Abstract
♦ Background and Objectives:
Catheter-related infection, namely exit-site infection (ESI) and peritonitis, is a major infectious complication and remains a main cause of technique failure for patients receiving peritoneal dialysis (PD). Topical application of antibiotic cream might reduce catheter-related infection but emergence of resistant or opportunistic organisms could be a concern. Optimal topical agents and regimens remain to be determined. We did a study to examine the effect of an alternating topical antibiotic regimen in preventing catheter-related infection.
♦ Method:
We performed a single-center, randomized, open-label study to compare daily topical application of gentamicin cream with a gentamicin/mupirocin alternate regimen to the exit site. Patients randomized to alternating regimen were asked to have daily application of gentamicin cream in odd months and mupirocin cream in even months. Primary outcomes were ESI and peritonitis. Secondary outcomes were catheter removal or death caused by catheter-related infection. A total of 146 patients (71, gentamicin group; 75, alternating regimen group) were enrolled with a total follow-up duration of 174 and 181 patient-years for gentamicin and alternating groups, respectively. All patients were followed up until catheter removal, death, transfer to another unit, transplantation or the end of the study on March 31, 2014. There were no significant differences in the age, sex, dialysis vintage, and rate of diabetes, helper-assisted dialysis and methicillin-resistant Staphylococcus aureus (MRSA) carriage state.
♦ Results:
No difference was seen in the time to first ESI or peritonitis. However, the time to first gram-negative peritonitis seemed longer for the gentamicin group (p = 0.055). The 2 groups showed a similar rate of ESI (0.17/yr vs 0.19/yr, p = 0.93) but P. aeruginosa ESI was less common in the gentamicin group (0.06/yr vs 0.11/yr, p < 0.001). There was no difference in the incidence of ESI due to non-tuberculous mycobacteria. Peritonitis rate was significantly lower in the gentamicin group (0.22/yr vs 0.32/yr, p < 0.001), with a striking decrease in gram-negative peritonitis (0.08/yr vs 0.14/yr, p < 0.001), and fungal peritonitis (0.006/yr vs 0.03/yr, p < 0.001), which was all antibiotics-related episodes with antecedent use of systemic antibiotics for the treatment of catheter-related infections. There was no significant difference in the catheter loss or death related to catheter-related infection.
♦ Conclusion:
Alternating gentamicin/mupirocin cream application appeared as effective as gentamicin alone in preventing ESI except for P. aeruginosa. However, it was inferior to gentamicin in the prevention of peritonitis episodes, especially for those caused by gram-negative organisms. It was also not useful in reducing catheter-related infection due to opportunistic organisms but instead associated with a higher incidence of antibiotic-related fungal peritonitis.
Keywords: Exit-site infection, peritonitis, catheter infection, prophylaxis, topical antibiotics, gentamicin, mupirocin
Catheter-related infection, including exit-site infection (ESI) and peritonitis, remains a major complication in patients receiving peritoneal dialysis (PD). It is a leading cause of technique failure and is associated with hospitalization and mortality in PD patients. In order to reduce the occurrence of this complication, various prophylactic strategies have been developed, and topical application of antibiotics is one of the well-studied measures.
Mupirocin cream applied to the exit site has been shown to be effective in reducing ESI and peritonitis caused by Staphyloccocus aureus in PD since the late 1990s (1,2). Subsequently, a randomized controlled study published in 2005 suggested that topical gentamicin was superior to its mupirocin counterpart as a prophylactic antibiotic in the prevention of catheter infection (3). In that study, apart from being as effective as mupirocin in preventing S. aureus infections, topical gentamicin also significantly reduced gram-negative ESI and peritonitis. However, a borderline increase in fungal ESI in the gentamicin group found in the study was a concern. Indeed, there were case series following this study reporting clustering of ESI with or without peritonitis caused by non-tuberculous mycobacteria (NTM) after regular application of gentamicin cream to the exit site (4,5). All these findings raise the question of whether regular use of gentamicin cream would increase the risk of catheter infection due to opportunistic organisms.
Furthermore, the superiority of topical gentamicin over mupirocin in preventing catheter-related infections was also challenged by subsequent observational studies, which reported no significant difference in efficacy between the 2 topical antibiotics in this respect (6,7). Therefore, while it is recommended that all PD patients should use topical antibiotic either at the catheter exit site, intranasally, or both, the optimal prophylactic topical antibiotic regimen at the exit site remains unclear (8).
As opposed to mupirocin, which is mainly active against gram-positive organisms, gentamicin is a broad-spectrum antibiotic with antimicrobial activity against both gram-positive and gram-negative organisms. It has been postulated that the emergence of infection caused by opportunistic organisms in patients receiving regular topical gentamicin might be related to prolonged broad-spectrum suppression of indigenous skin flora by a high concentration of topical gentamicin, resulting in selection pressure favoring the growth of atypical organisms (9).
