Abstract
♦ Background:
To investigate patient survival and technical failure of patients with prior stroke receiving continuous ambulatory peritoneal dialysis (CAPD) in Southern China.
♦ Methods:
This was a retrospective study. All subjects were recruited from the peritoneal dialysis center in The First Affiliated Hospital of Sun Yat-sen University from 1 January 2006 to 31 December 2010. All eligible patients were assigned to stroke group and non-stroke group according to a history of stroke before receiving CAPD. The primary outcomes were all-cause mortality and death-censored technical failure. Cox regression was used to estimate risk factors of all-cause mortality and death-censored technique failure.
♦ Results:
Of the 1,068 recruited patients, 75 (7.0%) patients had a previous history of stroke. The all-cause mortality and death-censored technique failure were significantly higher in the stroke group compared with the non-stroke group, respectively (odds ratio [OR] 2.67, 95% confidence interval [CI] 1.59 – 4.46 and OR 2.52, 95% CI 1.19 – 5.34). Older age (changed by 10 years, hazard ratio [HR] 1.90, 95% CI 1.07 – 3.38), lower body mass index (BMI 18.5 – 23.9 vs < 18.5 kg/m2 reference, HR 0.17, 95% CI 0.05 – 0.55) and time to the first episode of peritonitis (HR 0.93, 95% CI 0.89 – 0.96) were independently associated with increased risk of all-cause mortality in patients with prior stroke. In addition, time to the first episode of peritonitis was associated with decreased risk of death-censored technique failure (HR 0.91, 95% CI 0.84 – 0.99) in those with prior stroke.
♦ Conclusions:
Continuous ambulatory peritoneal dialysis patients with prior stroke had high rates of all-cause mortality and technique failure compared with those without prior stroke. Older age, lower BMI, and time to the first episode of peritonitis were independent risk factors of all-cause mortality in patients with prior stroke.
Keywords: Patient survival, technique failure, continuous ambulatory peritoneal dialysis, stroke
Peritoneal dialysis (PD) is a safe and effective modality of renal replacement therapy, and has been widely accepted for treatment of patients with end-stage renal disease (ERSD) (1). Although patient survival has greatly improved in the past decades, long-term patient survival remains poor, with approximately 11% of PD patients surviving more than 10 years (2). Cardiovascular disease (CVD) is the main cause of death in PD patients, accounting for 40% – 50% of all-cause mortality in both Chinese and Caucasian dialysis populations (3,4). Stroke is the second or third cause of death following cardiac disease, accounting for approximately 10% of death during continuous ambulatory PD (CAPD) therapy (5,6). The incidence of stroke in the dialysis population is 5- to 10-fold higher than that in the general population and is related to a worse prognosis (7–9).
To date, there were only few retrospective studies regarding all-cause mortality in PD patients with pre-existing cardiac diseases. Stack et al. reported that all-cause mortality in PD patients with congestive heart disease was higher than in those without congestive heart disease (10). Ganesh et al. reported that patient survival was significantly lower in PD patients with coronary artery disease than in those without coronary artery disease (11). Compared with patients without stroke, patients with prior stroke tend to be older and are more likely to have cardiac disease, hypertension, and other comorbidities (12,13). However, all-cause mortality in PD patients with prior stroke has received very little attention. In this study, we try to test the hypothesis that CAPD patients with prior stroke may have higher all-cause mortality compared with those without prior stroke.
Methods
Study Population
This was a retrospective single-center study. All eligible participants were recruited from the PD center in The First Affiliated Hospital of Sun Yat-sen University from 1 January 2006 to 31 December 2010. The inclusion criteria were: (i) incident patients who received stable CAPD for 3 months or more; (ii) aged from18 to 80 years old; (iii) no history of kidney transplantation; (iv) not transferred from hemodialysis. Eligible patients were assigned to the stroke group and non-stroke group based on the history of prior stroke presence before CAPD. Conventional PD solutions (Dianeal 1.5%, 2.5% or 4.25% dextrose; Baxter Healthcare, Guangzhou, China), Y-sets, and twin-bag systems were utilized in almost all of the CAPD patients. The study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University. Written informed consent forms were obtained from all participants. All patients were followed up until cessation of PD, death, or until December 31, 2011.
The primary outcomes were all-cause mortality and death-censored technical failure. Baseline characteristics and laboratory measurements in initiation of CAPD included age, gender, body mass index (BMI), measured glomerular filtration rate (mGFR), diabetes, hypertension, pre-existing cardiac diseases (coronary artery disease, myocardial infarction, angioplasty, coronary artery bypass, or congestive heart failure), mean arterial pressure (MAP), cause of end-stage renal disease (ESRD), hemoglobin, high sensitivity C-reactive protein (hs-CRP), serum albumin, cholesterol, triglyceride, high-density lipoprotein (HDL), and low-density lipoprotein (LDL). In addition, time to the first episode of peritonitis was also recorded, which was defined as the time from the initiation of CAPD to the first episode of peritonitis. Kt/v and assisted PD were also recorded during the follow-up periods.
