with CI were older (p < 0.001), were more likely to have only primary education or below (p < 0.001), suffered less commonly from hypertension (p = 0.03), and suffered more commonly from peripheral vascular disease (p = 0.013) (Table 2). Univariate analyses by logistic regression showed that age ≥ 65, primary education or below, hypertension, and peripheral vascular disease were significantly associated with CI (p < 0.001, < 0.001, 0.04, and 0.02, respectively) (Table 3). Multivariate analyses by logistic regression showed that age ≥ 65 years old (odds ratio [OR] 4.88, CI 1.79 – 13.28, p = 0.002) and primary education or below (OR 4.08, CI 1.30 – 12.81, p = 0.016) were independently associated with CI (Table 3).
TABLE 2.
Comparison of Baseline Characteristics Between Patients with Cognitive Impairment and Normal Cognitive Function
TABLE 3.
Logistic Regression Analyses for Risk Factors for Cognitive Impairment by MoCA
Peritonitis at 12-Month Follow-Up and Its Risk Factors
During the 12-month follow-up period, 30 patients (26.3%) developed PD peritonitis with a total of 44 episodes of PD-related peritonitis (1 episode per 31 patient-months). There was no relapsing PD peritonitis, defined as an episode that occurs within 4 weeks of completion of therapy of a prior episode with the same organism or 1 sterile episode, in the current patient sample (12). Twenty episodes of PD peritonitis were culture negative. Peritonitis caused by gram-positive, gram-negative, and fungal organisms accounted for 27.3% (n = 12), 22.7% (n = 10), and 2.3% (n = 1) of cases, respectively. One patient had peritonitis caused by mixed gram-positive and gram-negative organisms. One patient had peritonitis caused by Mycobacterium tuberculosis.
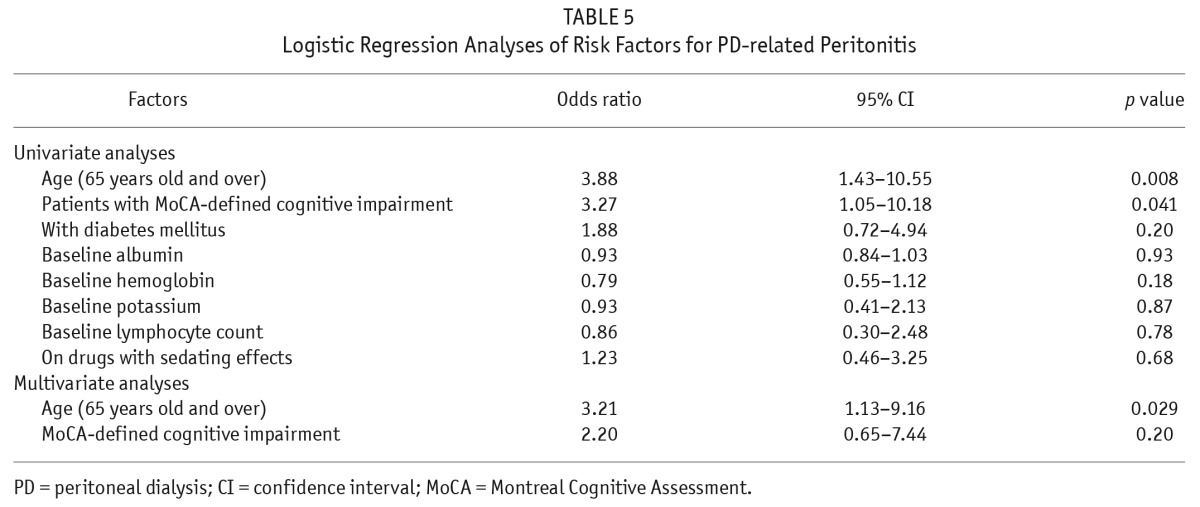
After excluding 20 PD patients on assisted PD, we compared the demographics, clinical features, and laboratory parameters of patients with and without PD-related peritonitis (Table 4). Patients with PD-related peritonitis were significantly older (p < 0.001) and more likely to have CI as defined by MoCA (p = 0.035). Univariate analyses by logistic regression using age ≥ 65, CI by MoCA, diabetes, albumin level, potassium level, lymphocyte count, and use of sedatives as independent variables, revealed that age ≥ 65 (OR 3.88, CI 1.43 – 10.55, p = 0.008) and CI defined by MoCA (OR 3.27, CI 1.05 – 10.18, p = 0.041) were associated with PD-related peritonitis. Sub-analyses on the MoCA subscores found that patients with PD-related peritonitis scored lower in the memory subscores than those without peritonitis (2 (IQR 0.75 – 4) vs 3 (IQR 2 – 4), p = 0.044). Multivariate analyses showed that only age was an independent risk factor for PD-related peritonitis (OR 3.21, CI 1.13 – 9.16, p = 0.029) (Table 5).