We hypothesized that a monthly alternating topical antibiotic regimen with mupirocin and gentamicin, hereby avoiding prolonged broad-spectrum suppression of indigenous skin flora, might reduce the emergence of catheter infection due to opportunistic organisms, especially NTM, without losing the efficacy in the prevention of PD catheter infections. To test this hypothesis, we performed a randomized controlled study to compare the effectiveness of this alternating regimen with gentamicin cream alone.
Materials and Methods
It was a single-center, randomized, open-label study conducted in a regional hospital in Hong Kong with a PD program of 280 prevalent patients. The study was approved by Hong Kong Hospital Authority Kowloon West Cluster Clinical Research Ethics Committee.
Enrollment was conducted during routine clinic visits. All prevalent and incident patients receiving PD aged 18 years or above and able to give consent were potential subjects. Excluded were those with a known allergy to either gentamicin or mupirocin, peritonitis or ESI in the previous 30 days, terminal illness with a life expectancy of less than 1 year, being expected to have PD lasting for less than 1 year including patients planning for elective renal transplantation or patients suffering from reversible acute kidney injury, pregnancy, non-compliance, and being involved in another clinical study.
After providing written informed consent, all patients had to undergo a 6-month washout period with no topical antibiotic applied to the catheter exit site before randomization as some individual patients had been previously receiving topical gentamicin or mupirocin as prescribed by their attending physicians. The participants were assigned into the 2 intervention groups in a 1:1 ratio using blocked randomization with a block size of 20, 10 for each intervention group. An independent clerical staff was solely responsible for the block size, intervention order, sequentially numbered sealed envelopes, all of which were blinded to the investigators who were responsible for the patient enrollment. The investigators were informed by the clerical staff of the intervention assignment. Diabetic and non-diabetic patients were stratified at randomization. Two sets of sealed envelopes, one for diabetic and another for non-diabetic patients, were used for allocation concealment.
Group A had daily exit-site application of 0.1% gentamicin cream. Group B patients employed an alternating regimen, where there was daily exit-site application of topical antibiotic, with 0.1% gentamicin cream for odd months and 2% mupirocin cream for even months.
Recruitment began in July 2008 and continued until September 2010, with follow-up to March 31, 2014. A total 259 patients were screened, and 112 were excluded or refused to participate. One patient withdrew after being randomized to the gentamicin group before beginning study cream. At baseline, culture swabs were taken from the exit site, bilateral axillae and nostrils of all studied patients to look for the presence of methicillin-resistant Staphylococcus aureus (MRSA).
Patients were asked to perform daily exit-site care by applying a small amount of cream around the catheter exit site using a cotton swab after daily dressing using swabsticks impregnated with 0.05% aqueous chlorhexidine gluconate solution. Catheters were anchored with tape and a small gauze dressing to prevent exit-site trauma. There was regular exit-site assessment by attending physicians every 3 months, with additional assessment whenever ESI was reported or clinically indicated.
Exit-site infection was defined as purulent discharge with erythema, tenderness or induration, and swabs were taken for bacterial, mycobacterial and fungal culture. Peritonitis was defined as cloudy effluent with ≥ 100/μL white cells with ≥ 50% polymorphonuclear cells, and fever or abdominal pain. Peritoneal dialysate effluent cultures were obtained with blood culture bottles. Antibiotic treatment was given according to the prevailing unit protocol. Patients on antibiotic therapy were routinely given antifungal prophylaxis with oral nystatin 500,000 units 4 times a day. The catheter was removed in the event of ESI or peritonitis refractory to antibiotic therapy.
Sample Size Estimation
We noted a dramatic increase in ESI due to NTM from a negligible level to 0.013 episodes per patient-year after regular exit-site application of topical gentamicin cream for some of our PD patients in the 2 years preceding this study. We estimated that if the alternating regimen could reduce the event rate of NTM ESI by 95% to the baseline, a total of 389 patient-years of follow-up would be required to ensure 80% power for detection of a treatment difference with a type 1 error of 0.05. However, when an interim analysis at 355 patient-years showed a significant difference in the peritonitis rate together with an absence of any identifiable trend towards a difference in NTM infection rate, it was decided to stop the study.
Study Outcomes and Follow-Up
The primary outcomes were time to first ESI, time to first PD-related peritonitis, rate of ESI and rate of peritonitis, in particular, ESI and peritonitis rates due to opportunistic organisms including fungal and NTM. The secondary outcomes were catheter loss from PD catheter-related infections, PD-catheter infection-related mortality and the composite of catheter loss or death related to catheter infections. All patients were followed up from the first day of study cream application until censored at catheter removal, death, transplantation, withdrawal from study, drop-out from study because of skin reaction to the study cream, transfer to another unit, and end of study on March 31, 2014.
