Abstract
♦ Background:
Although higher body mass index (BMI) is associated with better outcomes in hemodialysis patients, the relationship in peritoneal dialysis (PD) patients is less clear. We aimed to synthesize the results from all large and high-quality studies to examine whether underweight, overweight, or obesity is associated with any significantly different risk of death in peritoneal dialysis patients.
♦ Methods:
We searched MEDLINE, EMBASE, Web of Science, CINAHL, and Cochrane CENTRAL, and screened 7,123 retrieved studies for inclusion. Two investigators independently selected the studies using predefined criteria and assessed each study's quality using the Newcastle-Ottawa Quality Assessment Scale. We meta-analyzed the results of the largest studies with no overlap in their data sources.
♦ Results:
We included 9 studies (n = 156,562) in the systematic review and 4 studies in the meta-analyses. When examined without stratifying studies by follow-up duration, the results of the studies were inconsistent. Hence, we pooled the study results stratified based upon their follow-up durations, as suggested by a large study, and observed that being underweight was associated with higher 1-year mortality but had no significant association with 2- and 3- to 5-year mortalities. In contrast, being overweight or obese was associated with lower 1-year mortality but it had no significant association with 2-, and 3- to 5-year mortalities.
♦ Conclusion:
Over the short-term, being underweight was associated with higher mortality and being overweight or obese was associated with lower mortality. The associations of body mass with mortality were not significant over the long-term.
Keywords: Body mass index, mortality, peritoneal dialysis, meta-analysis, obesity paradox
Patients with end-stage renal disease undergoing maintenance dialysis therapy have a substantially higher risk of mortality (1). As in patients undergoing maintenance hemodialysis, cardiovascular disease is the main cause of death in patients receiving peritoneal dialysis (PD) (2). Even though in the general population obesity is associated with increased risk of cardiovascular disease and all-cause mortality, the risk of death is lower with increasing body mass (BMI), an indicator of obesity, in patients undergoing maintenance hemodialysis (3,4). Yet among patients undergoing PD, studies examining the association of body mass with mortality have been inconsistent; however, some studies have also linked obesity to lower mortality in this population (5,6). Given the inconsistent associations in studies published to date, we sought to synthesize the results from all large and high-quality studies to examine whether underweight, overweight, or obesity is associated with any significantly different risk of death in PD patients.
Methods
Search Strategies
We targeted studies investigating the link between BMI and mortality in all chronic kidney disease patients including those receiving PD. We searched MEDLINE (PubMed), Web of Science, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Cochrane Central Register of Controlled Trials (CENTRAL) up to July 2013 with no limitation in study type, language, and geographical area. To search PubMed, we used the following search query:
((Renal Insufficiency, Chronic) OR (chronic renal insufficien*) OR (Kidney Failure, Chronic) OR (chronic renal failure) OR (end?stage kidney disease*) OR (end?stage renal disease*) OR ESRD OR (chronic kidney disease*) OR (chronic renal disease*) OR (Renal Dialysis) OR ((renal OR kidney) AND dialys*) OR hemodialys* OR haemodialys* OR ((peritoneal OR extracorporeal) AND dialys*) OR Kidney Transplantation OR ((kidney OR renal) AND transplant*)) AND ((body mass index) OR BMI OR overweight OR obes*) AND (mortality OR (death rate*) OR (case fatality rate*) OR survival OR (reverse epidemiolog*) OR (obesity AND paradox*))
A similar query was used to search Web of Science, CINAHL, and CENTRAL. To search EMBASE, the above search query was tailored to match the searching keywords to EMTREE (the EMBASE's indexing thesaurus). Three field experts (RM, CPK, KK-Z) were consulted to identify any unidentified relevant study.
Study Selection
After importing the search results into EndNote software (Thomson Reuters, New York, NY, USA), we removed duplicated records. Two investigators (SFA, GZ), blinded to the study authors and journals, independently screened the studies for inclusion. The included studies had to describe data from case-control, cohort, or clinical trial studies to test the association of either baseline BMI or change in BMI with all-cause mortality in PD patients. Studies with mixed PD and hemodialysis patients were not included. Also, studies with fewer than 1,000 PD patients were not included as small studies are more likely to be influenced by publication (reporting) bias. Any discrepancies between the 2 reviewers on study eligibility were resolved by discussion and consensus.
