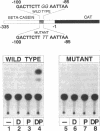
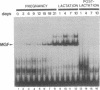
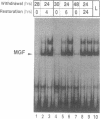
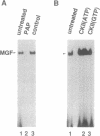
Abstract
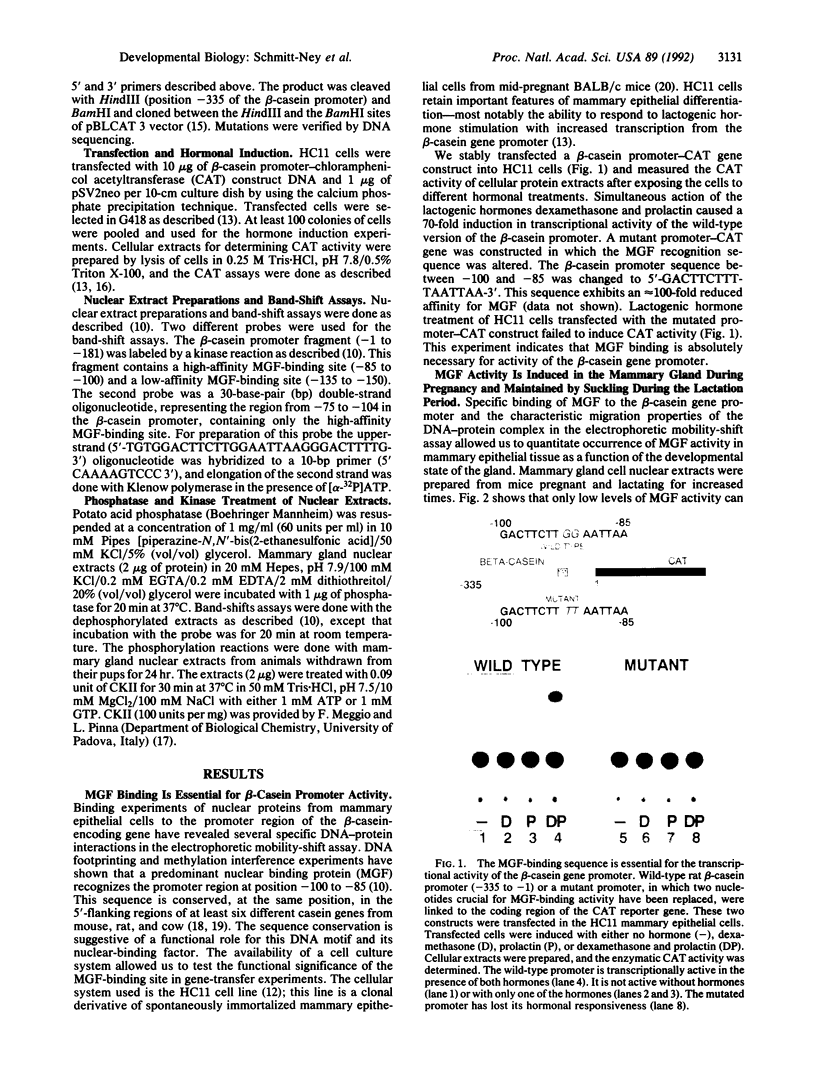
During the lactation period, mammary epithelial cells secrete large amounts of milk proteins. The coordinate regulation of milk protein expression is effected by the lactogenic hormones. We have investigated the activity of a mammary gland-specific transcription factor (MGF), which mediates hormonal influences at the level of a milk protein gene promoter. MGF-binding sites are present in the promoters of the most abundantly expressed milk protein genes. Mutation of the MGF-binding site in the beta-casein gene promoter completely abolishes responsiveness of the promoter to lactogenic hormones in cultured mammary epithelial cells. MGF activity is closely controlled in vivo. High MGF levels were found in mouse mammary gland nuclear extracts toward the end of pregnancy and during lactation. Withdrawal of suckling pups from their mothers during the lactation period caused a strong and rapid decrease of MGF activity. Readdition of pups to their mothers restored maximal MGF levels within 4 hr. We investigated MGF phosphorylation as a possible posttranslational modification responsible for regulation of the DNA-binding activity of MGF. Treatment of nuclear extracts from lactating mammary glands with potato acid phosphatase abolished MGF-binding activity. Casein kinase II phosphorylation of nuclear extracts from animals withdrawn from their pups for 24 hr enhanced MGF-binding activity. These results suggest that the reversible activation of MGF by suckling and withdrawal might be mediated by the action of kinases and phosphatases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Osheroff N. Regulation of casein kinase II activity by epidermal growth factor in human A-431 carcinoma cells. J Biol Chem. 1989 Jul 15;264(20):11958–11965. [PubMed] [Google Scholar]
- Ball R. K., Friis R. R., Schoenenberger C. A., Doppler W., Groner B. Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 1988 Jul;7(7):2089–2095. doi: 10.1002/j.1460-2075.1988.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Animal cell shape changes and gene expression. Bioessays. 1991 May;13(5):207–212. doi: 10.1002/bies.950130502. [DOI] [PubMed] [Google Scholar]
- Blackburn D. E., Hobbs A. A., Rosen J. M. Rat beta casein cDNA: sequence analysis and evolutionary comparisons. Nucleic Acids Res. 1982 Apr 10;10(7):2295–2307. doi: 10.1093/nar/10.7.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmann D. Transcription factor phosphorylation: a link between signal transduction and the regulation of gene expression. Cancer Cells. 1990 Nov;2(11):337–344. [PubMed] [Google Scholar]