Definitions
Stroke was defined as evidence of an acute disturbance of focal neurological function with symptoms lasting > 24 hours and considered to be due to intracerebral hemorrhage or ischemia as reported by the other investigators (14). Glomerular filtration rate was measured (mGFR) as the mean of urea and creatinine clearance, calculated from 24-hour urine collections and indexed for body surface area. Cardiovascular disease was defined as any diagnosis of coronary artery disease, myocardial infarction, angioplasty, coronary artery bypass, congestive heart failure, cerebrovascular disease, or peripheral arterial disease (15). Hypertension was defined as a systolic blood pressure of ≥ 140 mmHg, diastolic blood pressure of ≥ 90 mmHg, any use of antihypertensive medication in the last 2 weeks or any self-reported history of hypertension. Diabetes was defined as any self-reported history of diabetes, fasting plasma glucose of ≥ 7.0 mmol/L, or by hypoglycemic agents despite normal fasting plasma glucose. Body mass index was calculated as weight (kg)/height2 (m2) and was categorized as < 18.5, 18.5 – 23.9, 24.0 – 27.9, and ≥ 28.0 kg/m2 (16). Assisted PD is defined as a PD modality performed at the patients' home with the assistance of a family member or other assistants. Diagnosis of PD-associated peritonitis was made based on at least 2 of the following criteria: 1) abdominal pain or cloudiness of PD effluent; 2) white blood cell (WBC) count in PD effluent > 100/μL with > 50% polymorphonuclear leukocytes; and 3) a positive culture from PD effluent (17). Death-censored technique failure was defined as transfer to hemodialysis for more than 90 days, and it was censored for death, kidney transplantation, and spontaneous recovery of renal function, move to other center, or “still on CAPD” until December 31, 2011 (18).
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD), whereas categorical variables were presented as percentages or frequencies. Comparison of means was made using Student's t-test or the non-parametric Mann-Whitney test. Comparison of qualitative variables was performed with Pearson's χ2 test. Comparison of peritonitis rate was analyzed using a Poisson model. Time-to-event analysis of all-cause mortality and death-censored technique failure was calculated using Kaplan-Meier curves or Cox proportional hazards model. Factors analyzed by univariate analysis with p < 0.10 were included in multivariate Cox regression. Statistical significance was defined as p < 0.05 using 2-tailed tests. Statistical analyses were performed using SPSS 19.0 software (SPSS, Chicago, IL, USA).
Results
Baseline Characteristics
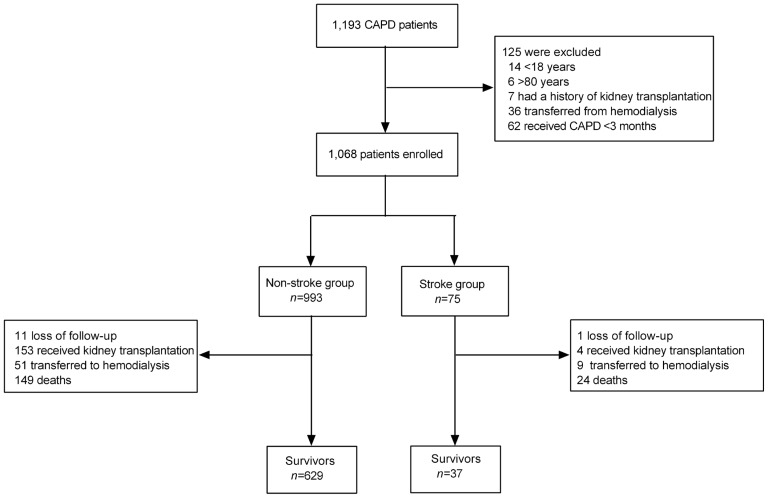
A total of 1,193 ESRD patients were investigated. Of them, 62 (5.2%) patients who received CAPD less than 3 months, 14 (1.2%) patients younger than 18 years old, 6 (0.5%) patients older than 80 years old, 7 (0.6%) patients with a history of kidney transplantation, and 36 (3.0%) patients transferred from hemodialysis before receiving CAPD were excluded. Finally, 1,068 patients who met the inclusion criteria were recruited, with an average age of 48.0 ± 15.4 years old and average PD duration of 27.4 ± 15.6 months. Of these eligible patients, 75 (7.0%) patients had prior stroke (stroke group) and 993 (93.0%) patients had no prior stroke (non-stroke group). The flow diagram is shown in Figure 1.
Figure 1 —
Flow diagram. CAPD = continuous ambulatory peritoneal dialysis.