Data Abstraction and Quality Assessment
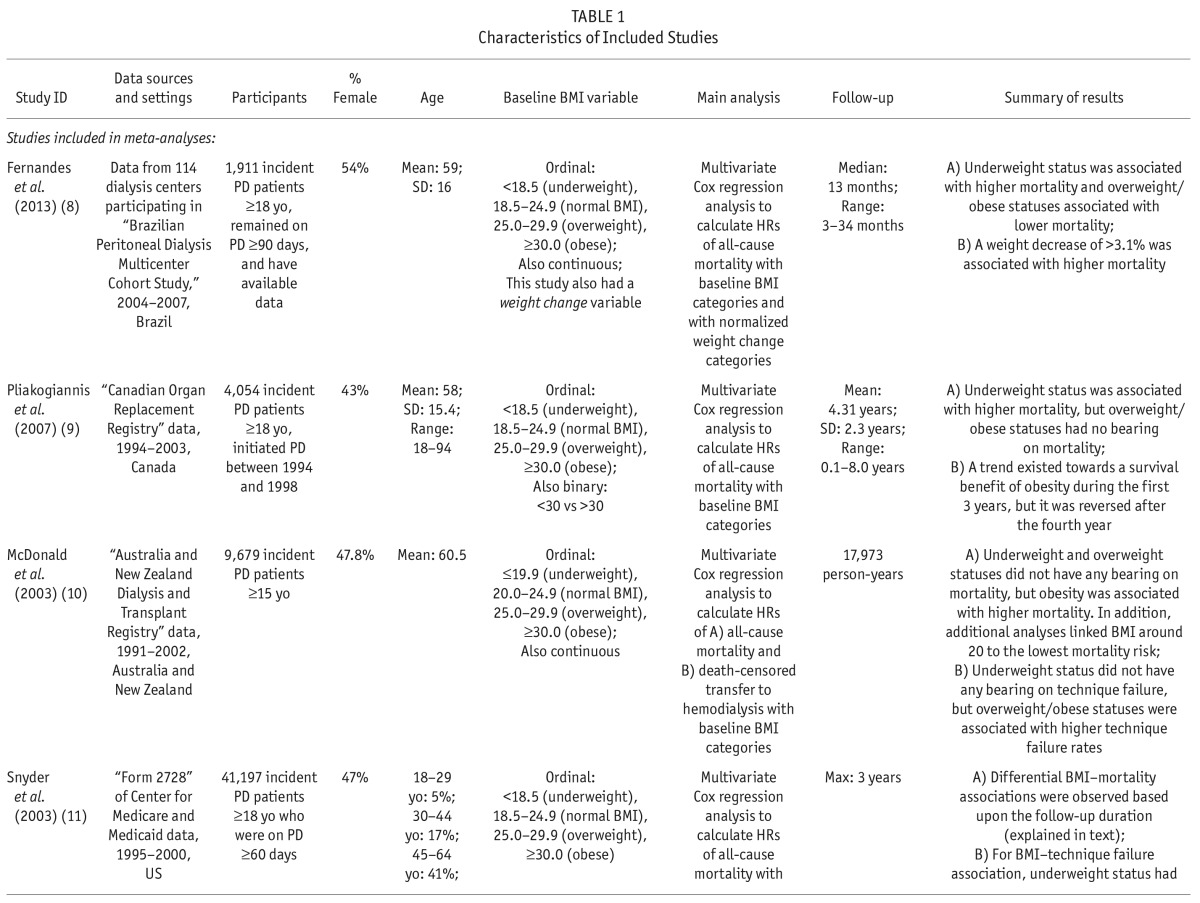
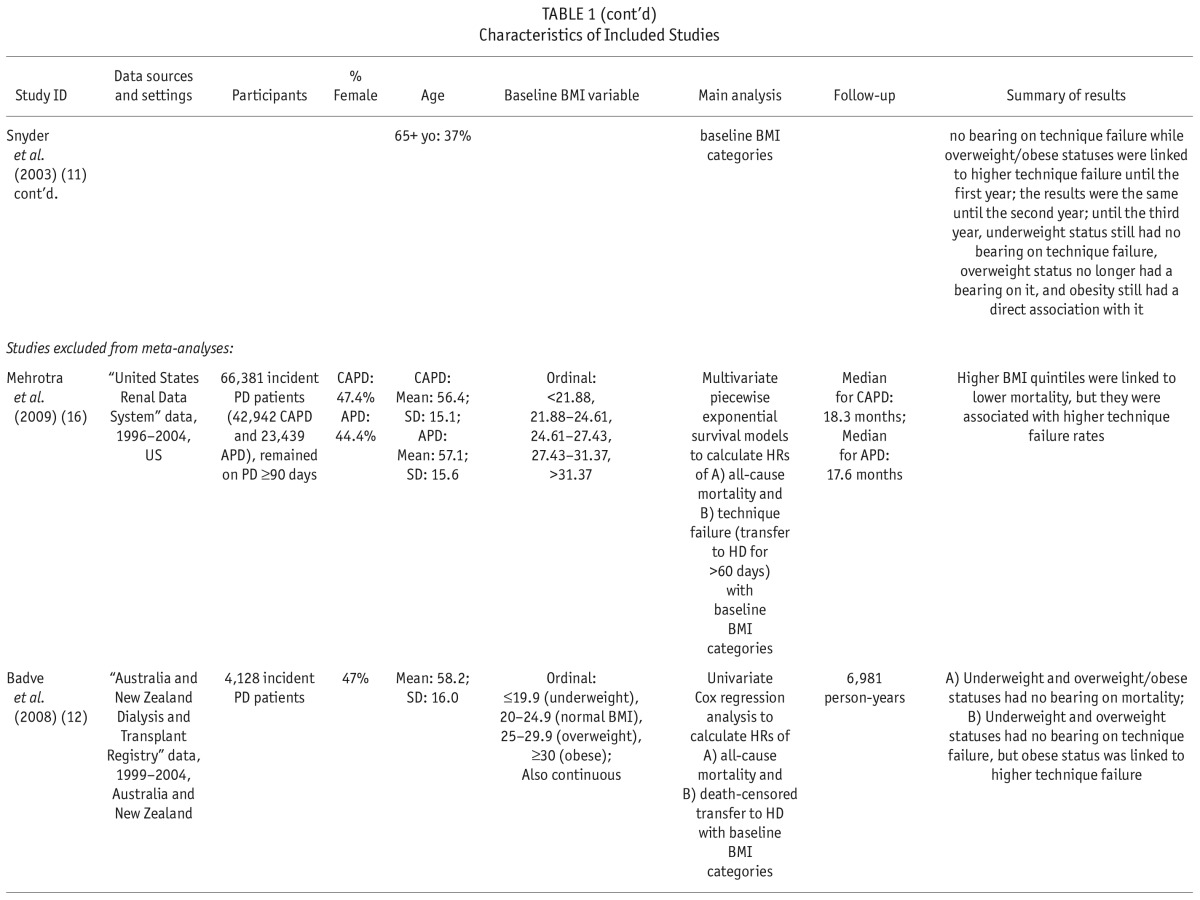
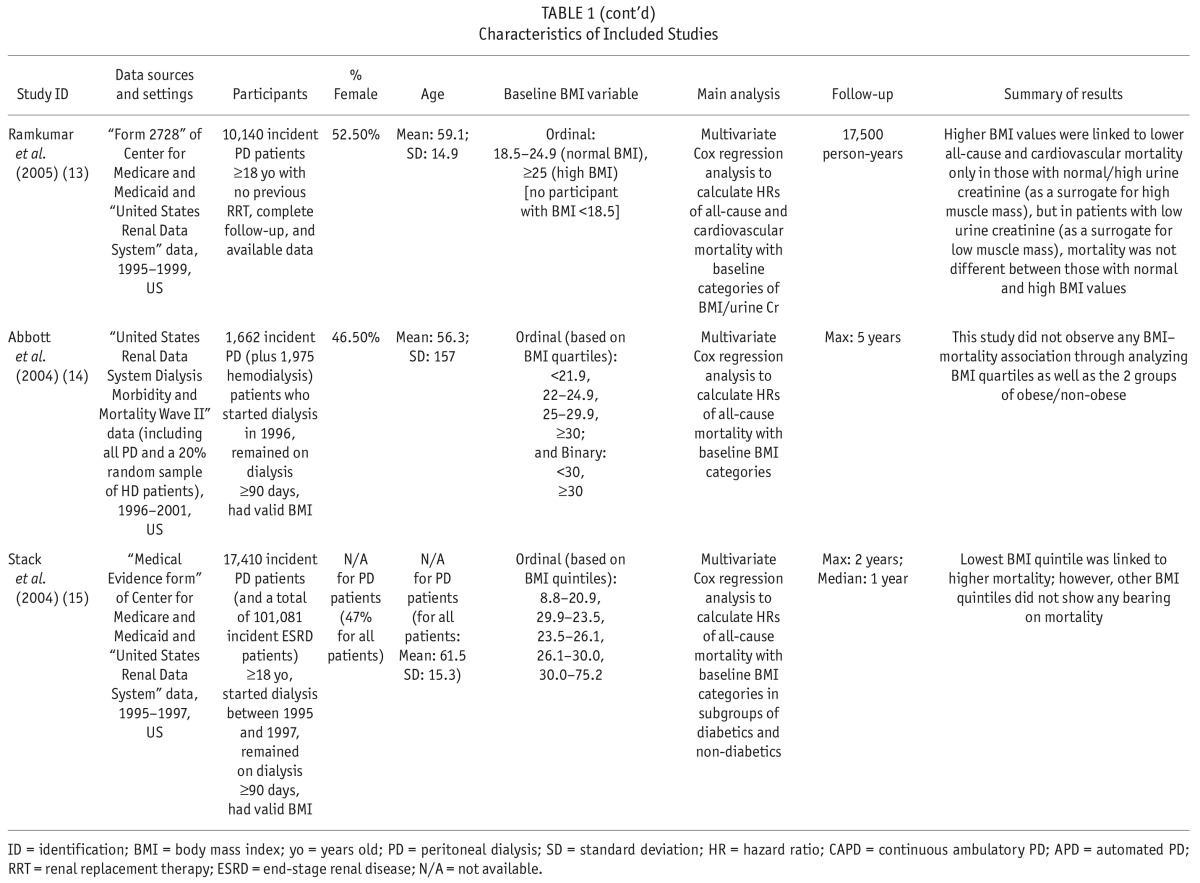
We extracted and tabulated the main characteristics and findings of the included studies (Table 1). In addition to all-cause mortality, we also extracted the results regarding technique failure (i.e. transfer to hemodialysis) when available. The corresponding authors of the studies with incomplete data were contacted in order to request further data. To assess the quality of the included studies, the same 2 investigators (SFA, GZ) independently applied the Newcastle-Ottawa Assessment Scale (7) assigning a quality score of 0 – 9 to each study (Supplementary Table S4). The quality score was based on 3 major components: selection of study participants (0 – 4 points), quality of the adjustment for confounding (0 – 2 points), and ascertainment of the exposure or outcome of interest in case-control studies or cohorts, respectively (0 – 3 points). The maximum score was 9 points, representing the highest methodological quality. Disagreements in the scores were resolved by discussion and consensus.