TABLE 4.
Comparison of Clinical Factors Between Subjects With and Without PD Peritonitis in Self-Care PD Patients
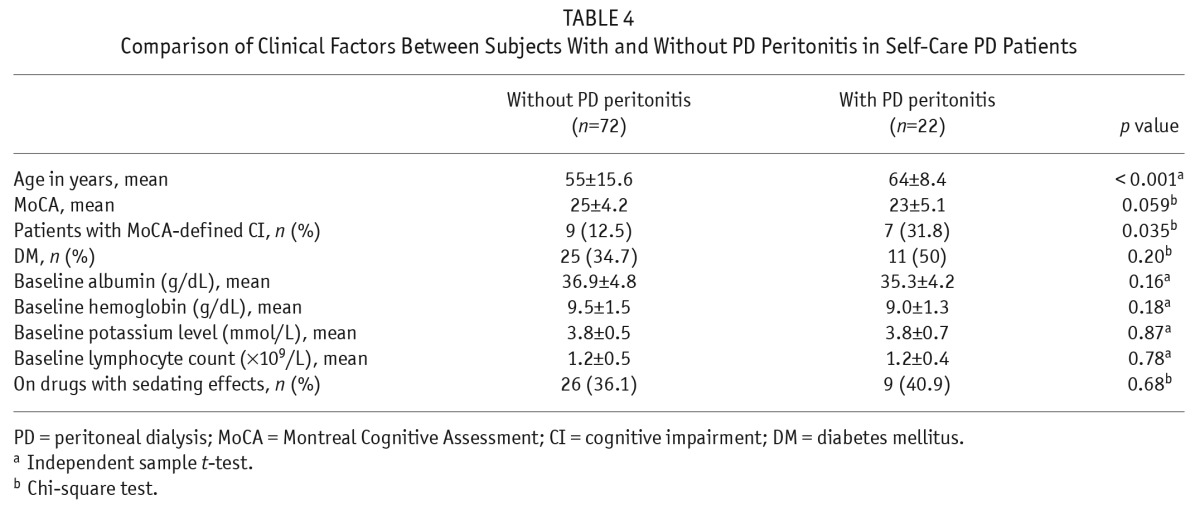
TABLE 5.
Logistic Regression Analyses of Risk Factors for PD-related Peritonitis
Patients with MoCA-Defined CI in Self-Care and Assisted PD
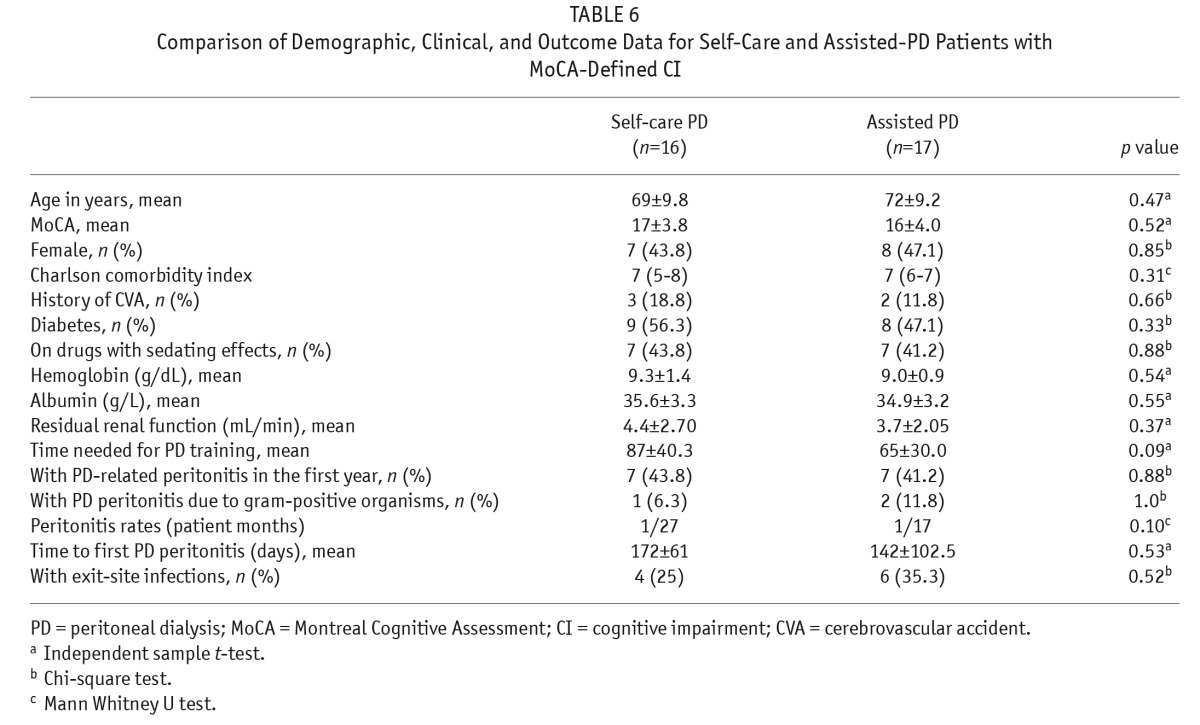
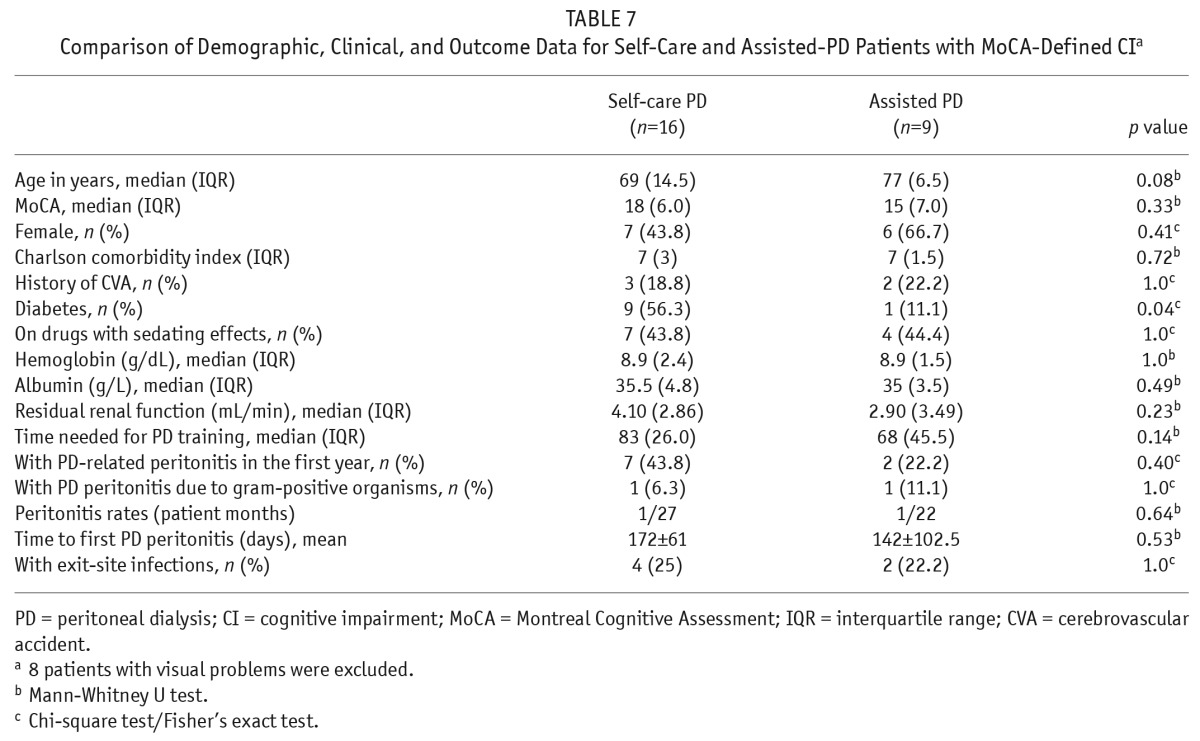
The demographics, comorbidities, and 1-year outcome of patients with MoCA-defined CI in self-care and assisted PD were compared (Table 6). There were no statistically significant differences between the 2 groups in terms of age, total MoCA scores, MoCA subscores, or CCI scores. There were also no statistically significant differences in terms of time required to complete PD training, 1-year PD-related peritonitis rates, or exit-site infection occurrence (Table 6). After exclusion of 8 patients with visual impairment contributing to the need for assisted PD, the results of the comparison of demographics, comorbidities, and 1-year outcome of patients with MoCA-defined CI between self-care and assisted PD remained similar (Table 7).