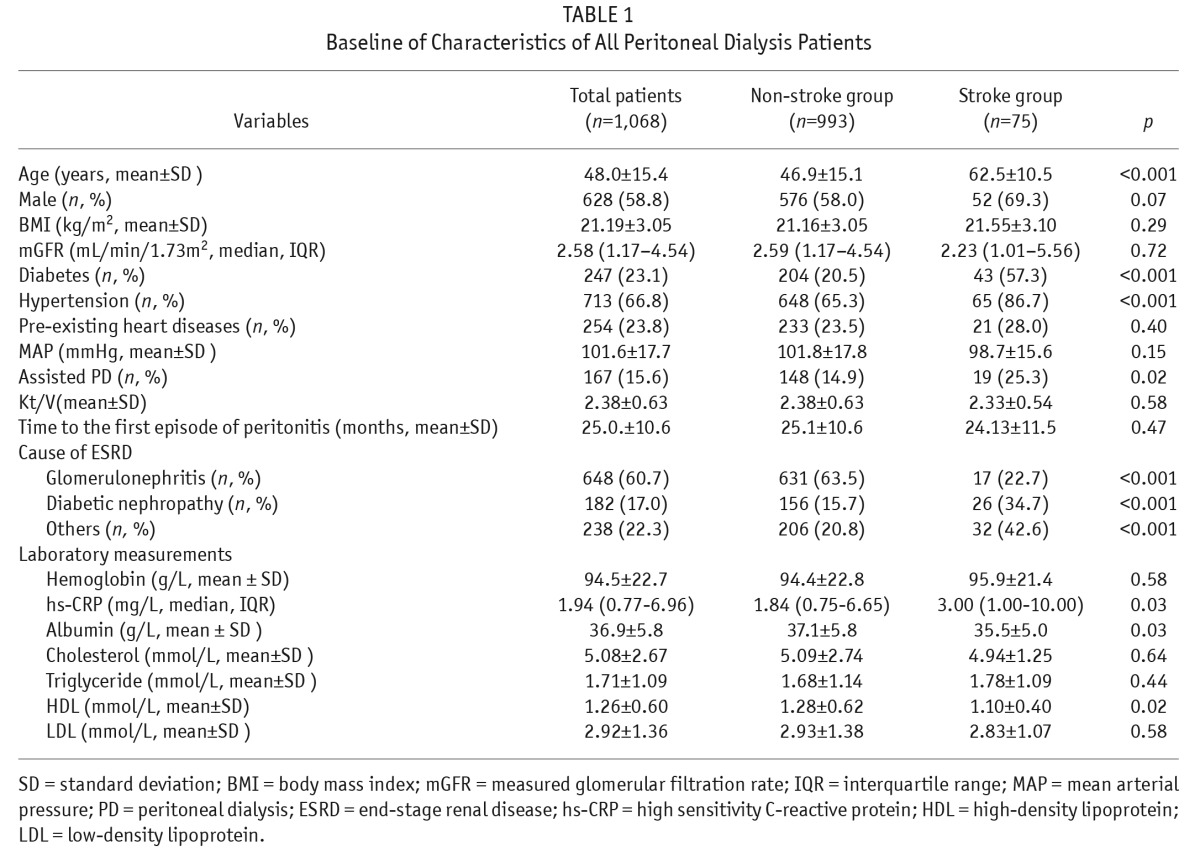
As shown in Table 1, 7.0% patients had a previous diagnosis of stroke, 23.1% with diabetes, 66.8% with hypertension, and 23.8% with pre-existing cardiac disease. Compared with those in the non-stroke group, patients in the prior-stroke group were older (p < 0.001), had a higher proportion of diabetes (p < 0.001), more hypertension (p < 0.001), and more diabetic nephropathy (p < 0.001); had higher hs-CRP (p = 0.03) lower HDL (p = 0.02) and serum albumin (p = 0.03), and less glomerulonephritis as the primary cause of ESRD (p < 0.001). In addition, the percentage of assisted PD was significantly higher in the stroke group compared with the non-stroke group (p = 0.02).
TABLE 1.
Baseline of Characteristics of All Peritoneal Dialysis Patients
All-Cause Mortality and Associated Risk Factors
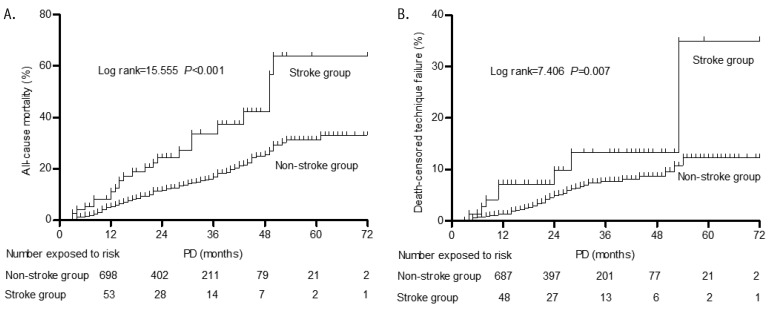
In this study, 173 (16.2%, 70.9/1,000 patient-years) patients died with a mean follow-up time of 21.7 ± 14.1 months. The cause of death is shown in Table 2. Cardiovascular disease (61.8%) was the major cause of death in this study. All-cause mortality rates were 149.8/1000 patient-years (32.0%, 24/75 patients) in the stroke group and 65.4/1000 patient-years (15.0%, 149/993 patients) in the non-stroke group. The all-cause mortality rate was significantly higher in the stroke group compared with the non-stroke group (odds ratio [OR] 2.67 95% confidence interval [CI] 1.59 – 4.46). The cumulative all-cause mortality rate at 1, 2, 3, and 5 years in the stroke group was significantly higher than in the non-stroke group (10% vs 5%, 15% vs 11%, 23% vs 17%, 69% vs 32%, log rank = 15.555, p < 0.001, Figure 2).
