Abstract
♦ Background:
Extending technique survival on peritoneal dialysis (PD) remains a major challenge in optimizing outcomes for PD patients while increasing PD utilization. The primary objective of the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS) is to identify modifiable practices associated with improvements in PD technique and patient survival. In collaboration with the International Society for Peritoneal Dialysis (ISPD), PDOPPS seeks to standardize PD-related data definitions and provide a forum for effective international collaborative clinical research in PD.
♦ Methods:
The PDOPPS is an international prospective cohort study in Australia, Canada, Japan, the United Kingdom (UK), and the United States (US). Each country is enrolling a random sample of incident and prevalent patients from national samples of 20 to 80 sites with at least 20 patients on PD. Enrolled patients will be followed over an initial 3-year study period. Demographic, comorbidity, and treatment-related variables, and patient-reported data, will be collected over the study course. The primary outcome will be all-cause PD technique failure or death; other outcomes will include cause-specific technique failure, hospitalizations, and patient-reported outcomes.
♦ Results:
A high proportion of the targeted number of study sites has been recruited to date in each country. Several ancillary studies have been funded with high momentum toward expansion to new countries and additional participation.
♦ Conclusion:
The PDOPPS is the first large, international study to follow PD patients longitudinally to capture clinical practice. With data collected, the study will serve as an invaluable resource and research platform for the international PD community, and provide a means to understand variation in PD practices and outcomes, to identify optimal practices, and to ultimately improve outcomes for PD patients.
Keywords: Dialysis Outcomes Practice Patterns Study, peritoneal dialysis, prospective observational cohort study, technique survival, survival
Peritoneal dialysis (PD) is an attractive treatment option for patients with end-stage renal disease wishing for increased treatment-related flexibility and autonomy. Patients who perform PD experience superior satisfaction and better preservation of kidney function (1–4). In many developed countries, PD patients have better early survival, and similar overall survival, when compared with patients treated with conventional in-center hemodialysis (HD). Additionally, PD is more cost-effective compared to center-based HD, with annualized treatment costs that are 60% – 70% of center-based HD (5–10).
Despite the potential advantages, PD use is highly variable across countries and in fact, the proportion of dialysis patients treated with PD declined by 5.3% (from 20.6% to 15.3%) among developed countries between 1997 and 2008. Peritoneal dialysis use currently represents 11% of the prevalent global dialysis population (11). Although the last decade saw an increase in PD utilization and improvements in PD patient survival in several countries, the relatively short technique survival compared to center-based HD remains a barrier towards increasing PD utilization (12–15). In many settings, a significant proportion of technique failure occurs in the first 2 years of treatment and necessitates a permanent transfer from PD to HD (12). Technique failure is itself associated with considerable cost, morbidity, and reduced quality of life. In a recent Canadian study, technique failure events within the first 3 years of PD resulted in similar dialysis-related costs to treatment with HD alone, thereby attenuating the economic benefits of PD over in-center HD as an initial dialysis modality (16). Because PD technique failure rates are highly variable, the occurrence of technique failure is in part driven by modifiable practice differences.
To better understand potentially modifiable causes of PD technique failure, the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS) has been established. The PDOPPS is a unique collaboration between Arbor Research Collaborative for Health (Arbor Research, the Data Coordinating Center for the Dialysis Outcomes and Practice Patterns Study [DOPPS] Program), the International Society for Peritoneal Dialysis (ISPD), participating countries that have secured local funding for data collection, and a consortium of industry support (primary sponsor: Baxter Healthcare).
Background
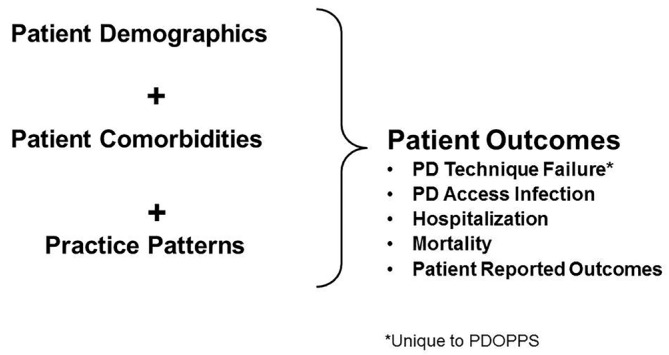
Since 1996, the DOPPS has studied in-center HD patients and practices and has served as an important resource to the nephrology community (17–20). Now entering its sixth phase and expanded to 21 countries, the DOPPS has identified key HD findings (21–26) that have helped shape international clinical practice guidelines and global HD practices, as well as inform country-level policy changes and initiatives to mitigate unwarranted practice variation to improve outcomes (19). As a logical extension of its goals and study design, the DOPPS Program has expanded into studies of chronic kidney disease (CKD) (CKDopps, for CKD stages 3 – 5) (27) and peritoneal dialysis (PDOPPS). The goals of the DOPPS Program are centered around the hypothesis that measurable differences in center practices influence patient outcomes. This same framework applies to PDOPPS (Figure 1).
Figure 1 —
Overall framework and approach for the DOPPS Program. DOPPS = Dialysis Outcomes and Practice Patterns Study; PDOPPS = Peritoneal Dialysis Outcomes and Practice Patterns Study; PD = peritoneal dialysis.
Objectives and Outcomes
The Peritoneal Dialysis Outcomes and Practice Patterns Study is a 3-year international prospective cohort study of PD practices and outcomes. The primary objective of PDOPPS is to identify modifiable and measurable PD practices that are associated with improved outcomes for patients, while simultaneously developing logical, scientific hypotheses for the differences seen. The primary study outcome is all-cause mortality or technique failure. Secondary study outcomes include cause-specific technique failure, hospitalization, and patient-reported outcomes.
Rationale
PDOPPS is Predicated on 3 Key Observations:
1) Wide Unexplained Variation Exists in Rates of Peritoneal Dialysis Technique Failure Within and Between Countries
Although not accounting for differences in the case-mix of patients internationally, national dialysis registries demonstrate considerable unexplained variability in the rates of PD technique failure between centers (Supplementary Figure 1). In Canada, unexplained interprovincial variability exists in the rates of technique failure after adjusting for case-mix differences and after accounting for competing events of transplantation and mortality (12) (Supplementary Figure 2).
