Abstract
Deciphering mechanisms of drug resistance is crucial to winning the battle against cancer. A new study points to an unexpected function of YAP in drug resistance and illuminates its potential role as a therapeutic target.
Cancer is a disease of the genome, in which mutations disrupt genes that control normal cellular proliferation and lead to malignant growth. In spite of the overwhelming complexity of cancer, recent years have brought significant advances in understanding its genetic landscape and the molecular mechanisms underlying tumor initiation and progression1. Several key ‘driver’ genes, central to cancer development, were identified and characterized2. Analysis of the molecular roles of these genes and the pathways in which they reside enabled the development of promising targeted therapies. One example is RAF and MEK inhibitors, which target oncogenic BRAF and its downstream effectors, preventing the abnormal activation of the mitogen-activated protein kinase (MAPK) pathway common to many malignancies3. Despite significant clinical responses to such targeted therapies, full remission is rarely durable, as nearly all tumors acquire resistance4. Resistance mechanisms, which are poorly understood and vary considerably, are extremely important to decipher, as an understanding of them allows the design of novel targeted therapies aimed at preventing resistance. In this issue of Nature Genetics, Trever Bivona and colleagues add another piece to the puzzle of cancer genetics by identifying YAP as a key survival input that mediates resistance by acting in parallel to other known pathways in tumor progression5.
YAP behind the wheel
The authors performed a genetic screen to identify factors increasing the efficacy of the RAF inhibitor vemurafenib in cancer cells harboring the BRAF V600E alteration. They identified YAP1 (encoding Yes-associated protein, YAP) as a key factor in resistance to RAF inhibitor therapy. YAP is one of the two main effectors of the Hippo tumor-suppressor pathway. Silencing of YAP1 increased cell sensitivity to RAF and MEK inhibitors, with this effect demonstrated across different genetic backgrounds and in a variety of tumor models—lung, melanoma, colon, thyroid and pancreatic cancer cell lines. Combined YAP and RAF or MEK inhibition was found to be lethal in both BRAF- and RAS-mutant tumors. Notably, these results suggest that the synthetic lethal relationship between YAP and MEK inhibitors is more significant in cells harboring mutations in BRAF than in RAS genes.
It takes two to tango
The authors suggest that YAP is a survival input that acts in parallel to the well-characterized MAPK signaling cascade, preventing apoptosis and enhancing overall survival signaling. According to this model, when both MAPK signaling and YAP are active, cells proliferate and resist apoptosis, leading to active disease progression. ‘Turning off’ only MAPK signaling results in decreased proliferation, but cells remain resistant to apoptosis and the response to treatment is thus incomplete. Only when both the MAPK pathway and YAP are inactivated do inhibition of proliferation and activation of apoptosis follow, leading to a complete response to treatment.
Assessment of YAP expression in tumors that harbored mutant BRAF showed increased YAP levels in patients with a worse response to RAF inhibitors. Furthermore, elevated YAP levels were found in tumors that acquired resistance in comparison to tumor samples obtained before treatment from the same patients. Thus, not only is YAP a biomarker of decreased response to RAF and MEK inhibitors in BRAF-mutant patients, but increased YAP levels also limit the clinical efficacy of RAF and MEK inhibitors.
It is well established that active YAP promotes cell proliferation6, epithelial-to-mesenchymal transition, invasion and metastasis7,8. But what triggers YAP activation? In KRAS-silenced pancreatic cancer cells, chromosomal amplifications increase YAP1 copy number9. However, no amplifications are found in colorectal and lung cancers, implying regulation of the YAP protein at the transcriptional level. In uveal melanoma, transcription is stimulated by translocation of YAP into the nucleus10. The transcriptional activity of YAP is mediated by different transcription factors, depending on the tumor type: in pancreatic cancer, YAP interacts with TEAD2 to regulate the cell cycle and DNA replication9, whereas in colon and lung cancers its interaction with FOS, a member of the AP-1 transcription factor family, induces epithelial-to-mesenchymal transition11. The findings of Lin et al. expand the role of YAP to mediating drug resistance, in the context of both oncogenic BRAF and NRAS. Although this and other recent studies highlight the complexity of YAP regulation, they also emphasize its value as a potential therapeutic target.