- Boxer L. M., Prywes R., Roeder R. G., Kedes L. The sarcomeric actin CArG-binding factor is indistinguishable from the c-fos serum response factor. Mol Cell Biol. 1989 Feb;9(2):515–522. doi: 10.1128/mcb.9.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Danielson K. G., Oborn C. J., Durban E. M., Butel J. S., Medina D. Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3756–3760. doi: 10.1073/pnas.81.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppler W., Groner B., Ball R. K. Prolactin and glucocorticoid hormones synergistically induce expression of transfected rat beta-casein gene promoter constructs in a mammary epithelial cell line. Proc Natl Acad Sci U S A. 1989 Jan;86(1):104–108. doi: 10.1073/pnas.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodetsky S. I., Tkach T. M., Kapelinskaya T. V. Isolation and characterization of the Bos taurus beta-casein gene. Gene. 1988 Jun 15;66(1):87–96. doi: 10.1016/0378-1119(88)90227-2. [DOI] [PubMed] [Google Scholar]
- Grosvenor C. E., Mena F., Whitworth N. S. The secretion rate of prolactin in the rat during suckling and its metabolic clearance rate after increasing intervals of nonsuckling. Endocrinology. 1979 Feb;104(2):372–376. doi: 10.1210/endo-104-2-372. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. A., Richards D. A., Kessler D. J., Rosen J. M. Complex hormonal regulation of rat casein gene expression. J Biol Chem. 1982 Apr 10;257(7):3598–3605. [PubMed] [Google Scholar]
- Horak I. D., Gress R. E., Lucas P. J., Horak E. M., Waldmann T. A., Bolen J. B. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves the activation of p56lck. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1996–2000. doi: 10.1073/pnas.88.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdebine L. M., Djiane J., Dusanter-Fourt I., Martel P., Kelly P. A., Devinoy E., Servely J. L. Hormonal action controlling mammary activity. J Dairy Sci. 1985 Feb;68(2):489–500. doi: 10.3168/jds.S0022-0302(85)80848-1. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Taverna D., Harwerth I. M., Ciardiello F., Salomon D. S., Yamamoto T., Groner B. Epidermal growth factor receptor, but not c-erbB-2, activation prevents lactogenic hormone induction of the beta-casein gene in mouse mammary epithelial cells. Mol Cell Biol. 1990 Aug;10(8):4027–4034. doi: 10.1128/mcb.10.8.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarlund J. K., Czech M. P. Insulin-like growth factor I and insulin rapidly increase casein kinase II activity in BALB/c 3T3 fibroblasts. J Biol Chem. 1988 Nov 5;263(31):15872–15875. [PubMed] [Google Scholar]
- Koprowski J. A., Tucker H. A. Bovine serum growth hormone, corticoids and insulin during lactation. Endocrinology. 1973 Sep;93(3):645–651. doi: 10.1210/endo-93-3-645. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Macleod R. M. Regulation of prolactin secretion at the level of the lactotroph. Physiol Rev. 1990 Apr;70(2):279–318. doi: 10.1152/physrev.1990.70.2.279. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manak J. R., Prywes R. Mutation of serum response factor phosphorylation sites and the mechanism by which its DNA-binding activity is increased by casein kinase II. Mol Cell Biol. 1991 Jul;11(7):3652–3659. doi: 10.1128/mcb.11.7.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manak J. R., de Bisschop N., Kris R. M., Prywes R. Casein kinase II enhances the DNA binding activity of serum response factor. Genes Dev. 1990 Jun;4(6):955–967. doi: 10.1101/gad.4.6.955. [DOI] [PubMed] [Google Scholar]