Across the initial countries participating in PDOPPS, reported technique failure rates are higher in the United Kingdom (UK) and Australia, but much lower in Japan (28–30). In Japan, infection-related technique failure is lowest, while failure of the peritoneal membrane to adequately remove fluid and small solutes is more common (28). Australia has one of the highest reported PD technique failure rates in the world (across centers, half to two-thirds of PD patients discontinued therapy after 3 years), and peritonitis was a leading or contributing factor in > 50% of permanent transfers to HD (29). While differences in the global rates of PD technique failure and its causes may be partially explained by residual patient-level confounding (i.e., patient health status independent of PD), center characteristics such as PD center size have consistently emerged as strong predictors of PD technique failure (31–33). Center size is an important predictor of PD outcomes across many countries. Patients treated in Canadian centers with a cumulative patient count of > 500 patients face a 27% lower risk of technique failure over the period of observation compared with patients treated at a center with a cumulative patient count of < 100 patients over 33,937 person-years of follow-up (34). Moreover, in Australia, 10-fold variability exists in PD peritonitis rates across dialysis centers (0.2 – 2 episodes per patient-year), and is not explained by measured differences in case-mix (29). A recent international survey of nephrologists demonstrated that PD center size was associated with stated adherence to national and international PD clinical practice guidelines (35). Together, significant inter-center and international differences exist in the rates and causes of technique failure.
2) Standardized Definitions for Peritoneal Dialysis Technique Failure Are Lacking
Our review of national and regional registries and large population-based observational cohort studies has revealed significant variability across definitions related to technique failure (36). This variability makes international comparisons challenging (Table 1; Supplementary Figure 2). Across registries, the PD start date can be defined as either the first PD exchange performed or the completion of PD training. More commonly, no clear definition of the start of PD is provided whatsoever (36). Approaches to collecting the cause for technique failure differ markedly and appear to depend on whether registries allow for multiple or a single reason to be recorded. Lastly, only one registry defines the minimum period of time that a patient must be off of PD to qualify as technique failure. Across the majority of registries, transitions to HD from PD are not categorized as temporary or permanent, or planned or unplanned (36).
TABLE 1.
Variability in Data Definitions and Data Capture for Peritoneal Dialysis Technique Failure
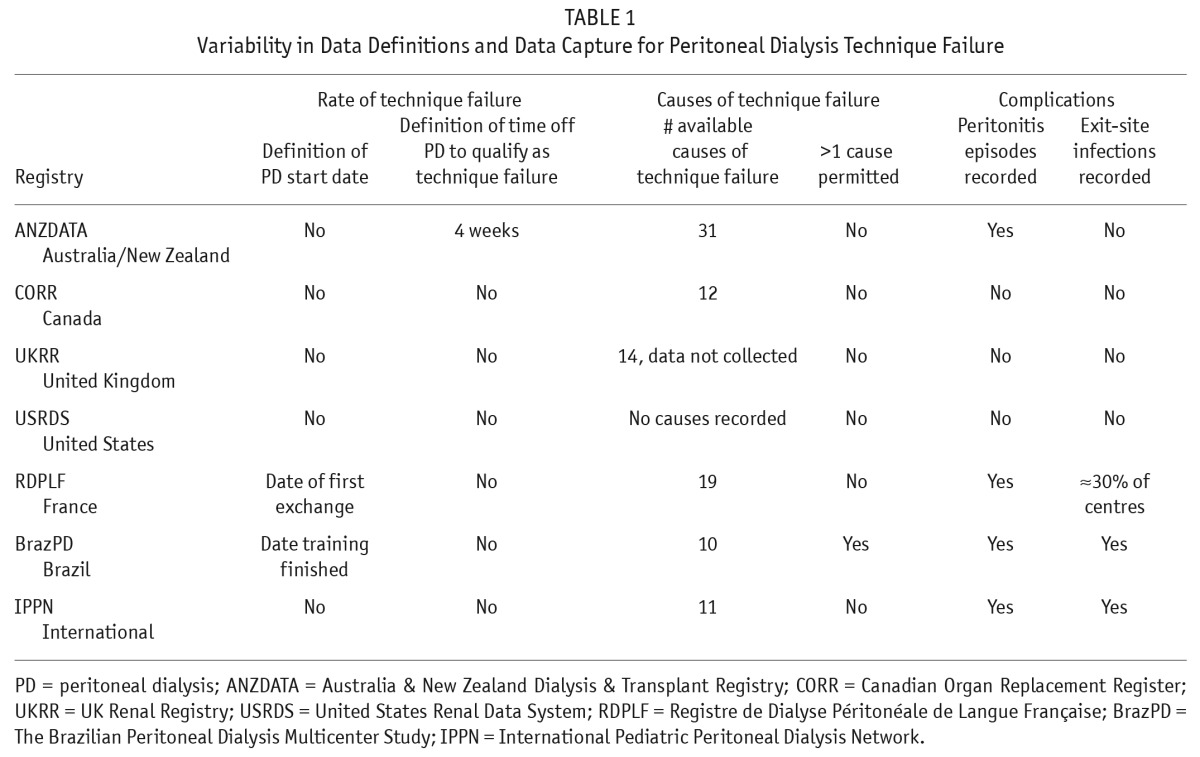
Variability also exists in analytic approaches for describing PD technique failure. Employing a definition of technique failure as ‘’any PD-related complication that leads to the permanent cessation of the therapy” can lead to the use of a combined outcome of either PD technique failure or death while on PD therapy. These analyses of combined outcomes can be used to identify practices that lead to improved patient outcomes overall. However, combined outcomes may be less useful when attempting to identify practices that contribute to specific causes of PD technique failure but may not directly contribute to death while still on PD. To properly account for informative censoring, analyses of cause-specific PD failure should be performed with techniques such as inverse weighting to account for the relationship between PD failure and death (12,37).