TABLE 1.
Characteristics of Included Studies
Data Analysis and Synthesis
We quantified the inter-rater agreement for selection of studies and assessment of quality.
For meta-analyses, we pooled the summary estimates of association between BMI and mortality. The summary estimates of association between BMI and technique failure were not pooled together as technique failure was a post-hoc outcome in our study and it was not targeted in our search strategies. We assessed statistical heterogeneity using I2 statistic. Summary statistics with a corresponding I2 ≤ 25% were pooled using fixed-effects meta-analysis while summary statistics with a corresponding I2 > 25% were pooled using a random-effects model. The small number of studies included in the meta-analyses prevented us from investigating the risk of publication bias using funnel plots and Egger's tests of asymmetry. A 95% confidence interval (CI) with no overlap with the null effect value (hazard ratio [HR] = 1) was considered significant in our study. For statistical procedures, we used Stata 12 (StataCorp, College Station, TX, USA).
Results
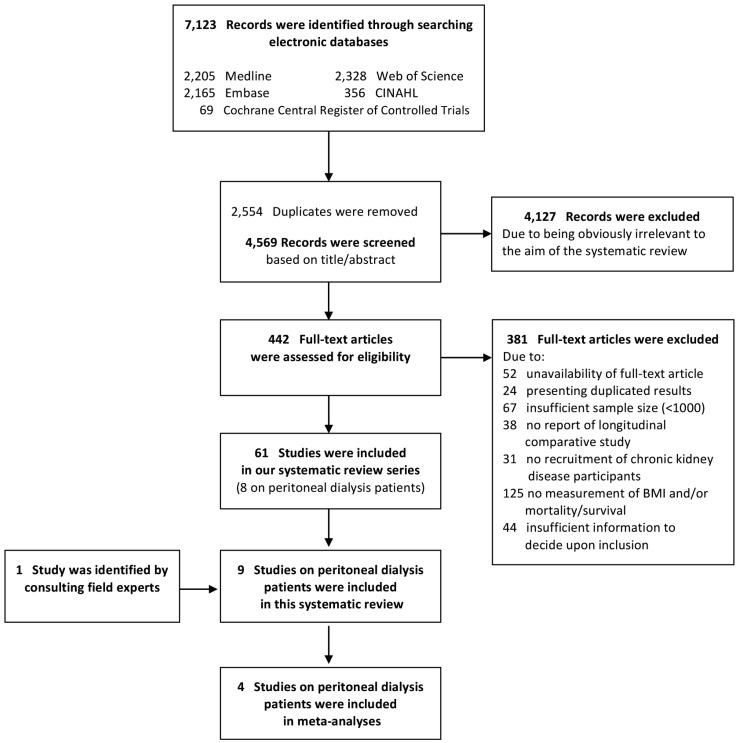
The retrieved 7,123 citations were screened for inclusion based on their titles/abstracts after duplicated records were removed. Subsequently, 442 citations were evaluated based on their full text article (Figure 1). We finally included 61 studies of the association of BMI with mortality in chronic kidney diseases, and segregated the 8 studies of patients who received PD (Table 1) (8–15). An additional study was identified through consulting field experts (16). All included studies comprised cohorts from pre-existing registry data and reported hazard ratios (HRs) from survival regression models. The utilized registries included the United States Renal Data System (USRDS) (11,13–16), the Australian and New Zealand Dialysis and Transplantation Registry (ANZDATA) (10,12), the Canadian Organ Replacement Register (CORR) (9), and the Brazilian Peritoneal Dialysis Multicenter Study (BRAZPD) (8). All included studies had a low risk of bias with a quality score range of 7 to 9 out of 9 (Supplementary Table S2). Agreement between the 2 investigators was 94% (Kappa: 0.77) for the study selection and 94% (Kappa: 0.78) for the quality assessment.
Figure 1 —
Study flow diagram. BMI = body mass index.
Association of BMI With All-Cause Mortality
Numerical results are summarized in Supplementary Table S5. Badve et al. (12) and Abbott et al. (14) reported baseline BMI to have no significant association with all-cause mortality in PD patients. On the other hand, McDonald et al. (10) reported that in comparison with BMI of 20.0 – 24.9 (normal), only BMI ≥ 30.0 (obese) was associated with higher mortality while there was no significant difference in risk for death among the other groups of BMI. In addition, there was no demonstrable association between BMI and mortality in the subset of their participants with Maori/Pacific Islander racial origins. In contrast, Pliakogiannis et al. (9) demonstrated an association only of BMI < 18.5 (underweight) with a higher mortality compared to BMI of 18.5 – 24.9 (normal). The trend for better survival in those with BMI ≥ 30 (obese) did not reach statistical significance. Similarly, Stack and Molony (15) reported that in both subgroups of diabetics and non-diabetics, BMI < 20.9 (lowest quintile) was associated with significantly higher mortality compared with BMI of 23.5 – 26.1 (middle quintile), while there was no significant difference in risk for death among individuals in other BMI quintiles. Also, Fernandes et al. (8) reported that in comparison with BMI of 18.5 – 24.9 (normal), BMI of < 18.5 (underweight) was associated with higher mortality while BMI of 25.0 – 29.9 (overweight) and BMI of ≥ 30.0 (obese) were associated with lower mortality. In the largest included study, Mehrotra et al. (16) showed that compared with the lowest quintile of BMI, all higher quintiles were associated with significantly lower mortality. However, if the second quintile (BMI of 21.88 – 24.61) was set as the reference group, only the third quintile (BMI of 21.88 – 24.61) would be associated with a significantly lower mortality. Additionally, Ramkumar et al. (13) reported that BMI ≥ 25.0 (overweight/obese) was associated with lower all-cause and cardiovascular mortality but only in patients with urine creatinine of > 0.64 grams/day (g/d) as a surrogate of high muscle mass. In contrast, in patients with urine creatinine of ≤ 0.64 g/d, all-cause and cardiovascular mortality were not significantly different for BMI of 18.5 – 24.9 (normal) and BMI ≥ 25.