TABLE 2.
Cause of Death in All Participants
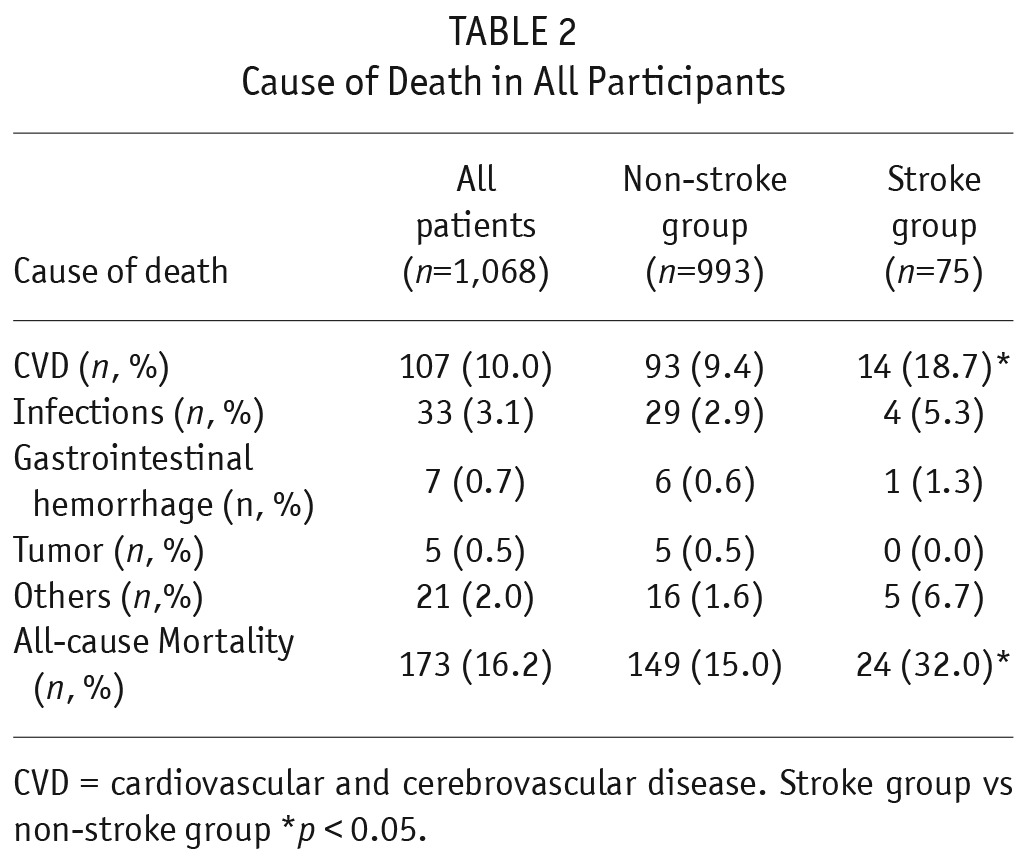
Figure 2 —
Kaplan-Meier curves of patients at risk for all-cause and death-censored technique failure. A: Survival curves for all-cause mortality (log rank=15.555, p<0.001). B: Survival curves for death-censored technique failure (log rank=7.406, p=0.007). PD = peritoneal dialysis.