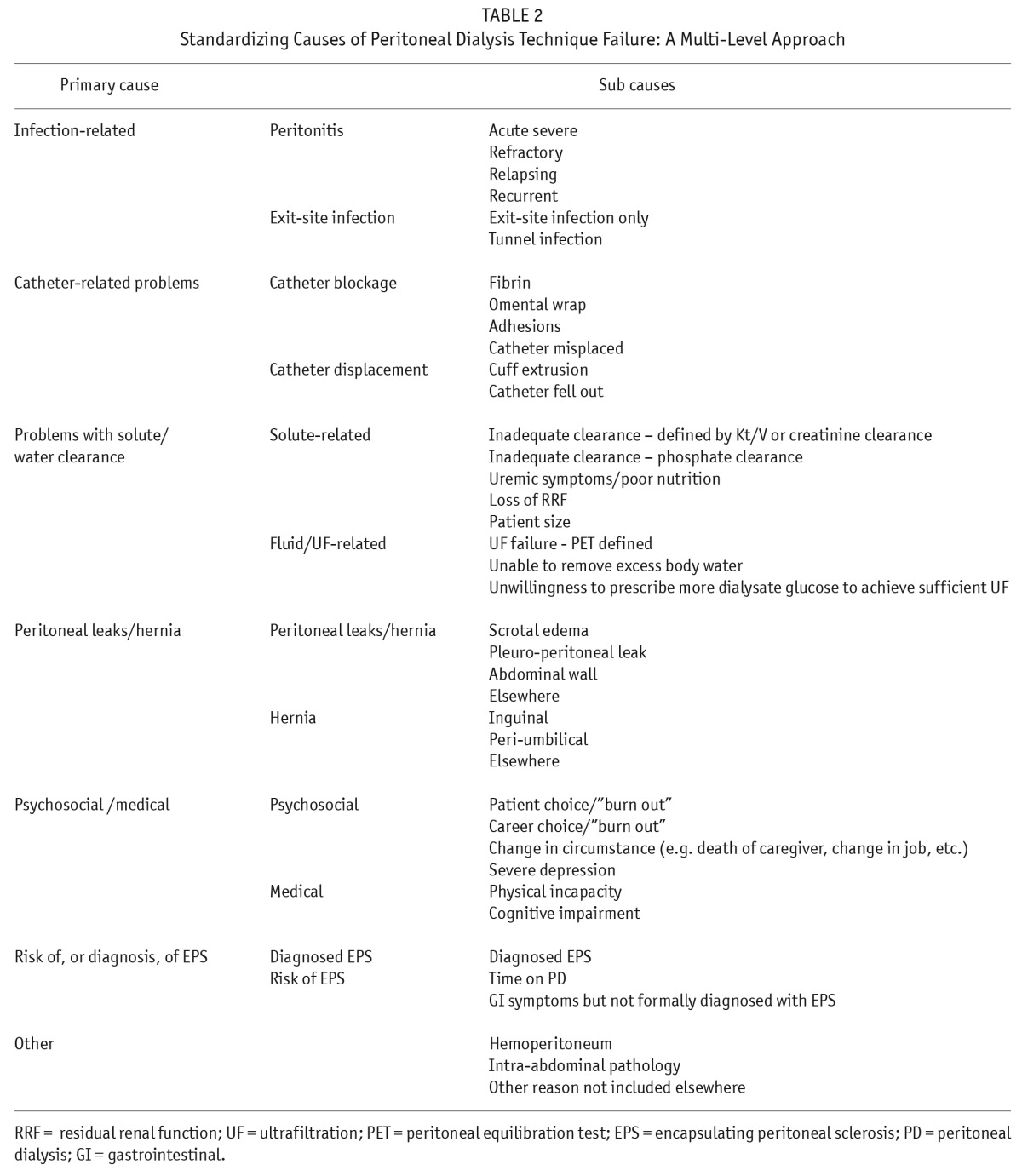
In collaboration with the ISPD, PDOPPS aims to develop consensus definitions and nomenclature for PD technique failure, and a standard set of approaches to analyze the risk of PD technique failure and its component causes. It is our hope that these definitions and analytic approaches can be broadly applied across all PD-related research and contribute to standardizing international registry data collection and reporting. Within this framework, a multi-level approach to technique failure, endorsed by the ISPD PDOPPS committee, was developed with identification of 7 primary causes of technique failure (Table 2) (36). Within these primary causes, additional detailed sub-causes were elaborated. Detailed ascertainment of the cause and component sub-causes will be ascertained for each case of technique failure. Documentation of additional secondary and tertiary causes will also be included.
TABLE 2.
Standardizing Causes of Peritoneal Dialysis Technique Failure: A Multi-Level Approach
3) Significant Practice Pattern Variability Exists Between PD Centers and Across Countries
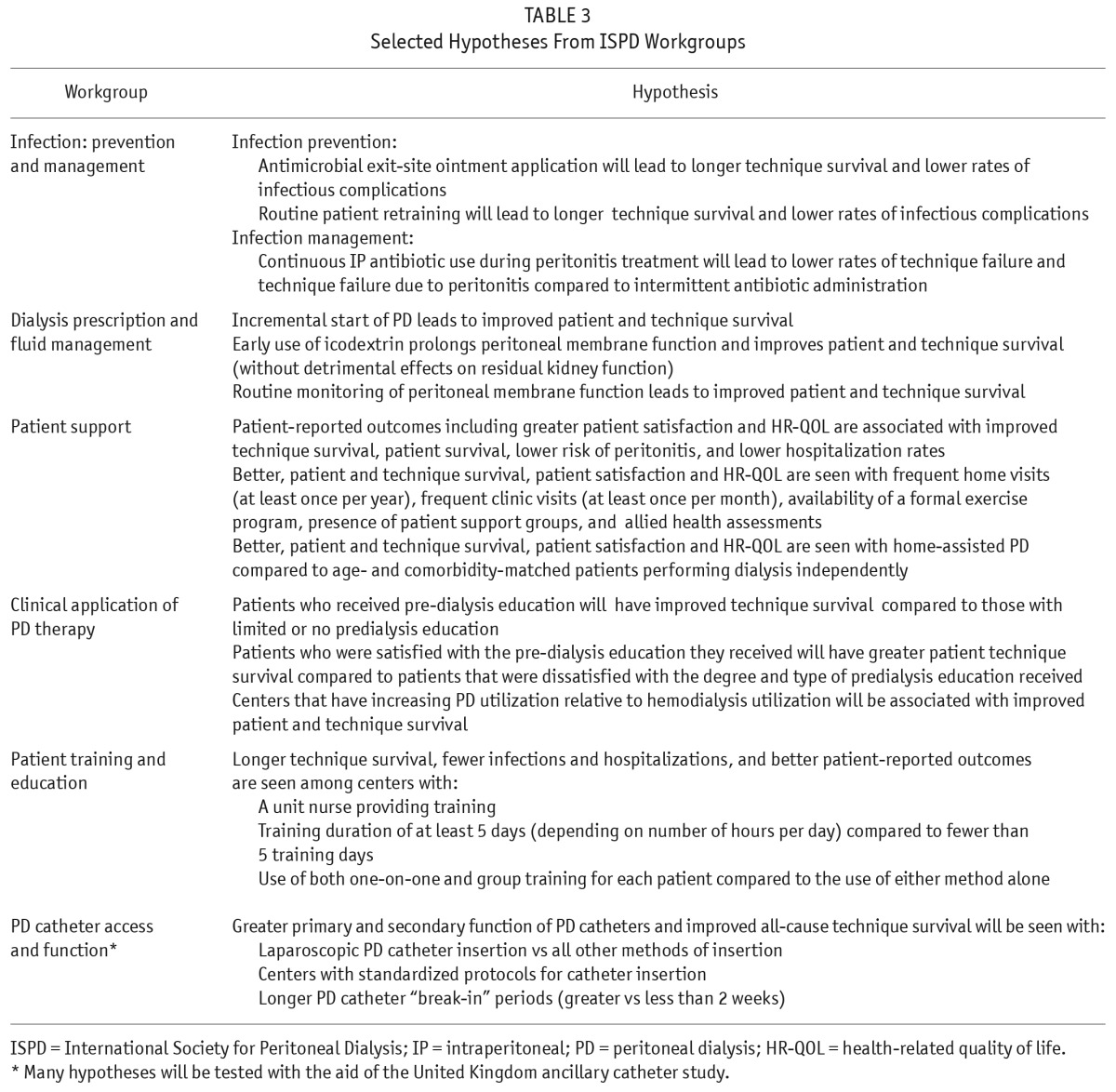
Evidence supporting many PD treatment practices is weak and therefore, significant variability exists across several key domains of practice. Moreover, the impact of such practice pattern variability on patient outcomes remains uncertain. As a result, in collaboration with the ISPD, the PDOPPS Steering Committee established 6 workgroups comprised of international leaders in PD research to study key domains of PD clinical practice, guideline development, and variation in practice. Hypotheses have been developed by each workgroup (Table 3), and research questions of highest priority were identified for testing via the PDOPPS study.