In the study by Snyder et al. (11), the association of baseline BMI with 1-, 2-, and 3-year mortality risk were shown to be different. The investigators categorized BMI values into < 18.5 (underweight), 18.5 – 24.9 (normal; the reference group), 25.0 – 29.9 (overweight), and ≥ 30.0 (obese), and showed that during the first year of PD, being underweight was associated with higher mortality while being overweight and obese were associated with lower mortality. Considering the first 2 years, being underweight continued to be associated with higher mortality and being overweight continued to be associated with lower mortality; however, being obese was no longer associated with significantly different mortality. In a 3-year follow-up, being underweight or overweight were no longer associated with a differential mortality risk while being obese was associated with significantly higher mortality in contrast to its 1-year effect.
Fernandes et al. (8) also assessed the association of weight change (in addition to the baseline BMI) with all-cause mortality in PD patients. They observed that a ≥ 3.1% decrease in normalized weight was associated with a significantly higher mortality.
Meta-Analysis of All-Cause Mortality Results
Four studies were included in meta-analyses (8–11). The selected studies used the WHO BMI classification system (17) and reported corresponding hazard ratios (HRs) of all-cause mortality for underweight, overweight, and obese BMI classes at baseline compared with those with normal BMI. The studies excluded from meta-analyses had substantial overlap in the utilized data sources with the selected studies: Badve et al. (12) overlapped with McDonald et al. (10), and the remaining 4 studies (13–16) overlapped with Snyder et al. (11).
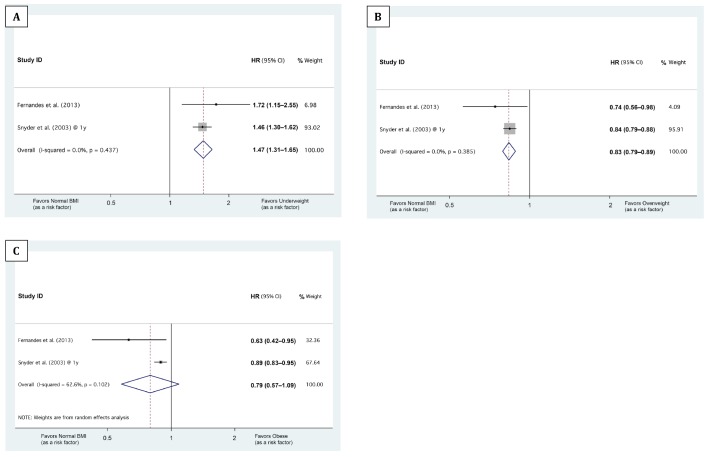
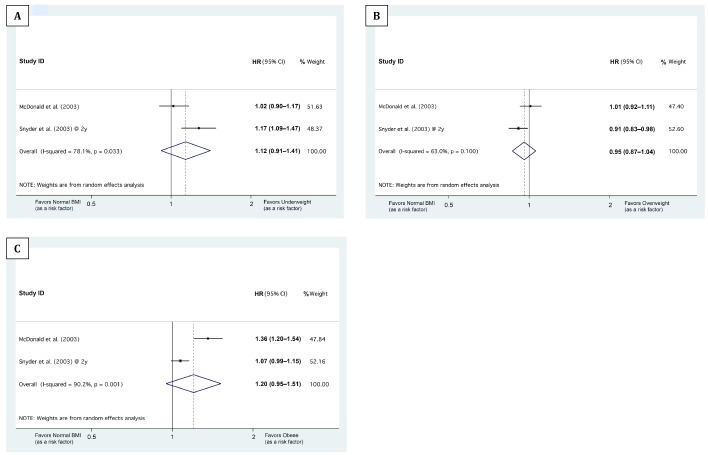
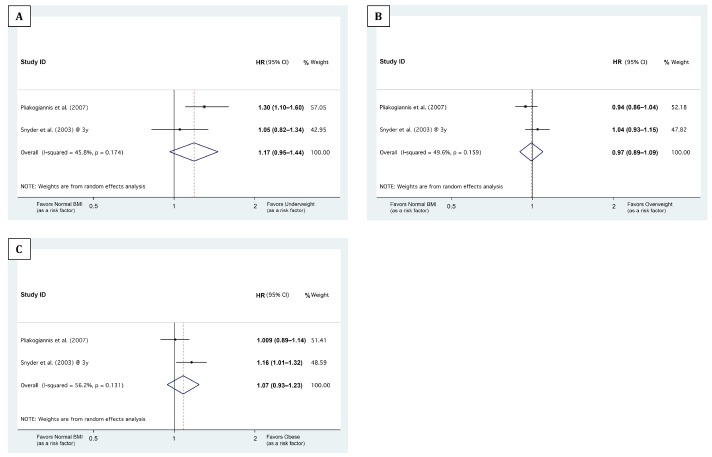
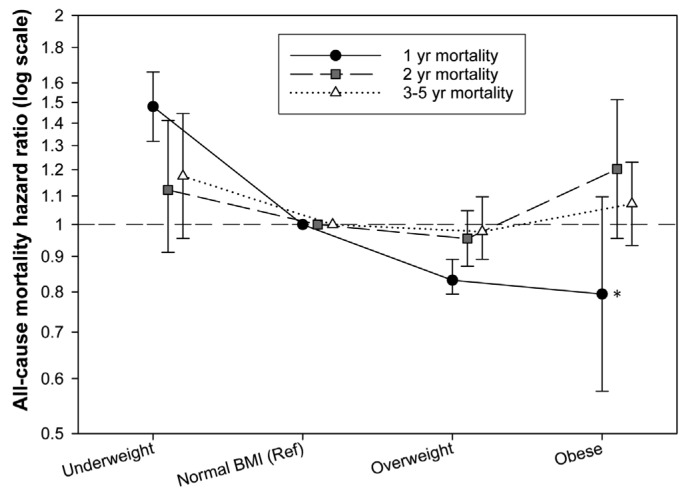
As Snyder et al. (11) reported differential associations of baseline BMI with 1-, 2-, and 3-year mortalities, we stratified the meta-analyses based on the follow-up durations: The median/mean follow-up durations of the studies by Fernandes et al. (8), McDonald et al. (10), and Pliakogiannis et al. (9) were 1.08, 1.86, and 4.31 years, respectively. Therefore, the HRs from these studies were pooled with the Snyder et al. (11) 1-, 2-, and 3-year HRs, respectively (Figures 2, 3 and 4). Our meta-analyses showed that being underweight at baseline was associated with higher 1-year mortality, being overweight with lower 