YAP in the bull’s-eye
As active YAP promotes resistance to RAF- and MEK-targeted inhibitor therapy, disrupting YAP activity is predicted to enhance the response to RAF and MEK inhibitors in patients with an activated MAPK pathway. This includes patients who harbor either BRAF or RAS gene mutations. As activating mutations in RAS genes are among the most frequent events in numerous human carcinomas12 and no inhibitors targeting active RAS proteins are available, further investigating this new avenue of concurrently targeting MEK and YAP is essential (Fig. 1). Indeed, specific inhibitors targeting YAP-TEAD complexes have already been developed13 and show some promise in animal models14. Importantly, this work, as well as other studies15, emphasizes the need to develop drugs targeting master transcriptional regulators, common to a wide range of malignancies.
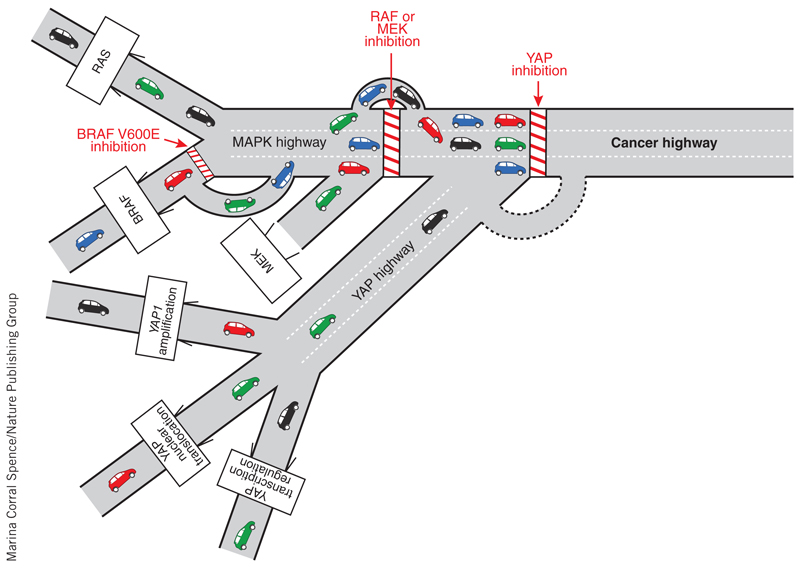
Figure 1.
Bypassing barriers on the cancer highway. Inhibition of YAP resensitizes a wide range of malignancies (cars) to BRAF and MEK inhibitors and prevents the progression of resistant lesions. The dotted line indicates potential resistance.
Finally, this report emphasizes the fact that in many cases different mutations activate similar pathways and may be treated by similar drugs, regardless of the tissue in which the tumor originated. However, the full complexities of cancer-associated genetic alterations and the intricate interactions between different players, as well as various drug resistance mechanisms, still elude us. Although the road to efficient treatment is still long, these findings are likely to encourage further research into targeting YAP in tumors and are likely to have clinical impact.
Acknowledgments
Work in the laboratory of Y.S. is supported by the Israeli Science Foundation (1604/13; 877/13), the European Research Council (StG-335377), and the Knell and Hamburger families.
Footnotes
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Vogelstein B, et al. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garraway LA, Lander ES. Cell. 2013;153:17–37. doi: 10.1016/j.cell.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty KT, Hodi FS, Fisher DE. Nat Rev Cancer. 2012;12:349–361. doi: 10.1038/nrc3218. [DOI] [PubMed] [Google Scholar]
- 4.Emery CM, et al. Proc Natl Acad Sci USA. 2009;106:20411–20416. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin L, et al. Nat Genet. 2015;47:250–256. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang K, Degerny C, Xu M, Yang XJ. Biochem Cell Biol. 2009;87:77–91. doi: 10.1139/O08-114. [DOI] [PubMed] [Google Scholar]
- 7.Overholtzer M, et al. Proc Natl Acad Sci USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamar JM, et al. Proc Natl Acad Sci USA. 2012;109:E2441–E2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapoor A, et al. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng X, et al. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shao DD, et al. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downward J. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 13.Stanger BZ. Genes Dev. 2012;26:1263–1267. doi: 10.1101/gad.196501.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu-Chittenden Y, et al. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung CH, Chan DS, Ma VP, Ma DL. Med Res Rev. 2013;33:823–846. doi: 10.1002/med.21266. [DOI] [PubMed] [Google Scholar]