TABLE 3.
Selected Hypotheses From ISPD Workgroups
Methods
Study Synopsis and Timeline
The PDOPPS is a prospective cohort study that applies the research design and methods used in the DOPPS and aims to recruit at least 5,000 adults in the 5 initial countries. These countries provide geographic diversity, large variation in practices and outcomes, and relatively large numbers of PD patients. Each country has secured country-level funds for data collection. Additional countries are under consideration, pending identification of appropriate funding. Recruitment started in the fourth quarter of 2013 with an estimated initial study duration of 3 years.
Selection of Centers
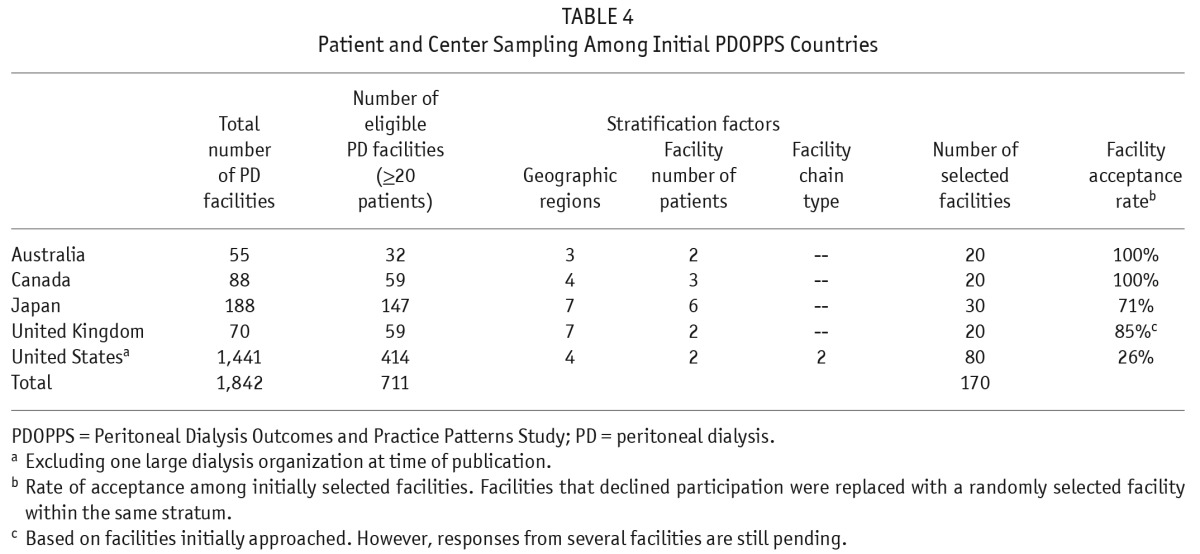
Initially, a sampling frame of all centers with at least 20 PD patients was constructed based on data from all the participating countries. Within each country a minimum of 20 centers were selected from this sampling frame, stratified by geographic region and center size (Table 4). This requirement of a minimum of 20 study sites per country is similar to the DOPPS approach that has yielded samples representative of national data (18,38). Although sites are initially randomly selected, those that decline to participate are replaced with sites falling within similar sampling strata, which may limit the national representativeness of the sample. However, as in the HD DOPPS study, acceptance rates have traditionally been high across the majority of countries, but lower in the United States (Table 4), and US data therefore need to be interpreted within the context of this limitation. An additional limitation in developing a nationally representative sample in the US is the lack of participation in PDOPPS at the time of publication by 1 of the 2 largest dialysis organizations in the US.
TABLE 4.
Patient and Center Sampling Among Initial PDOPPS Countries
Participant Selection and Follow-Up
At study start, centers complete a census of all PD patients. A random sample of 20 – 30 prevalent patients is drawn from each center census independent of the center's size. The sampling at the largest centers included more patients for improved estimates. This plan represents a substantial improvement over 1) a sampling plan that is completely proportionate to a center's size, which would have very small samples at small centers, making estimates of their practices unstable, or 2) equal-sized samples in every center, which would limit the sample to the number of patients at the smallest participating center.
Prevalent patients are defined as patients receiving PD at home (or at a nursing home) under the care of the center at the time of the census creation. All participants must be at least 18 years of age and receiving chronic maintenance PD. Random selection from the whole patient population should provide a representative sample of PD patients at the center and limits potential selection bias.
In addition, a sample of consecutive incident patients will be enrolled until a maximum of 25 incident patients per site is reached. Incident PD patients are defined as patients new to PD (first PD treatment at home within 30 days of the date recorded on the PDOPPS census) and having received at least one PD treatment outside of the center, either at home or in a nursing home under the center's care.
Enrolled patients will be followed until study end or until a terminal event (death, kidney transplantation, transfer to another dialysis center, recovery of kidney function so as to not require maintenance dialysis therapy, permanent (> 4 months) transfer to another dialysis modality with the discontinuation of PD). Some patients in Japan and other countries receive both PD and HD (i.e., hybrid therapy), and these patients will be followed until PD is permanently discontinued, defined as an initially permanent transition to HD or at least 4 months of HD with an initially intended temporary transition (see section on country specific considerations). For patients who leave the study, vital status and modality status will be determined 60 days after study departure. Annually, patients departing the study will be replaced by a random selection of patients having joined the center since the last sampling period. For all patients, informed consent will be obtained after the center has undergone local/national ethics board approval in accordance with the Declaration of Helsinki.