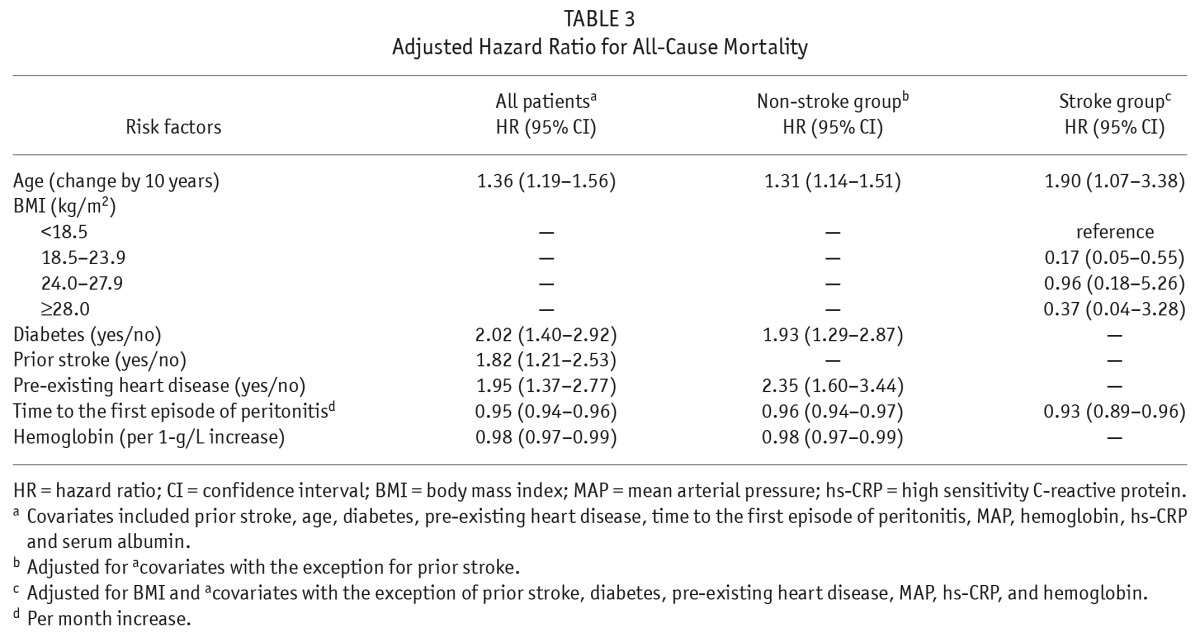
By a univariate analysis, older age, diabetes, prior stroke, pre-existing heart diseases, time to first peritonitis, MAP, lower hemoglobin, higher hs-CRP and lower serum albumin (all p values < 0.05) were determined to be risk factors of all-cause mortality among the total population (Table 3). After adjustment for MAP, hs-CRP, and albumin, older age (increased by 10 years [hazard ratio] HR 1.36, 95% CI 1.19 – 1.56), diabetes (HR 2.02, 95% CI, 1.40 – 2.92), prior stroke (HR 1.82, 95% CI 1.21 – 2.53), pre-existing cardiac disease (HR 1.95, 95% CI 1.37 – 2.77), time to first peritonitis (HR 0.95, 95% CI 0.94 – 0.96), and lower hemoglobin (HR 0.98, 95% CI 0.97 – 0.99) were independently associated with increased risk of all-cause mortality in these patients. Older age, BMI, time to the first episode of peritonitis, MAP and serum albumin (all p values < 0.05) were associated with all-cause mortality in the stroke group using univariate analysis. After adjustment for MAP and serum albumin, older age (changed by 10 years, HR 1.90, 95% CI 1.07 – 3.38), lower BMI (18.5 – 23.9 vs < 18.5 kg/m2 reference, HR 0.17, 95% CI 0.05 – 0.55), and time to the first episode of peritonitis (1-month increase of time to the first episode of peritonitis was associated with a 7% decreased risk of mortality in patients with prior stroke, HR 0.93, 95% CI 0.89 – 0.96) were independent risk factors of all-cause mortality in the stroke group.
TABLE 3.
Adjusted Hazard Ratio for All-Cause Mortality
Death-Censored Technique Failure and Associated Risk Factors
A total of 60 (5.6%, 24.6/1000 patient-years) patients developed death-censored technique failure with a mean follow-up time of 20.9 ± 12.6 months. The causes of technique failure were peritonitis (26 patients, 43.3%), inadequate dialysis (9 patients, 15.0%), ultrafiltration failure (3 patients, 5.0%), thoracoabdominal fistula (3 patients, 5.0%), edema of scrotum (4 patients, 6.7%), patient's choice (8 patients, 13.3%), and other causes (7 patients, 11.7%). Technique failure rates were 56.2/1000 patient-years (12.0%, 9/75) in the patients with previous stroke and 22.4/1000 patient-years (5.1%, 51/993) in the patients without stroke. The death-censored technique failure rate was significantly higher in the stroke group compared with the non-stroke group (OR 2.52, 95% CI 1.19 – 5.34). Of note, the peritonitis rate was 1/60.1 patient-months in the stroke group, whereas it was 1/71.3 patient-months in the non-stroke group (OR 1.12, 95% CI 1.04 – 1.28).
The cumulative death-censored technique failure rates at 1, 2, 3, and 5 years were 7%, 9%, 16%, and 31% in the stroke group, while those in the non-stroke group were 1%, 4%, 7%, and 12%, respectively. The cumulative death-censored technique failure rate was significantly higher in the stroke group compared with the non-stroke group (log rank = 7.406, p = 0.007, Figure 2).