1-year mortality (Figures 2 and 5). Although the pooled association of the obese BMI class with 1-year mortality was not significant, both meta-analyzed studies (8,11) showed that being obese at baseline was associated with lower 1-year mortality (Figure 2C). The meta-analyses also showed that compared with the normal BMI class, the trend of the association of underweight, overweight, and obese statuses at baseline with 2-year and 3- to 5-year mortality risk did not reach statistical significance (Figures 3, 4, and 5).
Figure 2 —
Forest plots showing the association of baseline BMI classes with 1-year mortality. A, B, and C illustrate the meta-analysis of the hazard ratios (HRs) of 1-year mortality in ‘underweight,’ ‘overweight,’ and ‘obese’ BMI classes, respectively, compared with normal BMI. The 3 BMI cut-points between the ‘underweight,’ ‘normal BMI,’ ‘overweight,’ and ‘obese’ classes were 18.5, 25.0, and 30.0, respectively. The horizontal axes are in logarithmic scale. ID = identification; HR = hazard ratio; BMI = body mass index.
Figure 3 —
Forest plots showing the association of baseline BMI classes with 2-year mortality. A, B, and C illustrate the meta-analysis of the hazard ratios (HRs) of 2-year mortality in ‘underweight,’ ‘overweight,’ and ‘obese’ BMI classes, respectively, compared with normal BMI. The 3 BMI cut-points between the ‘underweight,’ ‘normal BMI,’ ‘overweight,’ and ‘obese’ classes were 18.5 (in McDonald et al.) or 20.0 (in Snyder et al.), 25.0, and 30.0, respectively. The horizontal axes are in logarithmic scale. ID = identification; HR = hazard ratio; BMI = body mass index.
Figure 4 —
Forest plots showing the association of baseline BMI classes with 3- to 5-year mortality. A, B, and C illustrate the meta-analysis of the hazard ratios (HRs) of 3- to 5-year mortality in ‘underweight,’ ‘overweight,’ and ‘obese’ BMI classes, respectively, compared with normal BMI. The 3 BMI cut-points between the ‘underweight,’ ‘normal BMI,’ ‘overweight,’ and ‘obese’ classes were 18.5, 25.0, and 30.0, respectively. The horizontal axes are in logarithmic scale. ID = identification; HR = hazard ratio; BMI = body mass index.
Figure 5 —
Sigma plot comparing the pooled association of baseline BMI with 1-year, 2-year, and 3- to 5-year mortalities. *Although our meta-analysis yielded a non-significant pooled association of obesity with 1-year mortality, the association was significant in both meta-analyzed studies (see Figure 2C). In this case, the pooled association is considered significant. BMI = body mass index.