Study Data and Data Collection Instruments
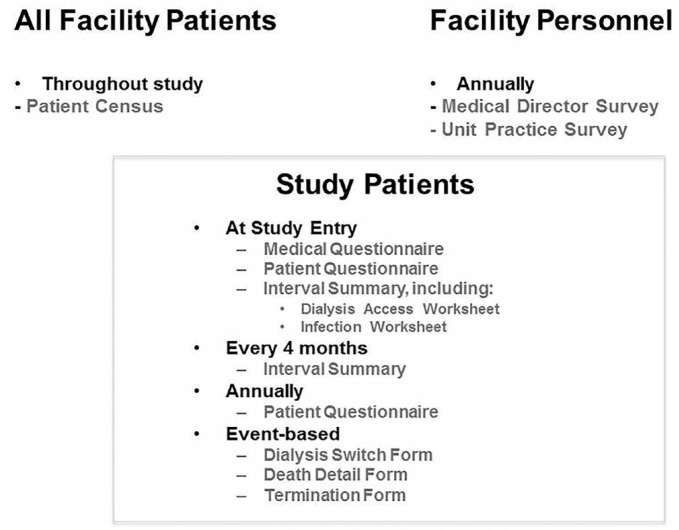
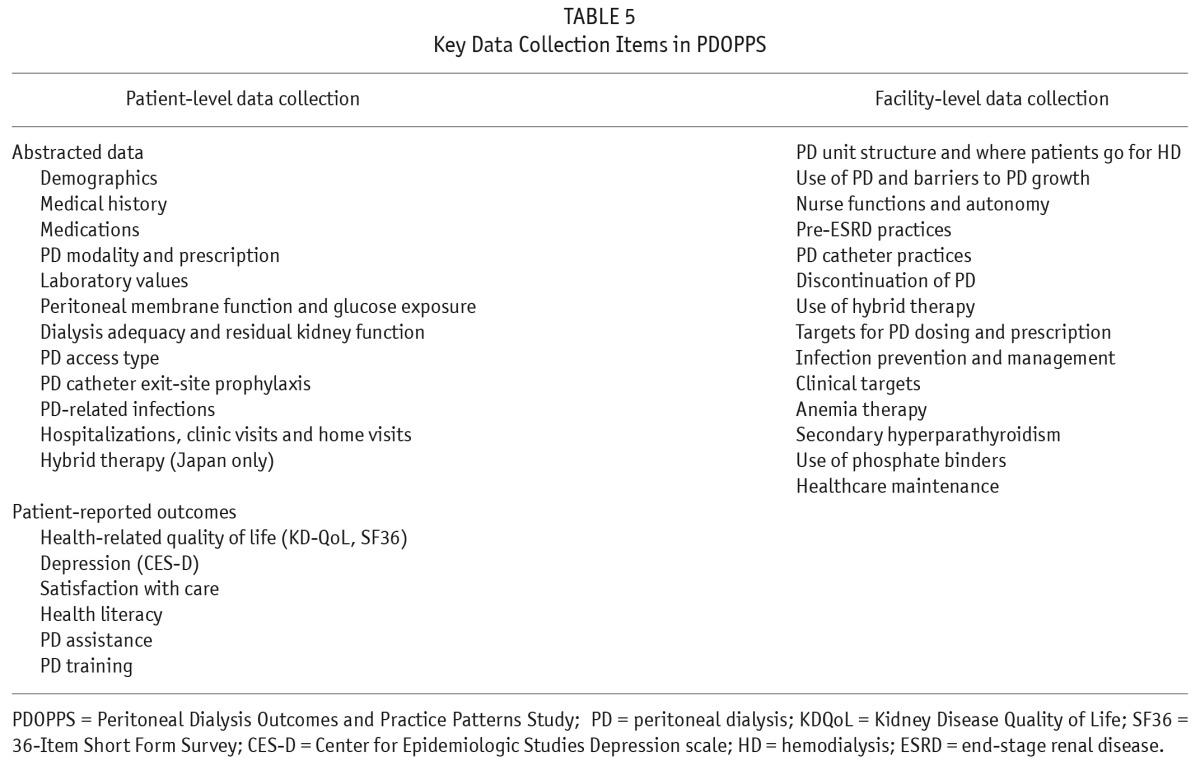
The extensive PDOPPS data collection occurs at both the patient level and center level (Figure 2). Patient-level data collection involves demographic information, comorbidities and medical history, medication use, and PD treatment. This information is updated every 4 months, including the capture of PD treatment changes and events, PD-related infections, hospitalization episodes, and PD access-related events and procedures. A questionnaire is also completed annually by the patient that includes a standardized assessment of quality of life, patient satisfaction, and an assessment of other key patient reported outcomes. Once per year, center-level questionnaires are completed by the Medical Director and Nurse Manager (Table 5) to assess specific center practices and targets, clinical opinion, center environmental elements (e.g. staffing, local reimbursement policies, quality improvement programs, etc.), and other factors.
Figure 2 —
Structure of the PDOPPS data collection instruments. PDOPPS = Peritoneal Dialysis Outcomes and Practice Patterns Study.
TABLE 5.
Key Data Collection Items in PDOPPS
Data quality is maintained through standardized protocols, data collection forms, training, and close interaction of data-reporting staff at each study site, the PDOPPS data coordinating center, and a local clinical research associate who will interact closely with study sites in their local language. All data are entered at each site electronically into a customized web-based data entry system (PDOPPSLink), programmed with consistency checks, and data are transmitted electronically to the data coordinating center at Arbor Research Collaborative for Health.
Data collection instruments were developed by workgroups comprised of international leaders in PD research, established by the ISPD and the PDOPPS Steering Committee. Collection of census and patient-level data, via the PDOPPS web-based data collection system (PDOPPSLink), was assessed through a 2-month pilot phase that enrolled a small sample of both incident and prevalent patients from selected pilot sites.
Analytic Methods
Associations between outcomes (e.g., economic, intermediate such as laboratory values, and clinical outcomes) and practice indicators or processes of care will be analyzed at the patient level and, if appropriate, at the center level. Most data will be analyzed at both the patient and center levels, providing the opportunity to monitor practice and policy changes and their effects on clinical outcomes such as time on PD therapy, mortality, hospitalization, and quality of life. The analyses will use Poisson distribution, logistic regression, proportional hazards, log-linear, or linear regression models, as appropriate, controlling for patient demographic and comorbidity differences. Patient-level left-truncated proportional hazards models may be used to model time to death, time to technique failure, and the combined primary outcome. Models for patient-level analyses will account for the effects of center-level clustering on the variability between patients through either random effects models with linear, logit, log links, or robust standard estimates based on the sandwich estimator for proportional hazards models. Analyses of longitudinal trends in patient characteristics, practice indicators, and outcomes will use repeated measures models to account for serial measures over successive years (time-dependent covariates). In many instances, depending on the event of interest, analyses will use techniques to account for competing risks, which may include techniques such as multivariable competing risks modeling, as well as inverse probability weighting (37,39). In order to further address bias introduced by unmeasured confounders, we will also apply instrumental-variable analyses as in other published DOPPS research regarding HD practices (23).