Statistical analyses were performed using the SPSS Statistics 22.0 for Windows (IBM, Armonk, NY, USA). Data are expressed as means with standard deviation (SD). The Chi-square test, Fisher's exact test, Student's t-test and the Mann-Whitney U test were used when appropriate. Comparison between the 2 groups in terms of time to first ESI and peritonitis, and catheter infection-related catheter loss or mortality were done using Kaplan-Meier curves, with log-rank test used for comparison. Multivariate Cox proportional hazards model was used to evaluate the effect of intervention allocation, age, sex, vintage on dialysis, diabetic status, MRSA carriage, helper-assisted dialysis and serum albumin. Exit-site infection and peritonitis rates were calculated as the number of events occurring divided by the total follow-up time and they were compared by Poisson regression analysis. All probabilities were 2-tailed and statistical significance was assumed at a p value < 0.05.
Results
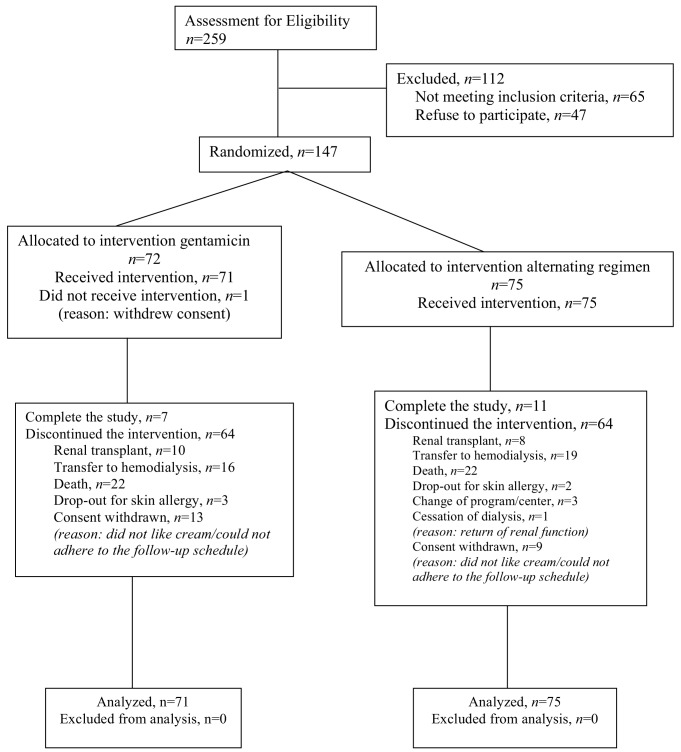
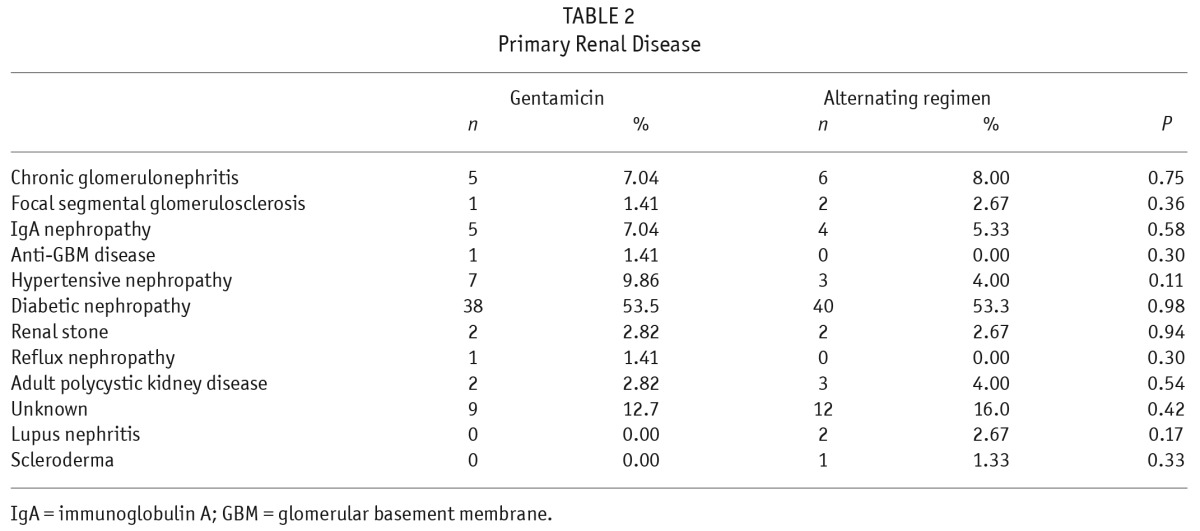
A total of 147 patients were randomly allocated to gentamicin (n = 72) and alternating regimen (n = 75), but 1 patient in the gentamicin group subsequently withdrew before starting the study cream (Figure 1). In total, 146 patients took part in the study, with 71 and 75 in gentamicin and alternating groups, respectively. There was no difference in groups by age, sex ratio, mean duration of follow-up, serum albumin, total weekly Kt/V, PD system, percentage with diabetes, MRSA carriage, automated PD or helper-assisted dialysis (Table 1). There was no difference in primary renal disease between the 2 groups (Table 2). The total follow-up durations were 174 and 181 patient-years for gentamicin and alternating groups, respectively. All the drop-outs were due to skin allergy to the study cream. The median duration of follow-up was 29.5 ± 19.6 months in gentamicin group and 29.0 ± 22.1 months in alternating group.