Association of BMI With Technique Failure
Four included studies also investigated the association of baseline BMI with technique failure, i.e. transfer to hemodialysis (Supplementary Table S6) (10–12,16). Mehrotra et al. (16) reported that compared with the lowest BMI quintile, higher BMI quintiles were associated with higher risks of technique failure. Also, Badve et al. (12) reported that technique failure was more likely for a BMI ≥ 30 (obese) compared with a BMI of 20.0 – 24.9 (normal). In the study by McDonald et al. (10) BMI of 25.0 – 29.9 (overweight) and ≥ 30 (obese) were observed to be associated with a higher risk of technique failure. Similarly, Snyder et al. (11) reported that a BMI of 25.0 – 29.9 (overweight) and a BMI ≥ 30.0 (obese) were associated with higher risks of 1- and 2-year technique failure. Also, a BMI ≥ 30.0 (obese) was still associated with a higher risk of 3-year technique failure.
Discussion
To the best of our knowledge, this is the first meta-analysis of published studies evaluating the association of BMI with mortality in PD patients. When examined without stratifying studies by follow-up duration, the results of the included studies were inconsistent. Hence, we pooled study results derived from similar follow-up durations and observed that being underweight at baseline was associated with a higher 1-year mortality but had no significant association with 2- and 3- to 5-year mortalities. Being overweight or obese at baseline was associated with lower 1-year mortality but had no significant association with 2-, and 3- to 5-year mortalities. It is worth noting that although our meta-analysis yielded a non-significant pooled association of obesity with 1-year mortality, the association was significant in both meta-analyzed studies (Figure 2C). (8,11) This phenomenon rarely happens when, in spite of the similar direction of association, the magnitude of association is considerably different among the pooled results. In this case, the pooled confidence interval may be disproportionately widened. However, the pooled association should be considered significant. Unlike BMI–mortality association, technique failure (i.e. transfer to hemodialysis) rates were reportedly higher with incremental BMI values.
A number of studies have demonstrated a lower risk of death in obese individuals in certain populations such as incident hemodialysis patients, hospitalized patients, elderly nursing home residents, and those with stroke or congestive heart failure (5,18–24). This phenomenon is termed the “obesity paradox” or “reverse epidemiology” and there are several possible explanations for it (5,23). We observed that in PD patients, being overweight or obese at baseline was associated with lower risk of death over the short term. This finding may potentially be due to the contrasting long- and short-term consequences of obesity: Although obesity generally increases the risk of cardiovascular mortality over the long term, it may attenuate the short-term risk of death due to malnutrition, inflammation, and protein-energy wasting (25). Notably, one of the included studies reported that in PD patients, weight loss was associated with a higher risk of death (8). However, this study did not examine whether weight loss was due to the loss of adipose tissue or muscle mass. Studies of hemodialysis patients (26,27) suggest that concurrent weight loss and muscle mass gain is associated with greater survival compared with concurrent weight gain and muscle mass loss. Also, an included study (13) suggested that the protective effect of a baseline BMI ≥ 25 may be due to higher muscle mass rather than higher adiposity. However, in PD patients, the relative influence of muscle mass or adiposity is difficult to evaluate because of limited epidemiologic evidence. In hemodialysis patients, a few studies have examined whether adiposity and muscle mass have differential associations with mortality; however, their results have not provided a consistent association (5). Also, since BMI can substantially change over time, the use of BMI at baseline rather than BMI as a time-varying covariate may have contributed to the loss of significant associations over longer follow-up durations. For technique failure, on the other hand, the association with BMI was rather direct and not paradoxical. This is possibly because malnutrition does not have a significant bearing on the common causes of technique failure such as mechanical and infectious complications as well as the inadequate solute clearance and/or ultrafiltration (28).
As our results are derived from observational studies, they cannot be used to drive weight-management interventions. However, in the absence of clinical trials, our findings can inform clinical practice by emphasizing that individual patient characteristics should influence decision-making regarding weight management, for example in obese PD patients with short life expectancy, it may not be necessary to advocate weight loss, whereas this may potentially be helpful in those with longer life expectancy.
In this systematic review, we searched multiple databases using sensitive search strategies. Moreover, we only included large studies (i.e. those with ≥ 1,000 patients undergoing PD) that provided higher accuracy and lower likelihood of publication bias. Furthermore, our study selection and quality assessment were carried out in duplicate. However, our study possesses the inherent limitations of systematic reviews of observational studies. Although the included studies represent the largest and highest-quality observational studies on the subject, their results were adjusted for only recognized and measured confounders. In addition, the included studies were from different geographical areas and they had different ranges of BMI values. Moreover, we were not able to run formal tests of publication bias due to the limited number of included studies in each meta-analysis. Furthermore, we used BMI as a surrogate of obesity even though it does not provide accurate information about adiposity or body composition. Nevertheless, BMI is still used in clinical practice to drive weight management and hence our results have clinical relevance.
In conclusion, over short-term follow-up, being underweight was associated with a higher risk of death and being overweight or obese with a lower risk of death. The associations were not significant over long-term follow-up. It is plausible that, despite its long-term consequences, obesity may in fact favorably impact short-term outcomes by attenuating the risk of malnutrition.