Power Calculations
Power calculations assume that there will be an average of 3 years of study follow-up in PDOPPS centers, with an average of 10% annual loss to follow-up because of delays in recruitment and replacement. Other losses (e.g., transition to HD, transplantation, etc.) will be accounted for by recruitment of replacement patients. With these assumptions, PDOPPS has 80% power to detect an association between a clinical practice experienced by half of the centers (e.g. a comparison of centers above versus below the median of a continuous measure) with various clinical outcomes. This will provide a hazard ratio in the range of 1.1 – 1.2 for the combined outcome of technique failure/mortality (1.3 for mortality alone), hospitalization, or peritonitis in analyses of the entire cohort. Within-country analyses would have sufficient power to detect hazard ratios closer to 1.5 – 1.9 for outcomes other than mortality alone.
Country Specific Considerations
Across the initial PDOPPS countries, efforts are underway to work with local experts to localize and customize the questionnaires to cultural diversity, health care, financial reimbursement systems, national practice guidelines, etc. to ensure the questionnaires are culturally and nationally relevant and easily understood.
Given this framework, opportunities exist to develop country-specific data collection modules to test country-specific hypotheses and to explore unique policies and distinct practices specific to a participating country. For example, in 2011, of the 9,138 prevalent PD patients in Japan, approximately 19% were receiving some form of hybrid therapy (http://www.jsdt.or.jp) (40). To better understand utilization and outcomes associated with this practice, patients who transition from PD to hybrid therapy will continue to be observed over the course of the study. Moreover, specific questions related to the clinical indications, prescription and outcomes with hybrid therapy have been included as part of center-level and patient-level PDOPPS questionnaires in Japan.
Study Oversight and Guidance
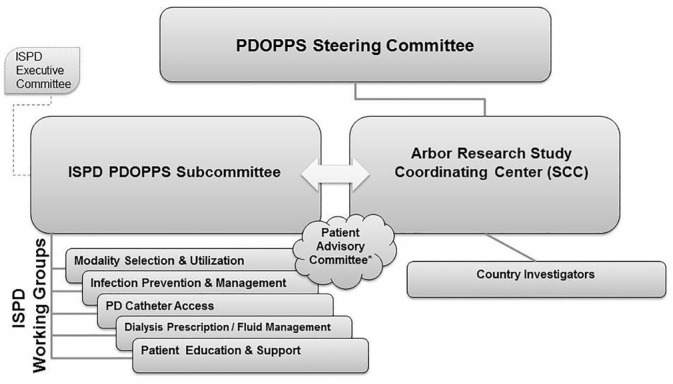
The PDOPPS is a collaborative study between Arbor Research Collaborative for Health, the ISPD, and a consortium of industry sponsors without restrictions on publications, as well as local country investigators (Figure 3). Representation from each group has been aligned to form the study's steering committee. The PDOPPS Steering Committee purview includes: 1) scientific and operational oversight in conjunction with feedback from country investigators, ISPD workgroups and other advisory groups (e.g., PDOPPS US Advisory Group, the Japanese Society for Peritoneal Dialysis [JSPD], PDOPPS committee), and patient groups in different countries; 2) approval of ancillary studies, with guidance from an Ancillary Studies Committee; 3) approval of research and manuscripts intended for peer-reviewed publication, as guided by the study's publication policy; 4) review and approval of new country-level participation in the study by evaluating funding support, operational feasibility, and alignment with overall study goals; and 5) promotion of PDOPPS as a resource for diverse stakeholders across the PD community.
Figure 3 —
Scientific leadership. ISPD = International Society for Peritoneal Dialysis; PDOPPS = Peritoneal Dialysis Outcomes and Practice Patterns Study; PD = peritoneal dialysis.
Opportunities for Expansion Beyond Currently Participating Countries
Expansion of the PDOPPS countries beyond the initial 5 could significantly enhance the scientific merits and reach of PDOPPS. In addition to the broader value of contributing to the international study, participation in PDOPPS is expected to yield direct benefits to participating countries, such as enrollment of a national sample of PD centers that will facilitate regional and national comparisons to understand local variation in PD practices and outcomes. This should also afford a perspective of trends over time and international comparisons for benchmarking purposes. These findings can directly inform local practice guidelines, quality improvement initiatives, and policy. Country investigators are encouraged to formulate research hypotheses that are both relevant to local practice and may not be easily addressed within the country alone. Collection of additional data elements specific to a participating country can be considered, as described above. Furthermore, PDOPPS data can be used to evaluate the reliability of national and regional registry data, as well as providing a forum to expand upon such data by collecting patient-reported outcomes that may be particularly germane to the needs of the country.
Raising funds locally is the most efficient route to participation in PDOPPS. Countries obtaining full funding are very likely to be approved by the PDOPPS Steering Committee for study participation. Further details for interested country investigators are at http://www.ispd.org.
Opportunities for Ancillary Studies
The international reach of the PDOPPS, coupled with the central and well-established infrastructure for data collection and analysis across the DOPPS Program, has created a unique opportunity for the development and implementation of investigator-initiated ancillary studies. Ancillary study approval is at the discretion of the PDOPPS Steering Committee and may include proposals for new PDOPPS data collection and/or analyses of existing PDOPPS data.
To date, there have been 3 successfully funded ancillary studies. The first is “The Empowering Patients On Choices for Renal Replacement Therapy Study” (EPOCH-RRT) funded by the Patient Centered Outcomes Institute (PCORI) in the US (www.choosingdialysis.org). The EPOCH-RRT primarily aims to compare the effectiveness of HD and PD with respect to patient-centered outcomes. The EPOCH-RRT will collect this information from PDOPPS, DOPPS (HD), and advanced chronic kidney disease patients using modules developed by formal qualitative research methods. Findings will be incorporated into a decision tool for patients faced with selection of dialysis modality.
The second ancillary study, called Biological Determinants of Peritoneal Dialysis Outcomes (BIO-PD), is funded by the National Institutes of Health (NIH) (Ancillary Studies to Large Ongoing Clinical Projects) in the US. This study seeks to establish a genetic and bio-repository of plasma and dialysate effluent among patients in Canada, the UK, and the United States to better understand genetic determinants of baseline and longitudinal changes in peritoneal membrane function. It will also draw on additional established bio-banked material from other cohort studies (e.g. the UK's National Institute of Health Research [NIHR] funded PD-CRAFT study) http://www.keele.ac.uk/pd-craft/.