Figure 1 —
Allocation and course of study participants.
TABLE 1.
Patient Characteristics
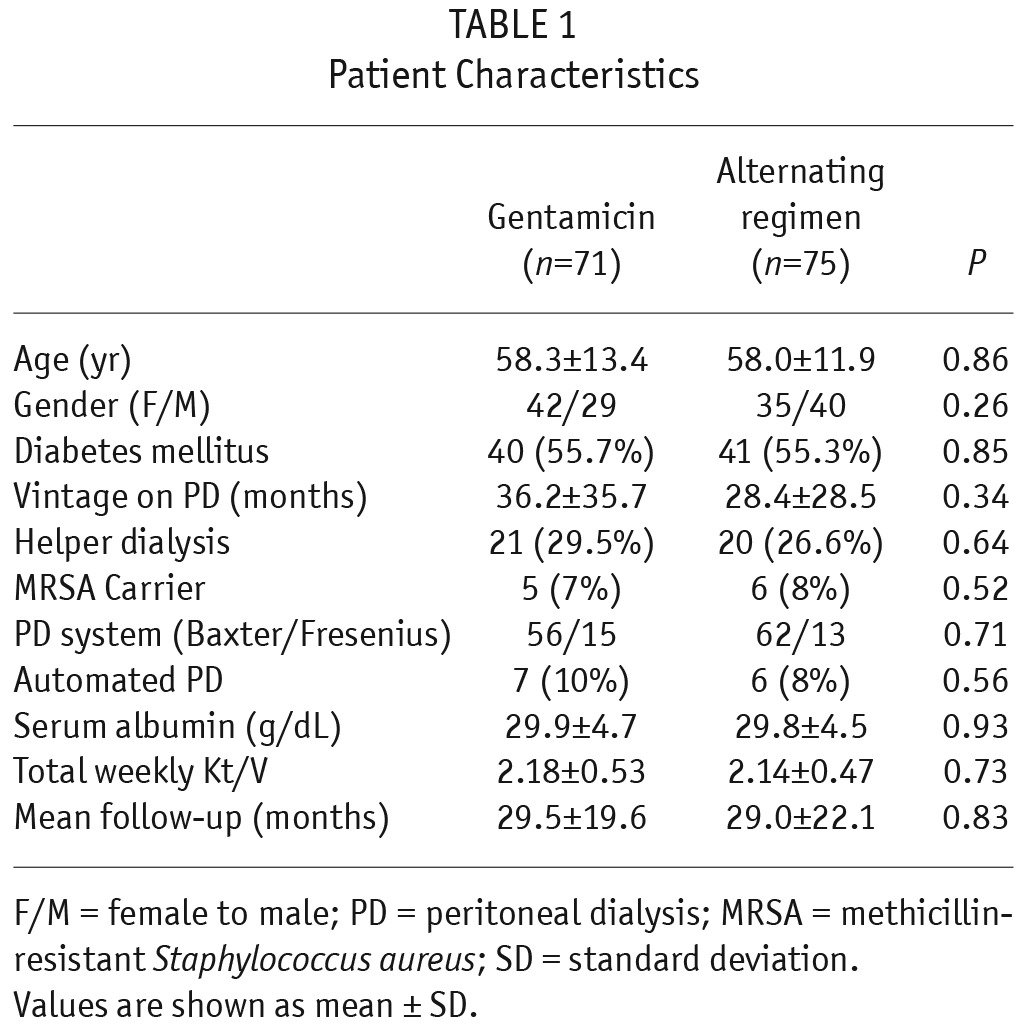
TABLE 2.
Primary Renal Disease
There was no significant difference in censoring reasons between the 2 groups. These included renal transplantation (14.1% vs 10.7%), transfer to hemodialysis (22.5% vs 25.3%), death (31% vs 29.3%), drop-out due to skin allergy (4.23% vs 2.67%), cessation of dialysis (0% vs 1.33%), withdrawal of consent (18.3% vs 12%), transfer to another PD unit (0% vs 4%), and end of study (9.86% vs 14.7%), respectively, in the groups using gentamicin vs alternating regimen. The only side effect reported in either group was skin reaction leading to drop-out and withdrawal of 3 patients in the gentamicin group and 2 in the alternating group.