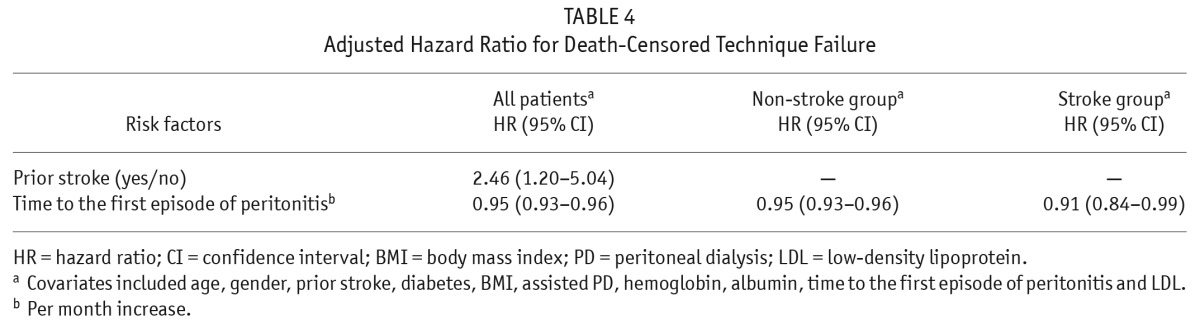
Death-censored technique failure was associated with prior stroke, time to the first episode of peritonitis, and lower LDL (all p values < 0.05) among this study population in a univariate Cox analysis. Furthermore, after adjustment for age, gender, diabetes, BMI, assisted PD, hemoglobin, and albumin, the results showed that prior stroke (HR 2.46, 95% CI 1.20 – 5.04) and time to the first peritonitis (HR 0.95, 95% CI 0.93 – 0.96) were independent risk factors for technique failure in the same study population using multivariate Cox regression analysis (Table 4). In patients with prior stroke, a 1-month increase in the time to the first episode of peritonitis was associated with a 9% decreased risk of death-censored technique failure (HR 0.91, 95% CI 0.84 – 0.99) after adjustment for the same key factors.
TABLE 4.
Adjusted Hazard Ratio for Death-Censored Technique Failure
Discussion
The results of this study show that all-cause mortality and death-censored technique failure were significantly higher in CAPD patients with prior stroke compared with those without prior stroke. Older age, lower BMI, and time to the first episode of peritonitis were independently associated with an increased risk of all-cause mortality in patients with prior stroke.
Han et al. reported that the incidence of all-cause mortality and technique failure was 98.8/1,000 patient-years and 42.8/1,000 patient-years in those patients undergoing conventional dialysis treatments, respectively (19). Kircelli et al. reported that the incidence of all-cause mortality and technique failure was 48.4/1,000 patient-years and 95.5/1,000 patient-years in those PD patients under strict volume control (20). Michels et al. reported that the incidence of all-cause mortality and technique failure was 110/1,000 patient-years and 279/1,000 patient-years in CAPD patients, respectively (21). In our study, the incidence of all-cause mortality and technique failure was 70.9/1,000 patient-years and 24.6/1,000 patient-years in this cohort, respectively. However, the current study results showed that patients with prior stroke had higher incidence of all-cause mortality and technique failure (149.8/1,000 patient years and 56.2/1,000 patient years, respectively), compared with those of patients without prior stroke (65.4/1,000 patient-years and 22.4/1,000 patient-years, respectively).
It is well known that older age is independently associated with increased risk of all-cause mortality in dialysis patients (22). We reported previously that older age may be inversely related to survival in patients with diabetes (23). In the present study, older age was also independently associated with all-cause mortality in all CAPD patients, including those with a history of stroke. Compared with patients without prior stroke, those with prior stroke had advanced age (62.5 ± 10.5 years) and the greatest risk of death (HR 1.90, 95% CI 1.07 – 3.38), which no doubt contributed to the higher rate of mortality for the patients with prior stroke.
Malnutrition is well recognized as a strong risk factor for all-cause mortality in patients on PD (24,25). Several authors have suggested that malnutrition in ESRD patients reflects not merely poor nutrient intake, but also the effects of a chronic inflammatory state, which may contribute to CVD mortality (26,27). In patients on hemodialysis, higher BMI is associated with better survival, whereas lower BMI and weight loss are associated with increased mortality (28,29). In a prospective study, Snyder et al. reported an association between mortality and BMI changes over time, with better survival among overweight and obese patients until the third year of PD (30). These findings are in contrast to the general population, in which obesity, rather than malnutrition, is positively correlated with an increased risk of mortality (31,32). In our study, low mortality rates were observed in stroke PD patients with a BMI of 18.5 – 23.9, compared with those patients with a BMI of < 18.5 kg/m2, 24.0 – 27.9, or ≥ 28.0 kg/m2. But no differences were observed among these 3 groups, including patients with a BMI of < 18.5 kg/m2, 24.0 – 27.9, or ≥ 28.0 kg/m2, which indicated that these patients should keep their body weight in a normal range.
A previous study reported that the risk of infection may be attributed to stroke-induced immunodepression syndrome (33). In our study, CAPD patients with prior stroke had higher rates of peritonitis than those without a history of stroke. Infections (peritonitis and catheter-related) were the leading cause of switching from PD to hemodialysis (34). In the present study, peritonitis events also were the main cause of switching from PD to hemodialysis. Of note, we found that every 1-month increase in time to the first episode of peritonitis was associated with a 7% decreased risk of patient mortality (HR 0.93; 95% CI, 0.89 – 0.96) and a 9% decreased risk of death-censored technique failure (HR 0.91; 95% CI 0.84 – 0.99), which indicated that early peritonitis events were closely associated with increased risks of all-cause mortality and technique failure.