The third ancillary study, “Optimising Early Dialysis Catheter Function,” is funded by the NIHR in the UK. The study is motivated by marked variation in success of PD catheter placement across centers in the UK, and will seek to better understand the determinants of early PD access function and means for improved outcomes. The study will also help inform future data collection instruments relating to PDOPPS in the core study. The study will enroll participants immediately prior to PD access insertion, followed by enrollment into PDOPPS at initiation of home dialysis.
Together, the core PDOPPS study and its ancillary studies should bring success and excitement in understanding practices associated with better outcomes in PD. Central to the success of PDOPPS in participating countries is local engagement of patient advisors (e.g., for EPOCH in the US and the PD access ancillary study in the UK) where patient advisors have been actively engaged in the studies' design and implementation. In other PDOPPS countries, similar plans are underway to recruit patient advisory groups. Diverse opportunities exist for other ancillary studies (http://www.ispd.org), which are encouraged and stand to strengthen the reach and impact of the PDOPPS initiative.
Summary
The PDOPPS is a visible resource to the PD community, providing a much needed infrastructure and forum to promote effective international collaborative clinical research. The detail and breadth of data collected uniformly across participating countries will serve as an extremely valuable source of PD practice and outcomes data. Launch is effectively underway in 5 countries to date. Based on the long-term success of the DOPPS across more than 20 countries, the introduction of PDOPPS has been met with enthusiasm in efforts to understand and improve outcomes for patients on PD worldwide.
Disclosures
JP has received speaking honoraria from Baxter Healthcare, Amgen Canada and has consulting fees from Baxter Healthcare, Shire, and Takeda as well as previous research support from Baxter Healthcare and currently receives salary support from Arbor Research Collaborative for Health. DWJ has previously received consultancy fees, speakers' honoraria, travel sponsorship, and research funds from Baxter Healthcare and Fresenius Medical Care. He is a current recipient of a Queensland Government Health Research Fellowship. SJD has received research funding from Baxter Healthcare and Fresenius Medical Care and serves on an advisory panel and has received a speakers honoraria from Fresenius Medical Care. JAS and SP are employees of Baxter Healthcare Corporation. FT is supported in part by Award Number K01DK087762 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. FT has received honoraria from Amgen, Dialysis Clinic Inc. and Renal Research Institute. BMR has received a speaker's fee from Kyowa Hakko Kirin. RLP has received speaker's fees from Amgen, Kyowa Hakko Kirin, and Vifor and has served on an advisory panel for Merck. The other authors declare that they have no other relevant financial interests.
The PDOPPS is supported by Baxter Healthcare, the Japanese Society for Peritoneal Dialysis (JSPD), Canadian Institutes of Health Research (CIHR), and the National Health & Medical Research Council (NHMRC) in Australia. The DOPPS Program is supported by Amgen, Kyowa Hakko Kirin, AbbVie Inc., Sanofi Renal, Baxter Healthcare, and Vifor Fresenius Medical Care Renal Pharma, Ltd. Additional support is provided for specific projects and countries and described at www.DOPPS.org. All support is provided without restrictions on publications.
Supplementary Material
Acknowledgments
The authors would like to thank participating center medical directors, nurses, adminstrators, and research coordinators as well as the following individuals for their invaluable contributions in the design, implementation, and questionnaire development for the PDOPPS study: Arbor Research Collaborative for Health: Doug Fuller, Kristen Jones, Stephen Kress, Lalita Subramanian, Sayori Suda-Wilson. International Society for Peritoneal Dialysis workgroups (*workgroup leader): Clinical application of PD therapy: Raj Mehrotra*, Mathew Oliver, Helen Hurst, Rachael Morton, Alfonso Cueto Manzano, Dusit Lumlertgul; PD catheter access and function: Martin Wilkie*, Richard Fluck, Bak Leong Goh, Elaine Bowes, John Crabtree, Mark Marshall, Isaac Teitelbaum; Infection: prevention and management: David Johnson*, Fiona Brown, Beth Piraino, Judy Bernadini, Sharon Nessim, Neil Boudville, CC Szeto, John Collins; Dialysis prescription and fluid management: Angela Wang*, Simon J Davies*, Graham Woodrow, Andreas Vychytil, Wim Van Biesen, Hideki Kawanishi, Thyago de Moraes, Gillian Brunier; Patient training and education: Ana Figueiredo*, Valerie Price; Patient support: Fred Finkelstein*, Edwina Brown, Vanita Jassal, Fang Wei, Susanne Ljungman. Country Investigational Teams (*country principal investigator): Australia: David Johnson*, Sunil Badve, Neil Boudville, Fiona Brown, Stephen Holt, Peter Kerr, Laura Robison, Rowan Walker; Canada: Jeffrey Perl*, Denise Gaudet, Arsh Jain, Brendan Mcormick, Sharon J Nessim, Rob Quinn, Andrea Rathe, Manish Sood; Japan: Hideki Kawanishi*, Tadashi Tomo, Hidetomo Nakamoto, Kenji Tsuchida, Ikuto Masakane, Akihiro Yamashita, Susumu Takahashi, Kousaku Nitta; United Kingdom: Simon J Davies*, Fergus Caskey, Mark Lambie, Hugh Rayner, Martin Wilkie; United States (Advisory Group): John Burkart, Kristin Corapi, Dugan Maddux, Raj Mehrotra, Joseph Pulliam, James A Sloand.