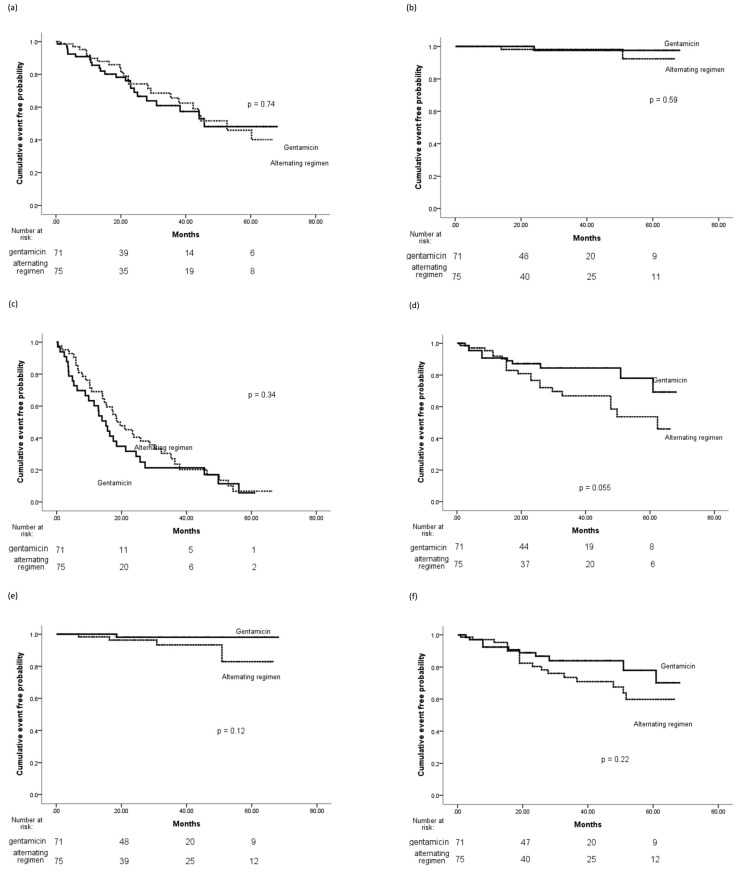
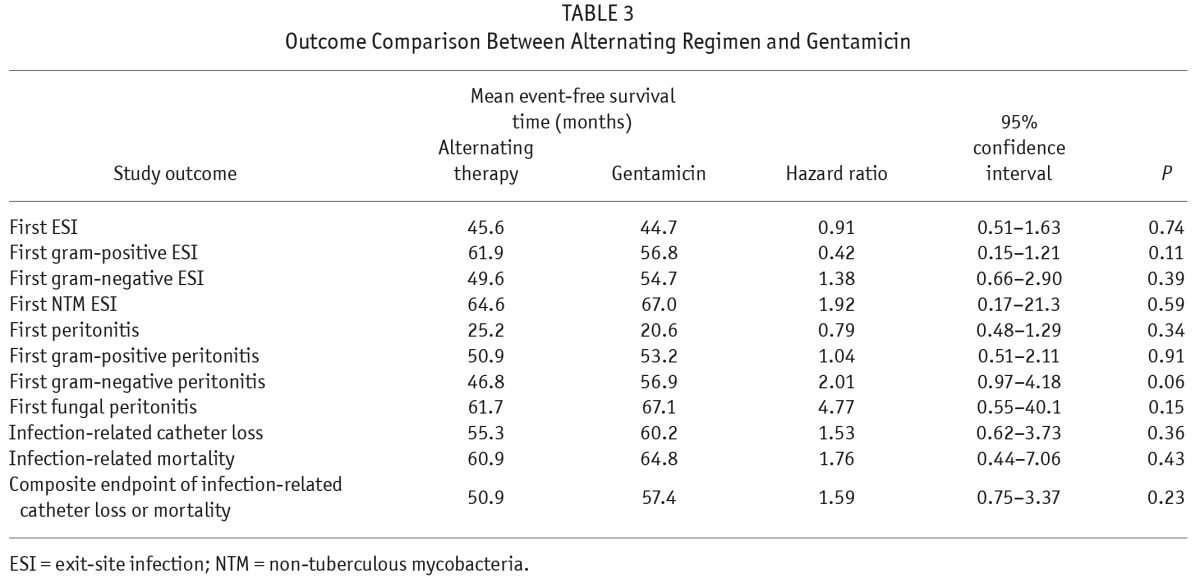
There was no significant difference in time to first ESI and times to first ESI due to gram-positive organisms, gram-negative organisms, or NTM (Figure 1, Table 3). Multivariate Cox regression analysis of time to first ESI showed that helper-assisted dialysis was a significant independent predictor for ESI (hazard ratio [HR], 2.0; 95% confidence interval [CI], 1.07 – 3.75; p = 0.03), while the presence of MRSA carriage was a significant risk factor for gram-positive ESI (HR, 4.0; CI, 1.19 – 13.5; p = 0.025). No significant independent predictors were identified for gram-negative ESI.
TABLE 3.