In addition, patients with prior stroke needed a higher percentage of assisted PD compared with those without stroke. The reasons may include the following: (i) CAPD patients with prior stroke were older and more likely to have comorbidities compared with those without previous stroke (12,13); (ii) stroke patients often suffer from deficits in physical, psychosocial, and cognitive functioning (35), which commonly causes these patients to need assisted PD. Cheng et al. reported that older assisted PD patients had poorer survival and technique survival rates than those of self-care patients (36). However, there were no significant differences in patient survival and technique failure between assisted PD patients and those with self-care patients in our study (data not shown).
There were some limitations to this study. Firstly, a small sample size of 75 CAPD patients with prior stroke were included and analyzed. We failed to do subgroup analyses with regard to mortality and technique failure in those with prior stroke subtypes such as hemorrhagic or ischemic stroke, due to the small sample size of these patients. Thus, further prospective studies with large sample sizes regarding those with prior stroke subtypes are needed, which may provide more information about the outcomes of the subgroups and how to take proper medical care for patients with different subgroup complications. In addition, there may have been some management bias in these CAPD patients from a single center.
In conclusion, this study found that CAPD patients with prior stroke had higher mortality and technique failure compared with those without prior stroke. Older age, lower BMI and time to the first episode of peritonitis are independently associated with an increased risk for all-cause mortality in those with prior stroke, which suggests that these kinds of patients need more intensive care and proper nutrition improvement.
Disclosure
The authors have no conflicts of interest to declare.
Acknowledgments
This project was supported by the National Basic Research Program of China (Grant No. 2011CB504005); National Key Technology Research and Development Program of the Ministry of Science and Technology of China (Grant No.2011BAI10B05); Key Clinical Program of the Ministry of Health, China (2010439); Guangdong Department of Science & Technology Translational Medicine Center (grant 2011A080300002). We thank all nurses and nephrologists in our PD center for their great contribution and for the excellent patient care and data collection, etc.
REFERENCES
- 1. Krishnan M, Thodis E, Ikonomopoulos D, Vidgen E, Chu M, Bargman JM, et al. Predictors of outcome following bacterial peritonitis in peritoneal dialysis. Perit Dial Int 2002; 22:573–81. [PubMed] [Google Scholar]
- 2. Kendrick J, Teitelbaum I. Strategies for improving long-term survival in peritoneal dialysis patients. Clin J Am Soc Nephrol 2010; 5:1123–31. [DOI] [PubMed] [Google Scholar]
- 3. Szeto CC, Wong TY, Chow KM, Leung CB, Li PK. Are peritoneal dialysis patients with and without residual renal function equivalent for survival study? Insight from a retrospective review of the cause of death. Nephrol Dial Transplant 2003; 18:977–82. [DOI] [PubMed] [Google Scholar]
- 4. National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39:S1–266. [PubMed] [Google Scholar]
- 5. Gokal R, Jakubowski C, King J, Hunt L, Bogle S, Baillod R. Outcome in patients on continuous ambulatory peritoneal dialysis and haemodialysis: 4-year analysis of a prospective multicentre study. Lancet 1987; 2:1105–9. [DOI] [PubMed] [Google Scholar]
- 6. Maiorca R, Edward FV, Cavalli P, Amedeo DV, Giangrande A, La Giuseppe G, et al. A multicenter, selection-adjusted comparison of patient and technique survivals on CAPD and hemodialysis. Perit Dial Int 1991; 11:118–27. [PubMed] [Google Scholar]
- 7. Kawamura M, Fijimoto S, Hisanaga S, Yamamoto Y, Eto T. Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis 1998; 31:991–6. [DOI] [PubMed] [Google Scholar]
- 8. Seliger SL, Gillen DL, Longstreth WT, Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int 2003; 64:603–9. [DOI] [PubMed] [Google Scholar]
- 9. Sozio SM, Armstrong PA, Coresh J, Jaar BG, Fink NE, Plantinga LC, et al. Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: the choices for healthy outcomes in caring for ESRD (CHOICE) study. Am J Kidney Dis 2009; 54:468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stack AG, Molony DA, Rahman NS, Dosekun A, Murthy B. Impact of dialysis modality on survival of new ESRD patients with congestive heart failure in the United States. Kidney Int 2003; 64:1071–9. [DOI] [PubMed] [Google Scholar]
- 11. Ganesh SK, Hulbert-Shearon T, Port FK, Eagle K, Stack AG. Mortality differences by dialysis modality among incident ESRD patients with and without coronary artery disease. J Am Soc Nephrol 2003; 14:415–24. [DOI] [PubMed] [Google Scholar]