Footnotes
Supplemental material available at www.pdiconnect.com
REFERENCES
- 1. Rubin HR, Fink NE, Plantinga LC, Sadler JH, Kliger AS, Powe NR. Patient ratings of dialysis care with peritoneal dialysis vs hemodialysis. JAMA 2004; 291:697–703. [DOI] [PubMed] [Google Scholar]
- 2. Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 2000; 11:556-64. [DOI] [PubMed] [Google Scholar]
- 3. Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 2002; 62:1046–53. [DOI] [PubMed] [Google Scholar]
- 4. Lysaght MJ, Vonesh EF, Gotch F, Ibels L, Keen M, Lindholm B, et al. The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans 1991; 37:598–604. [PubMed] [Google Scholar]
- 5. McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol 2009; 20:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perl J, Wald R, McFarlane P, Bargman JM, Vonesh E, Na Y, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol 2011; 22:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011; 171:110–8. [DOI] [PubMed] [Google Scholar]
- 8. Klarenbach S, Manns B. Economic evaluation of dialysis therapies. Semin Nephrol 2009; 29:524–32. [DOI] [PubMed] [Google Scholar]
- 9. Liem YS, Wong JB, Hunink MG, de Charro FT, Winkelmayer WC. Comparison of hemodialysis and peritoneal dialysis survival in the Netherlands. Kidney Int 2007; 71:153–8. [DOI] [PubMed] [Google Scholar]
- 10. Quinn RR, Hux JE, Oliver MJ, Austin PC, Tonelli M, Laupacis A. Selection bias explains apparent differential mortality between dialysis modalities. J Am Soc Nephrol 2011; 22:1534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 2012; 23:533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perl J, Wald R, Bargman JM, Na Y, Jassal SV, Jain AK, et al. Changes in patient and technique survival over time among incident peritoneal dialysis patients in Canada. Clin J Am Soc Nephrol 2012; 7:1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehrotra R, Kermah D, Fried L, Kalantar-Zadeh K, Khawar O, Norris K, et al. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J Am Soc Nephrol 2007; 18:2781–8. [DOI] [PubMed] [Google Scholar]
- 14. Maiorca R, Vonesh E, Cancarini GC, Cantaluppi A, Manili L, Brunori G, et al. A six-year comparison of patient and technique survivals in CAPD and HD. Kidney Int 1988; 34:518–24. [DOI] [PubMed] [Google Scholar]
- 15. Serkes KD, Blagg CR, Nolph KD, Vonesh EF, Shapiro F. Comparison of patient and technique survival in continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis: a multicenter study. Perit Dial Int 1990; 10:15–9. [PubMed] [Google Scholar]
- 16. Chui BK, Manns B, Pannu N, Dong J, Wiebe N, Jindal K, et al. Health care costs of peritoneal dialysis technique failure and dialysis modality switching. Am J Kidney Dis 2013; 61:104–11. [DOI] [PubMed] [Google Scholar]
- 17. Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis 2004; 44:7–15. [DOI] [PubMed] [Google Scholar]
- 18. Robinson B, Fuller D, Zinsser D, Albert J, Gillespie B, Tentori F, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS) Practice Monitor: rationale and methods for an initiative to monitor the new US bundled dialysis payment system. Am J Kidney Dis 2011; 57:822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robinson BM, Bieber B, Pisoni RL, Port FK. Dialysis Outcomes and Practice Patterns Study (DOPPS): its strengths, limitations, and role in informing practices and policies. Clin J Am Soc Nephrol 2012; 7:1897–905. [DOI] [PubMed] [Google Scholar]
- 20. Tentori F, Fuller DS, Port FK, Bieber BA, Robinson BM, Pisoni RL. The DOPPS Practice Monitor for US dialysis care: potential impact of recent guidelines and regulatory changes on management of mineral and bone disorder among US hemodialysis patients. Am J Kidney Dis 2014; 63:851–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hecking M, Karaboyas A, Saran R, Sen A, Horl WH, Pisoni RL, et al. Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2012; 59:238–48. [DOI] [PubMed] [Google Scholar]
- 22. Jadoul M, Thumma J, Fuller DS, Tentori F, Li Y, Morgenstern H, et al. Modifiable practices associated with sudden death among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Clin J Am Soc Nephrol 2012; 7:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis 2009; 53:475–91. [DOI] [PubMed] [Google Scholar]
- 24. Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int 2002; 61:305–16. [DOI] [PubMed] [Google Scholar]
- 25. Okamoto K, Kobayashi S, Noiri E. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2006; 70:1877; author reply 1877–8. [DOI] [PubMed] [Google Scholar]
- 26. Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int 2006; 69:1222–8. [DOI] [PubMed] [Google Scholar]
- 27. de Oliveira RB, Lopes AA, Sesso R, de Campos LG, Mariani L, Lugon JR, et al. The Chronic Kidney Disease Outcomes and Practice Patterns Study Brazil (CKDopps-Brazil): design, data and methodology. J Bras Nefrol 2014; 36:96–101. [DOI] [PubMed] [Google Scholar]
- 28. Nakamoto H, Kawaguchi Y, Suzuki H. Is technique survival on peritoneal dialysis better in Japan? Perit Dial Int 2006; 26:136–43. [PubMed] [Google Scholar]
- 29. Jose MD, Johnson DW, Mudge DW, Tranaeus A, Voss D, Walker R, et al. Peritoneal dialysis practice in Australia and New Zealand: a call to action. Nephrology (Carlton) 2011; 16:19–29. [DOI] [PubMed] [Google Scholar]
- 30. Tangri N, Ansell D, Naimark D. Predicting technique survival in peritoneal dialysis patients: comparing artificial neural networks and logistic regression. Nephrol Dial Transplant 2008; 23:2972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Afolalu B, Troidle L, Osayimwen O, Bhargava J, Kitsen J, Finkelstein FO. Technique failure and center size in a large cohort of peritoneal dialysis patients in a defined geographic area. Perit Dial Int 2009; 29:292–6. [PubMed] [Google Scholar]
- 32. Schaubel DE, Blake PG, Fenton SS. Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int 2001; 60:1517–24. [DOI] [PubMed] [Google Scholar]
- 33. Huisman RM, Nieuwenhuizen MG, Th de Charro F. Patient-related and centre-related factors influencing technique survival of peritoneal dialysis in the Netherlands. Nephrol Dial Transplant 2002; 17:1655–60. [DOI] [PubMed] [Google Scholar]
- 34. Schaubel DE, Blake PG, Fenton SS. Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int 2001; 60:1517–24. [DOI] [PubMed] [Google Scholar]
- 35. Allen N, Schwartz D, Sood AR, Mendelssohn D, Verrelli M, Tanna G, et al. Perceived barriers to guidelines in peritoneal dialysis. Nephrol Dial Transplant 2011; 26:1683–9. [DOI] [PubMed] [Google Scholar]
- 36. Lambie M, Davies S, Perl J, Johnson D, Wilkie M, Pisoni R, et al. Standardising data definitions for peritoneal dialysis (PD):progress towards P-DOPPS. J Am Soc Nephrol 2012; 23:54A. [Google Scholar]
- 37. Schaubel DE, Wei G. Double inverse-weighted estimation of cumulative treatment effects under nonproportional hazards and dependent censoring. Biometrics 2011; 67:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mendelssohn DC, Ethier J, Elder SJ, Saran R, Port FK, Pisoni RL. Haemodialysis vascular access problems in Canada: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS II). Nephrol Dial Transplant 2006; 21:721–8. [DOI] [PubMed] [Google Scholar]
- 39. Zhou B, Fine J, Latouche A, Labopin M. Competing risks regression for clustered data. Biostatistics 2012; 13:371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kawanishi H, Moriishi M, Katsutani S, Sakikubo E, Tsuchiya S. Hemodialysis together with peritoneal dialysis is one of the simplest ways to maintain adequacy in continuous ambulatory peritoneal dialysis. Adv Perit Dial 1999; 15:127–31. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.