Outcome Comparison Between Alternating Regimen and Gentamicin
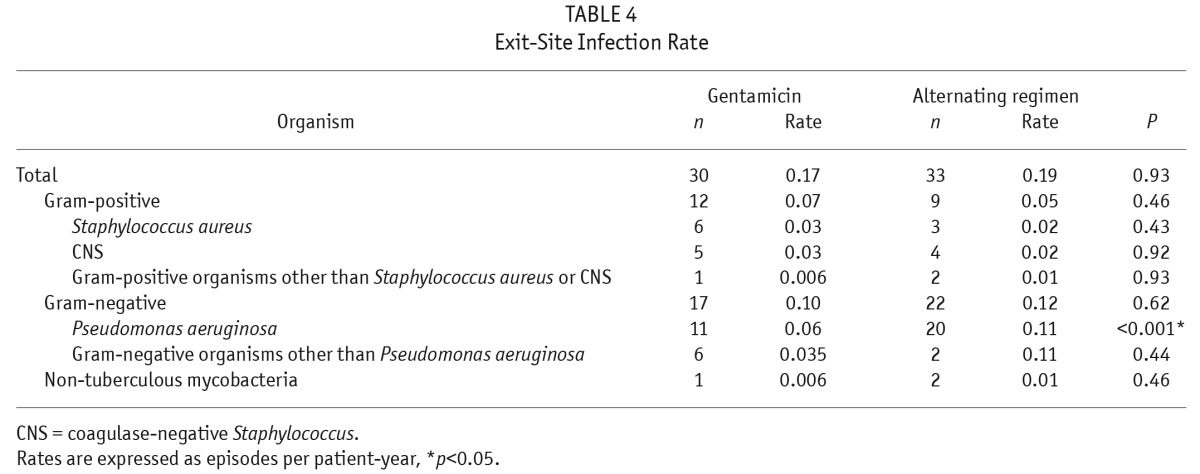
The overall ESI rates were the same in both groups (Table 4). The 2 groups also had similar incidences of ESI due to gram-positive organisms. However, Pseudomonas aeruginosa ESI rates were significantly lower in the gentamicin group, while there was no significant difference in overall gram-negative ESI rates between the 2 groups. There was no difference in NTM ESI rates, with 1 episode of NTM ESI in the gentamicin group and 2 episodes in the alternating group during the follow-up period. All 3 episodes of NTM ESI occurred within 1 month following exposure to broad-spectrum antibiotics for the treatment of preceding Pseudomonas aeruginosa ESIs.
TABLE 4.
Exit-Site Infection Rate
There was no significant difference in times to first peritonitis, first gram-positive peritonitis, first gram-negative peritonitis, or first fungal peritonitis (Figure 2, Table 3). Multivariate Cox regression analysis of time to first peritonitis showed that advancing age was a significant predictor for peritonitis (HR, 1.04; CI, 1.02 – 1.07; p = 0.002). No significant predictors were identified for gram-positive peritonitis, but there was a trend that gentamicin exit-site use gave rise to a lower risk of developing gram-negative peritonitis (HR, 2.01; CI, 0.97 – 4.18; p = 0.06).
Figure 2 —
Kaplan-Meier plots of primary and secondary endpoints. (a) Time to first ESI. (b) Time to first NTM ESI. (c) Time to first peritonitis. (d) Time to first gram-negative peritonitis. (e) Time to first fungal peritonitis. (f) Time to infection-related catheter removal or death. ESI = exit-site infection; NTM = non-tuberculous mycobacteria.
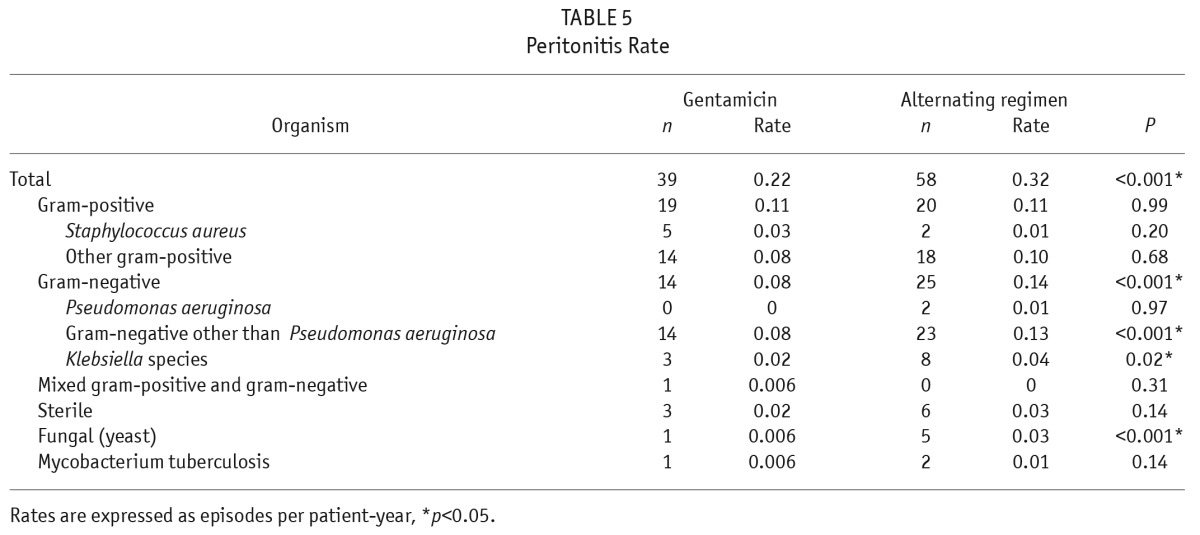
Overall peritonitis rates were lower using gentamicin, 0.22 per patient-year compared with the alternating regimen, 0.32 per patient-year (p < 0.001, Table 5). It was due to a reduced rate of gram-negative peritonitis using gentamicin. Fungal peritonitis rates were also strikingly lower in the gentamicin group compared with those using the alternating regimen (0.006 vs 0.03, p < 0.001). All 6 fungal peritonitis episodes (1 in gentamicin and 5 in alternating regimen) were caused by Candida species and were antibiotic-related, with prior exposure to systemic antibiotics within 1 month of occurrence. There was no difference in the incidence of NTM peritonitis, and there was only 1 episode in the gentamicin group and none in the alternating group.