TABLE 6.
Comparison of Demographic, Clinical, and Outcome Data for Self-Care and Assisted-PD Patients with MoCA-Defined CI
TABLE 7.
Comparison of Demographic, Clinical, and Outcome Data for Self-Care and Assisted-PD Patients with MoCA-Defined CIa
Discussion
We have shown that the prevalence of CI increased with age to 54.5% for those aged 65 and above, which was much higher than the previously reported prevalence of mild dementia among community Chinese elderly adults aged 70 years and above (8.9%) (13). Our prevalence is also greater than that reported in a previous local study which reported a prevalence of 32.1% (8). In both studies, increasing age and lower educational level are independent risk factors for CI in PD patients (8). Our higher prevalence is likely because MoCA detects executive dysfunction better than CMMSE (2). There was only 1 previous Western study on the prevalence of CI among 51 PD patients, which reported a prevalence of 66.7% (14). Our prevalence is slightly less than the Western study, due to the methods of assessment of CI. We used MoCA while Kalirao et al. employed more detailed cognitive assessments (including Hopkins Verbal Learning Test-Revised, Colors Trails 1 and 2, the Stroop Interference test, Brief Visuospatial Memory Test-Revised, the Controlled Oral Word Association Test, etc.), many of which have not been validated in Hong Kong. In addition, the utilization of these cognitive assessments may not be feasible in the busy day-to-day clinical setting.
To the best of our knowledge, this is the first study exploring the impact of CI on PD-related peritonitis. Although self-care PD patients with CI may be prone to PD-related peritonitis due to an inability to handle complex PD procedures, we were unable to identify CI as an independent risk factor for PD-related peritonitis after adjustment for age. There are several possible reasons. Firstly, the sample size in the logistic regression analysis of peritonitis was small and hence our study may have limited power in identifying CI as an independent risk factor for peritonitis. Secondly, age and MoCA are correlated, i.e. cognitively impairment is more likely with increasing age and it could therefore be argued that age could mask the effect of MoCA in the multivariate analysis of peritonitis. Nevertheless, it is better to adjust for age in order to assess CI as an independent risk factor for peritonitis. Thirdly, such results could be due to the renal specialist nurse being more likely to assign patients with CI to the assisted group during assessment of patients' ability to perform PD (despite not knowing the MoCA results). Previous studies on comparing self-care and assisted-PD patients found that there was no difference in the risk of technique failure or overall survival (15–18). Another possible explanation is that self-care PD patients with CI can have similar outcomes to self care non-CI patients if adequate training is provided. We further compared any differences in 1-year outcome between CI patients on self-care or assisted PD and found there were no differences in their PD-related peritonitis rates or exitsite infection occurrence (Tables 6 and 7), further suggesting that even with CI, the peritonitis rate in self-care PD can be similar to the rate in assisted PD, provided that adequate training is given.
Our subjects, with mean age of 59 ± 15 and the 3 most common causes of ESRF being diabetic nephropathy (31.6%), unknown cause (20.2%), and glomerulonephritis (27.2%), were comparable to the HK Renal Registry Report 2012, and hence our sample should be representative of PD patients in HK (7). Around 91% of our patients were on CAPD. Our study had certain limitations. We did not perform a detailed cognitive history taken from both patients and family members to further differentiate between mild CI and dementia. Since only 33 PD patients were suffering from CI, our study had a low statistical power to detect differences between CI patients on assisted PD and self-care PD; our results only provided pilot data, and larger studies are needed. Our patients should also be offered longer follow-up to see whether there is any difference in PD peritonitis or technique survival between self-care and assisted-PD patients with CI.