Disclosure
RM has served as an ad hoc consultant for Baxter Healthcare. CPK is an employee of the US Department of Veterans Affairs. Opinions expressed in this paper are those of the authors and do not represent the official opinion of the US Department of Veterans Affairs. GCF reports significant consulting for Novartis, and modest consulting for Amgen, Bayer, Gambro, Medtronic, and Janssen. KK-Z has received honoraria and/or research grants from Abbott, DaVita, Fresenius, Genzyme, and Shire. This work was supported by the NIH/NIDDK K24 DK091419 grant (KK-Z) and philanthropist grants from Mr. Harold Simmons and Mr. Louis Chang.
Supplementary Material
Footnotes
Supplemental material available at www.pdiconnect.com
REFERENCES
- 1. Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375(9731):2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krediet RT, Balafa O. Cardiovascular risk in the peritoneal dialysis patient. Nat Rev Nephrol 2010; 6(8):451–60. [DOI] [PubMed] [Google Scholar]
- 3. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006; 113(6):898–918. [DOI] [PubMed] [Google Scholar]
- 4. Prospective Studies Consortium. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373(9669):1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis 2014; 56:415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khawar O, Kalantar-Zadeh K, Lo WK, Johnson D, Mehrotra R. Is the declining use of long-term peritoneal dialysis justified by outcome data? Clin J Am Soc Nephrol 2007; 2(6):1317–28. [DOI] [PubMed] [Google Scholar]
- 7. Newcastle – Ottawa Quality Assessment Scale. [June 27, 2013]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf.
- 8. Fernandes NMD, Bastos MG, Franco MRG, Chaoubah A, Lima MD, Divino JC, et al. Body size and longitudinal body weight changes do not increase mortality in incident peritoneal dialysis patients of the Brazilian peritoneal dialysis multicenter study. Clinics 2013; 68(1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pliakogiannis T, Trpeski L, Taskapan H, Shah H, Ahmad M, Fenton S, et al. Reverse epidemiology in peritoneal dialysis patients: The Canadian experience and review of the literature. Int Urol Nephrol 2007; 39(1):281–8. [DOI] [PubMed] [Google Scholar]
- 10. McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J Am Soc Nephrol 2003; 14(11):2894–901. [DOI] [PubMed] [Google Scholar]
- 11. Snyder JJF, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int 2003; 64(5):1838–44. [DOI] [PubMed] [Google Scholar]
- 12. Badve SV, Hawley CM, McDonald SP, Mudge DW, Rosman JB, Brown FG, et al. Automated and continuous ambulatory peritoneal dialysis have similar outcomes. Kidney Int 2008; 73(4):480–8. [DOI] [PubMed] [Google Scholar]
- 13. Ramkumar N, Pappas LM, Beddhu S. Effect of body size and body composition on survival in peritoneal dialysis patients. Perit Dial Int 2005; 25(5):461–9. [PubMed] [Google Scholar]
- 14. Abbott KC, Glanton CW, Trespalacios FC, Oliver DK, Ortiz MI, Agodoa LY, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int 2004; 65(2):597–605. [DOI] [PubMed] [Google Scholar]
- 15. Stack AG, Murthy BVR, Molony DA. Survival differences between peritoneal dialysis and hemodialysis among “large” ESRD patients in the United States. Kidney Int 2004; 65(6):2398–408. [DOI] [PubMed] [Google Scholar]
- 16. Mehrotra R, Chiu Y-W, Kalantar-Zadeh K, Vonesh E. The outcomes of continuous ambulatory and automated peritoneal dialysis are similar. Kidney Int 2009; 76(1):97–107. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization Body Mass Index Classification. Sept. 5, 2013]. Available from: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 18. Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the Longitudinal Study of Aging. J Am Geriatr Soc 2001; 49(7):968–79. [DOI] [PubMed] [Google Scholar]
- 19. Andersen KK, Olsen TS. The obesity paradox in stroke: lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke 2013. [DOI] [PubMed] [Google Scholar]
- 20. Landi F, Onder G, Gambassi G, Pedone C, Carbonin P, Bernabei R. Body mass index and mortality among hospitalized patients. Arch Intern Med 2000; 160(17):2641–4. [DOI] [PubMed] [Google Scholar]
- 21. Herselman M, Esau N, Kruger JM, Labadarios D, Moosa MR. Relationship between body mass index and mortality in adults on maintenance hemodialysis: a systematic review. J Renal Nutr 2010; 20(5):281–92. [DOI] [PubMed] [Google Scholar]
- 22. Park J, Jin DC, Molnar MZ, Dukkipati R, Kim Y-L, Jing J, et al. Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clin Proc 2013; 88(5):479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr 2005; 81(3):543–54. [DOI] [PubMed] [Google Scholar]
- 24. Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol 2004; 43(8):1439–44. [DOI] [PubMed] [Google Scholar]
- 25. Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nature Clin Pract Nephrol 2007; 3(9):493–506. [DOI] [PubMed] [Google Scholar]
- 26. Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc 2010; 85(11):991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalantar-Zadeh K, Streja E, Molnar MZ, Lukowsky LR, Krishnan M, Kovesdy CP, et al. Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol 2012; 175(8):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quinn RR, Ravani P, Hochman J. Technique failure in peritoneal dialysis patients: insights and challenges. Perit Dial Int 2010; 30(2):161–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.