TABLE 5.
Peritonitis Rate
Kaplan-Meier survival analyses for infection-related catheter loss, infection-related death, and the composite of infection-related catheter loss or death showed no significant difference between the 2 groups (Figure 2, Table 3).
Discussion
Ever since Bernardini et al. published the results of their randomized controlled trial comparing exit-site application of gentamicin vs mupirocin in 2005, there has been no further data appearing in the literature supporting the superiority of gentamicin over mupirocin as a topical agent in the prevention of catheter-related infections (3). In contrast, a small prospective study and an observational study afterwards reported no difference in these 2 topical antibiotics in the prevention of catheter-related infections (6,7). An exceptionally intriguing finding in Bernardini's study was a significantly lower gram-negative peritonitis rate in the gentamicin group, for which the exact mechanism of action remains unclear, while possible prevention of subclinical exit-site or cuff infections have been suggested (10,11).
In this randomized controlled trial, it seemed that topical mupirocin and gentamicin were not equal in terms of their efficacy in the prevention of catheter-related infections, with the alternating group showing a higher gram-negative peritonitis rate (p < 0.001) and trend toward a shorter time to first gram-negative peritonitis (p = 0.055). It is indeed a corroboration of Bernardini's finding to support the argument that gentamicin is more effective than mupirocin in the prevention of gram-negative catheter infections.
One could argue that the inferior outcome in the alternating group in the present study might be caused by potentially lower adherence in this group due to its inherently complicated regimen. However, no non-adherence was suspected or documented in the patient records throughout the study. In addition, this argument could not readily explain why the difference was merely limited to gram-negative infections but spared its gram-positive counterparts.
Apart from being inferior in preventing gram-negative infections, the alternating regimen also appeared ineffective in preventing opportunistic infections. The alternating regimen was associated with a significantly higher rate of fungal peritonitis than gentamicin alone, although there was no significant difference in the incidences of ESI caused by NTM. It is noteworthy that all these fungal episodes were antibiotic-related episodes as a result of more frequent use of systemic antibiotics for the treatment of catheter-related infections, in particular, gram-negative infections. In fact, a similar trend could also be observed for NTM infections, where all NTM ESIs were preceded by exposure to systemic antibiotics for the treatment of Pseudomonas aeruginosa ESIs. In a previous case series reporting on clustering of NTM ESI in association with exit-site application of gentamicin cream, exposure to systemic antibiotics also appeared to be a common predisposing factor (4). Taken together, both topical and systemic antibiotics should contribute to the overall selective pressure in skin flora and overgrowth of opportunistic organisms.
In addition to leading us to reject our hypothesis, this study also illustrates that making a choice of topical agents is not straightforward. It involves striking a delicate balance in terms of spectrum of activity and potency. The agent chosen should be able to help prevent the occurrence of catheter-related infections due to common pathogenic organisms with reduction in the use of systemic antibiotics but not lead to undue suppression of normal skin flora or overgrowth of opportunistic organisms. As an example, a previous study demonstrated that PD patients receiving prophylactic use of intranasal mupirocin were associated with less exposure to systemic antibiotics than the controls (12). In this regard, an agent with these ideal characteristics remains elusive. Recent studies on 2 new topical agents including Polysporin Triple ointment and antibacterial honey were deemed disappointing (13,14). On the other hand, despite appearing safe and effective, there has been a concern that topical gentamicin daily at the exit site might eventually produce resistant organisms. However, no studies have been published demonstrating such resistance (15).
Helper-assisted dialysis and the presence of MRSA carriage were found to independently predict the risk of ESI and gram-positive ESI, respectively, in this study. Therefore, apart from the consideration of topical antibiotics, the importance of other preventive strategies in the prevention of catheter-related infections, especially education and training of patients and carers, could not be over-emphasized.