- 12. Kesarwani M, Perez A, Lopez VA, Wong ND, Franklin SS. Cardiovascular comorbidities and blood pressure control in stroke survivors. J Hypertens 2009; 27:1056–63. [DOI] [PubMed] [Google Scholar]
- 13. Bushnell CD, Lee J, Duncan PW, Newby LK, Goldstein LB. Impact of comorbidities on ischemic stroke outcomes in women. Stroke 2008; 39:2138–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stroke—1989 Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke 1989; 20:1407–31. [DOI] [PubMed] [Google Scholar]
- 15. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–305. [DOI] [PubMed] [Google Scholar]
- 16. Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012; 379:815–22. [DOI] [PubMed] [Google Scholar]
- 17. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. International Society for Peritoneal Dialysis. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423. [DOI] [PubMed] [Google Scholar]
- 18. Joshi U, Guo Q, Yi C, Huang R, Li Z, Yu X, et al. Clinical outcomes in elderly patients on chronic peritoneal dialysis: a retrospective study from a single center in china. Perit Dial Int 2014; 34:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han SH, Ahn SV, Yun JY, Tranaeus A, Han DS. Mortality and technique failure in peritoneal dialysis patients using advanced peritoneal dialysis solutions. Am J Kidney Dis 2009; 54:711–20. [DOI] [PubMed] [Google Scholar]
- 20. Kircelli F, Asci G, Yilmaz M, Sevinc Ok E, Demirci MS, Toz H, et al. The impact of strict volume control strategy on patient survival and technique failure in peritoneal dialysis patients. Blood Purif 2011; 32:30–7. [DOI] [PubMed] [Google Scholar]
- 21. Michels WM, Verduijn M, Boeschoten EW, Dekker FW, Krediet RT. Similar survival on automated peritoneal dialysis and continuous ambulatory peritoneal dialysis in a large prospective cohort. Clin J Am Soc Nephrol 2009; 4:943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wagner M, Ansell D, Kent DM, Griffith JL, Naimark D, Wanner C, et al. Predicting mortality in incident dialysis patients: an analysis of the United Kingdom Renal Registry. Am J Kidney Dis 2011; 57:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang X, Yi C, Liu X, Guo Q, Yang R, Cao P, et al. Clinical outcome and risk factors for mortality in Chinese patients with diabetes on peritoneal dialysis: a 5-year clinical cohort study. Diabetes Res Clin Pract 2013; 100:354–61. [DOI] [PubMed] [Google Scholar]
- 24. Avram MM, Sreedhara R, Fein P, Oo KK, Chattopadhyay J, Mittman N. Survival on hemodialysis and peritoneal dialysis over 12 years with emphasis on nutritional parameters. Am J Kidney Dis 2001; 37:S77–80. [DOI] [PubMed] [Google Scholar]
- 25. Fung F, Sherrard DJ, Gillen DL, Wong C, Kestenbaum B, Seliger S, et al. Increased risk for cardiovascular mortality among malnourished end-stage renal disease patients. Am J Kidney Dis 2002; 40:307–14. [DOI] [PubMed] [Google Scholar]
- 26. Stenvinkel P. Malnutrition and chronic inflammation as risk factors for cardiovascular disease in chronic renal failure. Blood Purif 2001; 19:143–51. [DOI] [PubMed] [Google Scholar]
- 27. Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis 2001; 38:1343–50. [DOI] [PubMed] [Google Scholar]
- 28. Chazot C, Gassia JP, Di Benedetto A, Cesare S, Ponce P, Marcelli D. Is there any survival advantage of obesity in Southern European haemodialysis patients? Nephrol Dial Transplant 2009; 24:2871–6. [DOI] [PubMed] [Google Scholar]
- 29. Kalantar-Zadeh K, Kopple JD. Obesity paradox in patients on maintenance dialysis. Contrib Nephrol 2006; 151:57–69. [DOI] [PubMed] [Google Scholar]
- 30. Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int 2003; 64:1838–44. [DOI] [PubMed] [Google Scholar]
- 31. Gu D, He J, Duan X, Reynolds K, Wu X, Chen J, et al. Body weight and mortality among men and women in China. JAMA 2006; 295:776–83. [DOI] [PubMed] [Google Scholar]
- 32. Klenk J, Nagel G, Ulmer H, Strasak A, Concin H, Diem G, et al. VHM&PP Study Group: body mass index and mortality: results of a cohort of 184,697 adults in Austria. Eur J Epidemiol 2009; 24:83–91. [DOI] [PubMed] [Google Scholar]
- 33. Zhang H, Li X. Correlation between inflammatory factors and post-stroke pneumonia in diabetic patients. Exp Ther Med 2013; 6:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jaar BG, Plantinga LC, Crews DC, Fink NE, Hebah N, Coresh J, et al. Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: a prospective study. BMC Nephrol 2009; 10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pang MY, Charlesworth SA, Lau RW, Chung RC. Using aerobic exercise to improve health outcomes and quality of life in stroke: evidence-based exercise prescription recommendations. Cerebrovasc Dis 2013; 35:7–22. [DOI] [PubMed] [Google Scholar]
- 36. Cheng CH, Shu KH, Chuang YW, Huang ST, Chou MC, Chang HR. Clinical outcome of elderly peritoneal dialysis patients with assisted care in a single medical centre: a 25 year experience. Nephrology (Carlton) 2013; 18:468–73. [DOI] [PubMed] [Google Scholar]