In conclusion, CI is common among local PD patients. But even with CI, peritonitis rates in self-care PD can be similar to the rate in non-CI patients on self-care PD and in CI patients on assisted PD, provided that adequate training is given.
Disclosures
The authors have no financial conflicts of interest to declare.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the Queen Mary Hospital Charitable Trust – Training and Research Assistance Scheme (TRAS) – Grant for supporting this study and the assistance of renal nurses of 2 dialysis centers.
Footnotes
Supplemental material available at www.pdiconnect.com
REFERENCES
- 1. Khatri M, Nickolas T, Moon YP, Paik MC, Rundek T, Elkind MSV, et al. CKD associates with cognitive decline. J Am Soc Nephrol 2009; 20(11):2427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurella Tamura M, Yaffe K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int 79(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson LS, Li S, et al. Cognitive impairment in hemodialysis patients is common. Neurology 2006; 67(2):216–23. [DOI] [PubMed] [Google Scholar]
- 4. Chiu HFK, Lam LCW, Chi I, Leung T, Li SW, Law WT, et al. Prevalence of dementia in Chinese elderly in Hong Kong. Neurology 1998; 50:1002–9. [DOI] [PubMed] [Google Scholar]
- 5. Burkart J. The future of peritoneal dialysis in the United States: optimizing its use. Clin J Am Soc Nephrol 2009; 4(Suppl 1):S125–31. [DOI] [PubMed] [Google Scholar]
- 6. Fenton SS, Desmeules M, Jeffery JR, Corman JL. Dialysis therapy among elderly patients; data from the Canadian Organ Replacement Register, 1981–1991. Adv Perit Dial 1993; 9:124–9. [PubMed] [Google Scholar]
- 7. Ho YW, Chau KF, Choy BY, Fung KS, Cheng YL, Kwan TH, et al. Hong Kong renal registry report 2012. Hong Kong J Nephrol 2013; 15:28–42. [Google Scholar]
- 8. Li JSC. Prevalence of cognitive impairment among elderly Chinese continuous ambulatory peritoneal dialysis patients. Hong Kong J Nephrol 2004; 6:22–30. [Google Scholar]
- 9. Smyth A, McCann E, Redahan L, Lambert B, Mellotte GJ, Wall CA. Peritoneal dialysis in an ageing population: a 10-year experience. Int Urol Nephrol 2012; 44(1):283–93. [DOI] [PubMed] [Google Scholar]
- 10. Wong A, Xiong YY, Kwan PW, Chan AY, Lam WW, Wang K, et al. The validity, reliability and clinical utility of the Hong Kong Montreal Cognitive Assessment (HK-MoCA) in patients with cerebral small vessel disease. Dement Geriatr Cogn Disord 2009; 28(1):81–7. [DOI] [PubMed] [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 12. Li PK, Szeto CC, Piraino B, et al. International Society for Peritoneal Dialysis. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30(4):393–423. [DOI] [PubMed] [Google Scholar]
- 13. Lam LC, Tam CW, Lui VW, Chan WC, Chan SS, Wong S, et al. Prevalence of very mild and mild dementia in community-dwelling older Chinese people in Hong Kong. Int Psychogeriatr 2008; 20(1):135–48. [DOI] [PubMed] [Google Scholar]
- 14. Kalirao P, Pederson S, Foley RN, Kolste A, Tupper D, Zaun D, et al. Cognitive impairment in peritoneal dialysis patients. Am J Kidney Dis 2011; 57(4):612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li PK, Law MC, Chow KM, Leung CB, Kwan BC, Chung KY, Szeto CC. Good patient and technique survival in elderly patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 2007; 27(Suppl 2):S196–201. [PubMed] [Google Scholar]
- 16. Xu R, Zhuo M, Yang Z, Dong J. Experiences with assisted peritoneal dialysis in China. Perit Dial Int 2012; 32(1):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dimkovic N, Majster Z, Davidovic Z, Dimkovic S. CAPD assisted by family member – a single-center experience. Perit Dial Int 2009; 29(2):238–9. [PubMed] [Google Scholar]
- 18. Lobbedez T, Moldovan R, Lecame M, Hurault de Ligny B, El Haggan W, Ryckelynck JP. Assisted peritoneal dialysis. Experience in a French renal department. Perit Dial Int 2006; 26(6):671–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.