There are several limitations to this study. First, there were no data in mupirocin resistance. Second, it was an open-label study where neither patients nor investigators were blind to the treatment regimen. In addition, the alternating regimen was inherently more complicated, which might lead to potential confusion of patients and non-adherence. Finally, this study was not powered to detect small and moderate differences in the predisposition for NTM infections.
In conclusion, this study showed that topical use of mupirocin and gentamicin in an alternating regimen was as effective as gentamicin alone in preventing ESIs except for P. aeruginosa. However, this regimen was inferior to gentamicin alone in the prevention of peritonitis, especially gram-negative episodes, and it did not seem to help avoiding the emergence of opportunistic infections but associated with a higher incidence of antibiotic-related fungal peritonitis.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
The authors thank the nursing staff of the renal unit of Kwong Wah Hospital for their dedicated care to the patients and the Hong Kong Society of Nephrology Research Grant 2008/09 in supporting this study.
REFERENCES
- 1. Gaosi Xu, Weiping Tu, Chengyun Xu. Mupirocin for preventing exit-site infection and peritonitis in patients undergoing peritoneal dialysis. Nephrol Dial Transplant 2010; 25:587–92. [DOI] [PubMed] [Google Scholar]
- 2. Bernardini J, Piraino B, Holley J, Johnston JR, Lutes R. A randomized trial of Staphylococcus aureus prophylaxis in peritoneal dialysis patients: mupirocin calcium ointment 2% applied to the exit site versus cyclic oral rifampin. Am J Kidney Dis 1996; 27:695–700. [DOI] [PubMed] [Google Scholar]
- 3. Bernardini J, Bender F, Florio T, Sloand J, Palmmontalbano L, Fried L, et al. Randomized, double-blind trial of antibiotic exit site cream for prevention of exit site infection in peritoneal dialysis patients. J Am Soc Nephrol 2005; 16:539–45. [DOI] [PubMed] [Google Scholar]
- 4. Tse KC, Lui SL, Cheng VC, Yip TP, Lo WK. A cluster of rapidly growing mycobacterial peritoneal dialysis catheter exit-site infections. Am J Kidney Dis 2007; 50(1):e1-5. [DOI] [PubMed] [Google Scholar]
- 5. Lo MW, Mak SK, Wong YY, Lo KC, Chan SF, Tong GM, et al. Atypical mycobacterial exit-site infection and peritonitis in peritoneal dialysis patients on prophylactic exit-site gentamicin cream. Perit Dial Int 2013; 33(3):267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chu KH, Choy WY, Cheung CCW, Fung KS, Tang HL, Lee W, et al. A prospective study of the efficacy of local application of gentamicin versus mupirocin in the prevention of peritoneal dialysis catheter-related infections. Perit Dial Int 2008; 28:505–8. [PubMed] [Google Scholar]
- 7. Mahaldar A, Weisz M, Kathuria P. Comparison of gentamicin and mupirocin in the prevention of exit-site infection and peritonitis in peritoneal dialysis. Adv Perit Dial 2009; 25:56–9 . [PubMed] [Google Scholar]
- 8. Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, Lye WC, et al. ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int 2011; 31(6):614–30. [DOI] [PubMed] [Google Scholar]
- 9. Pierce DA, Williamson JC, Mauck VS, Russell GB, Palavecino E, Burkart JM. The effect on peritoneal dialysis pathogens of changing topical antibiotic prophylaxis. Perit Dial Int 2012; 32: 525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piraino B, Bernardini J. Catheter-related peritonitis. Perit Dial Int 2013; 33(6):592–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Twardowski ZJ, Dobbie JW, Moore HL, Nichols WK, DeSpain JD, Anderson PC, et al. Morphology of peritoneal dialysis catheter tunnel: macroscopy and light microscopy. Perit Dial Int 1991; 11:237–51. [PubMed] [Google Scholar]
- 12. Davey P, Craig A, Hau C, Malek M. Cost-effectiveness of prophylactic nasal mupirocin in patients undergoing peritoneal dialysis based on a randomized placebo-controlled trial. J Antimicrob Chemother 1999; 43:105–12. [DOI] [PubMed] [Google Scholar]
- 13. McQuillan RF, Chiu E, Nessim S, Lok CE, Roscoe JM, Tam P, et al. A randomized controlled trial comparing mupirocin and polysporin triple ointments in peritoneal dialysis patients: the MP3 Study. Clin J Am Soc Nephrol 2012; 7:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson DW, Clark C, Isbel NM, Hawley CM, Beller E, Cass A, et al. Antibacterial honey for the prevention of peritoneal-dialysis-related infections (HONEYPOT): a randomised trial. Lancet Infect Dis 2014; 14:23–30. [DOI] [PubMed] [Google Scholar]
- 15. Piraino B, Bernardini J, Bender FH. An analysis of methods to prevent peritoneal dialysis catheter infections. Perit Dial Int 2008; 28:437–43. [PubMed] [Google Scholar]