- Meggio F., Perich J. W., Reynolds E. C., Pinna L. A. Phosphotyrosine as a specificity determinant for casein kinase-2, a growth related Ser/Thr-specific protein kinase. FEBS Lett. 1991 Feb 25;279(2):307–309. doi: 10.1016/0014-5793(91)80174-2. [DOI] [PubMed] [Google Scholar]
- Mieth M., Boehmer F. D., Ball R., Groner B., Grosse R. Transforming growth factor-beta inhibits lactogenic hormone induction of beta-casein expression in HC11 mouse mammary epithelial cells. Growth Factors. 1990;4(1):9–15. doi: 10.3109/08977199009011005. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Pinna L. A. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990 Sep 24;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Schmitt-Ney M., Doppler W., Ball R. K., Groner B. Beta-casein gene promoter activity is regulated by the hormone-mediated relief of transcriptional repression and a mammary-gland-specific nuclear factor. Mol Cell Biol. 1991 Jul;11(7):3745–3755. doi: 10.1128/mcb.11.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani Y., Oka T. Epidermal growth factor stimulates cell proliferation and inhibits functional differentiation of mouse mammary epithelial cells in culture. Endocrinology. 1983 Sep;113(3):871–877. doi: 10.1210/endo-113-3-871. [DOI] [PubMed] [Google Scholar]
- Taverna D., Groner B., Hynes N. E. Epidermal growth factor receptor, platelet-derived growth factor receptor, and c-erbB-2 receptor activation all promote growth but have distinctive effects upon mouse mammary epithelial cell differentiation. Cell Growth Differ. 1991 Mar;2(3):145–154. [PubMed] [Google Scholar]
- Topper Y. J., Freeman C. S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980 Oct;60(4):1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Vonderhaar B. K., Ziska S. E. Hormonal regulation of milk protein gene expression. Annu Rev Physiol. 1989;51:641–652. doi: 10.1146/annurev.ph.51.030189.003233. [DOI] [PubMed] [Google Scholar]
- Voogt J. L., Sar M., Meites J. Influence of cycling, pregnancy, labor, and suckling on corticosterone-ACTH levels. Am J Physiol. 1969 Mar;216(3):655–658. doi: 10.1152/ajplegacy.1969.216.3.655. [DOI] [PubMed] [Google Scholar]
- Watson P. A. Function follows form: generation of intracellular signals by cell deformation. FASEB J. 1991 Apr;5(7):2013–2019. doi: 10.1096/fasebj.5.7.1707019. [DOI] [PubMed] [Google Scholar]
- Wuarin J., Schibler U. Expression of the liver-enriched transcriptional activator protein DBP follows a stringent circadian rhythm. Cell. 1990 Dec 21;63(6):1257–1266. doi: 10.1016/0092-8674(90)90421-a. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Oka T. Isolation and structural analysis of the mouse beta-casein gene. Gene. 1989 May 30;78(2):267–275. doi: 10.1016/0378-1119(89)90229-1. [DOI] [PubMed] [Google Scholar]